Abstract
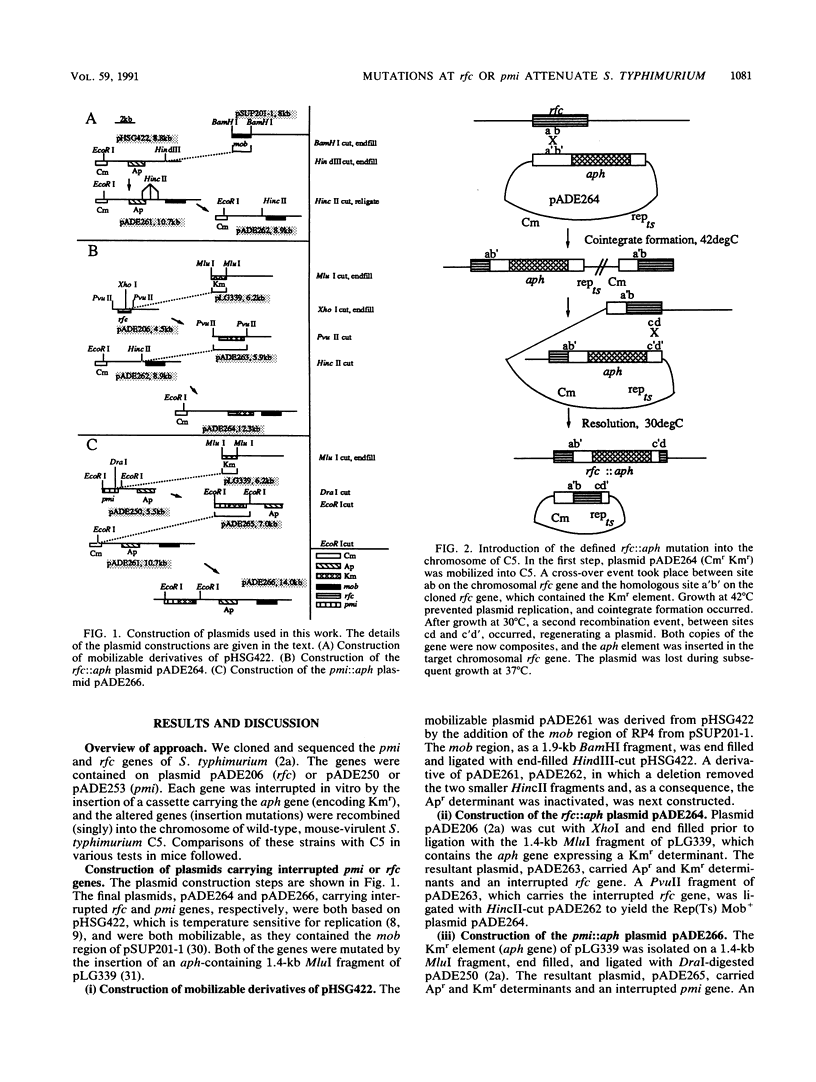
Insertion mutations were constructed in cloned pmi and rfc genes of Salmonella typhimurium, and these mutations were recombined (singly) into the chromosome of mouse-virulent S. typhimurium C5, displacing the wild-type alleles. Phage sensitivity profiles, lipopolysaccharide analysis, and DNA blotting all confirmed that the replacement events had occurred. The mutations were complemented by plasmid-borne wild-type alleles, as judged by the restoration of wild-type phage plaquing profiles and lipopolysaccharide production (both mutants) and the restoration of pmi-encoded enzyme production (pmi mutant). The virulence, persistence, and immunizing capacities of the mutants fed to mice were compared with those of the wild-type strain and complemented mutants. Both mutants were much reduced in virulence, with the rfc mutant being avirulent even at 10(9) bacteria per mouse. This mutant was also avirulent at up to 10(6) bacteria per mouse when administered intraperitoneally. Both the rfc and pmi mutant strains persisted in the Peyer's patches of the gut after feeding and were capable of colonizing the deeper tissues of the mice from such initial infective foci. Both mutant strains were effective as live oral vaccines (10(7) bacteria or more) against oral S. typhimurium challenge (10(4) 50% lethal doses; 6 x 10(8) bacteria) in mice.
Full text
PDF


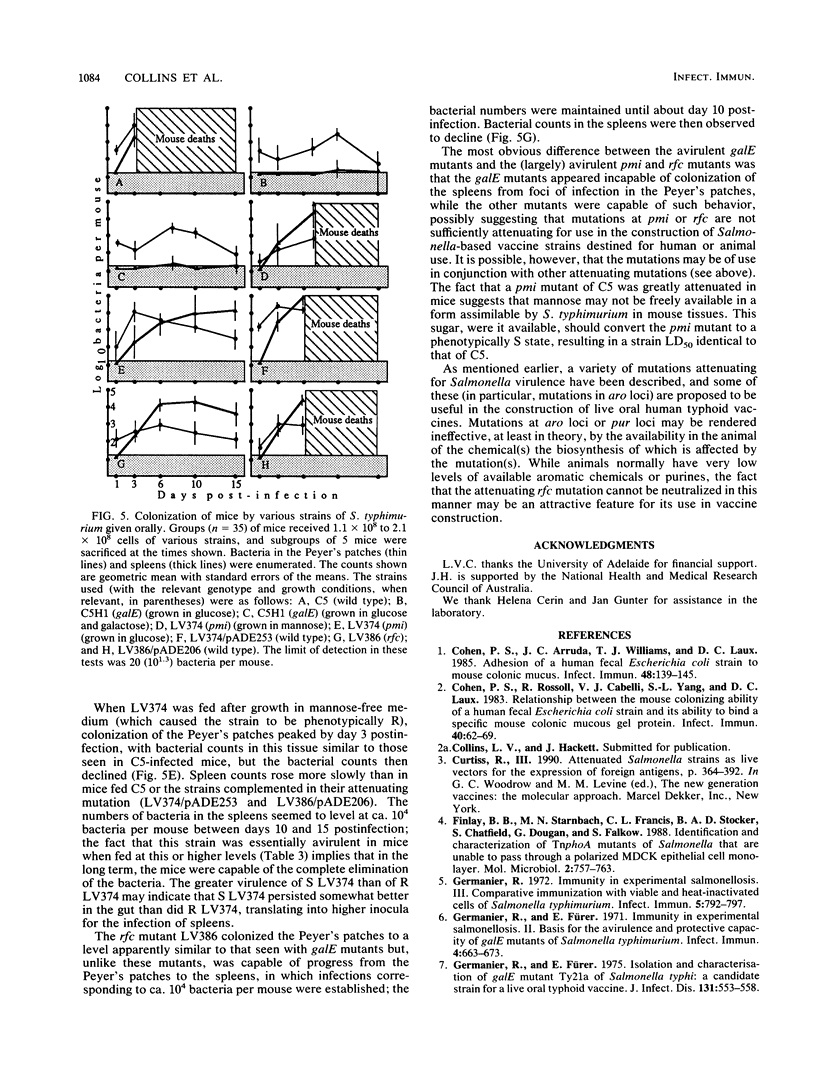

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Starnbach M. N., Francis C. L., Stocker B. A., Chatfield S., Dougan G., Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988 Nov;2(6):757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E. Immunity in experimental salmonellosis. II. Basis for the avirulence and protective capacity of gal E mutants of Salmonella typhimurium. Infect Immun. 1971 Dec;4(6):663–673. doi: 10.1128/iai.4.6.663-673.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in experimental salmonellosis. 3. Comparative immunization with viable and heat-inactivated cells of Salmonella typhimurium. Infect Immun. 1972 May;5(5):792–797. doi: 10.1128/iai.5.5.792-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Sekiguchi M. Mutations of temperature sensitivity in R plasmid pSC101. J Bacteriol. 1977 Aug;131(2):405–412. doi: 10.1128/jb.131.2.405-412.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D. M., Attridge S. R., Forrest B., Morona R., Daniels D., LaBrooy J. T., Bartholomeusz R. C., Shearman D. J., Hackett J. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988 May;56(5):1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D., Hackett J. Vaccination against enteric bacterial diseases. Rev Infect Dis. 1989 Nov-Dec;11(6):853–877. doi: 10.1093/clinids/11.6.853. [DOI] [PubMed] [Google Scholar]
- Hone D., Morona R., Attridge S., Hackett J. Construction of defined galE mutants of Salmonella for use as vaccines. J Infect Dis. 1987 Jul;156(1):167–174. doi: 10.1093/infdis/156.1.167. [DOI] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Markovitz A. Induction of capsular polysaccharide synthesis by rho-fluorophenylalanine in Escherichia coli wild type and strains with altered phenylalanyl soluble ribonucleic acid synthetase. J Bacteriol. 1967 Feb;93(2):584–591. doi: 10.1128/jb.93.2.584-591.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Steward J. P., Roantree R. J. Characterization of the virulence and antigenic structure of Salmonella typhimurium strains with lipopolysaccharide core defects. Infect Immun. 1976 Jun;13(6):1539–1542. doi: 10.1128/iai.13.6.1539-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz C. S., Deibel R. H. Effect of lipopolysaccharide mutations on the pathogenesis of experimental Salmonella gastroenteritis. Infect Immun. 1983 Apr;40(1):236–244. doi: 10.1128/iai.40.1.236-244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczenski-Wildey M. J., Di Fabio J. L., Cabello F. C. Invasion and lysis of HeLa cell monolayers by Salmonella typhi: the role of lipopolysaccharide. Microb Pathog. 1989 Feb;6(2):143–152. doi: 10.1016/0882-4010(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Myhal M. L., Cohen P. S., Laux D. C. Altered colonizing ability for mouse large intestine of a surface mutant of a human faecal isolate of Escherichia coli. J Gen Microbiol. 1983 May;129(5):1549–1558. doi: 10.1099/00221287-129-5-1549. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Valtonen V. V., Valtonen M. Role of O-antigen (lipopolysaccharide) factors in the virulence of Salmonella. J Infect Dis. 1973 Jul;128(Suppl):81–85. doi: 10.1093/infdis/128.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- NAIDE Y., NIKAIDO H., MAEKELAE P. H., WILKINSON R. G., STOCKER B. A. SEMIROUGH STRAINS OF SALMONELLA. Proc Natl Acad Sci U S A. 1965 Jan;53:147–153. doi: 10.1073/pnas.53.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- Nevola J. J., Stocker B. A., Laux D. C., Cohen P. S. Colonization of the mouse intestine by an avirulent Salmonella typhimurium strain and its lipopolysaccharide-defective mutants. Infect Immun. 1985 Oct;50(1):152–159. doi: 10.1128/iai.50.1.152-159.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]





