Abstract
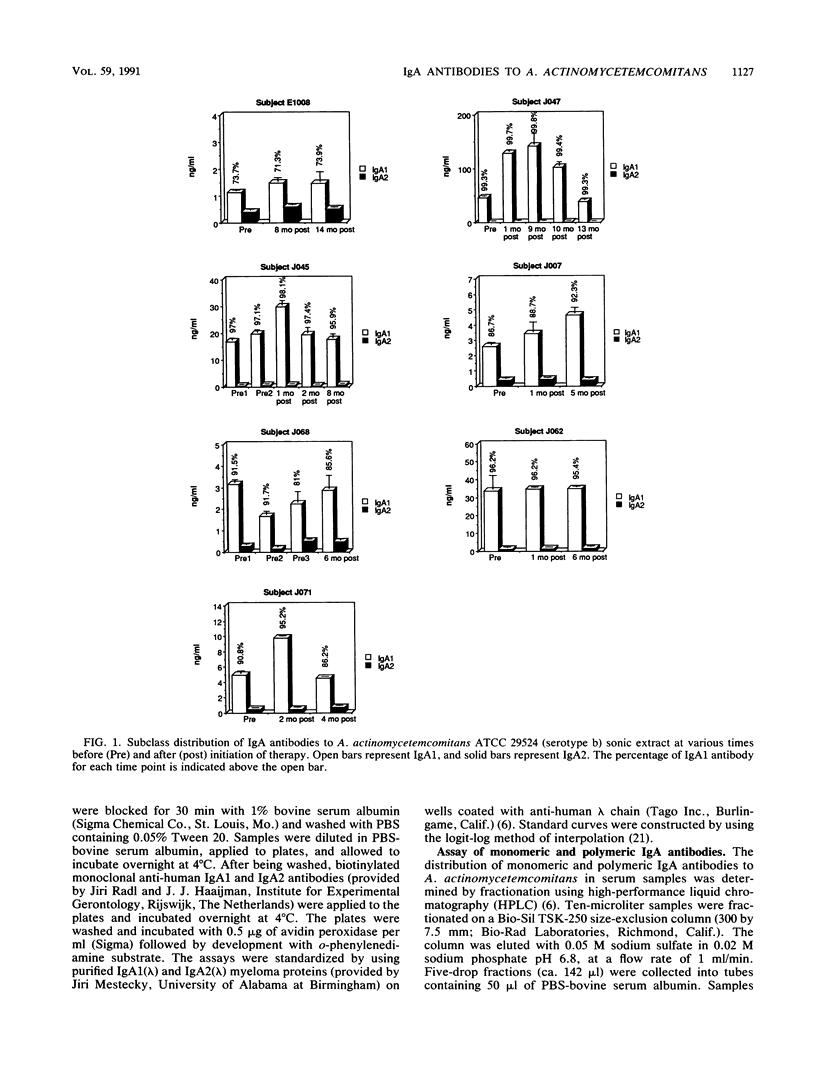
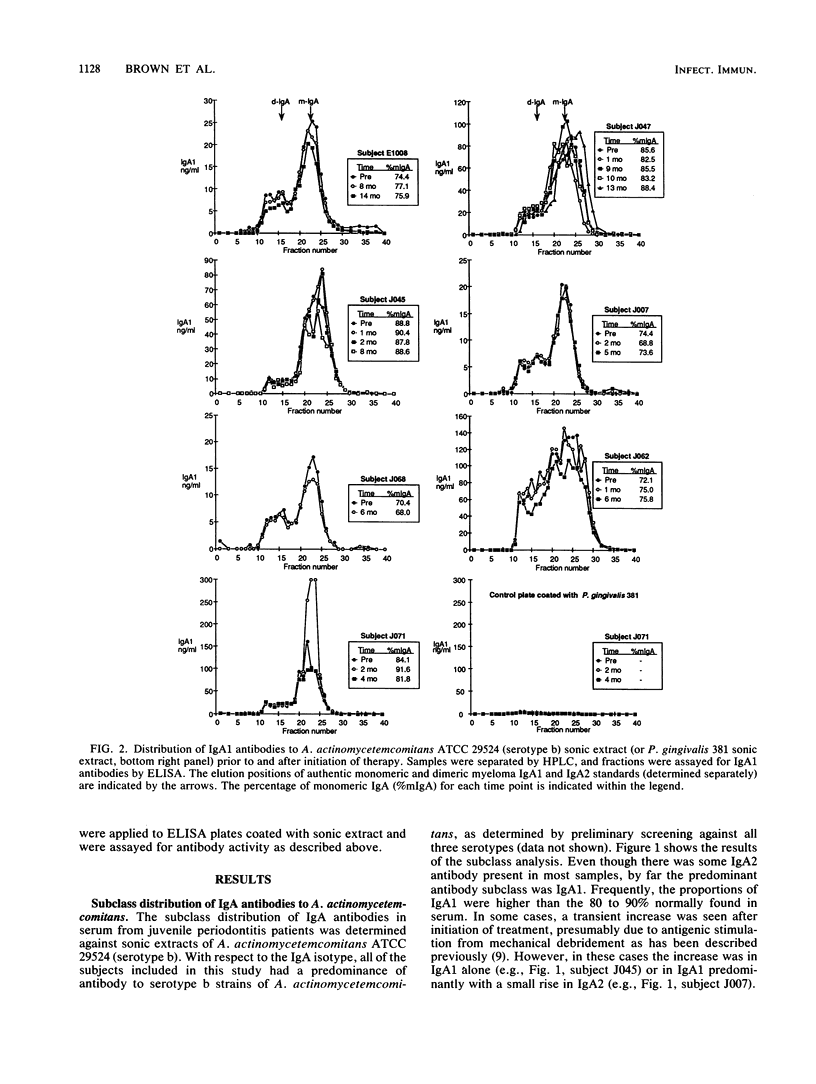
Patients with juvenile periodontitis frequently have elevated levels of serum immunoglobulin A (IgA) antibodies to antigens of Actinobacillus actinomycetemcomitans. IgA occurs in two subclasses, IgA1 and IgA2, and in monomeric and polymeric forms. Because IgA1 is susceptible to cleavage by IgA1 proteases produced by microorganisms found at mucosal sites and in the gingival crevice, we wished to determine the IgA subclass distribution of antibodies to antigens of A. actinomycetemcomitans. The molecular form was examined because it may indicate the origin of the IgA and because the form differs in acute and chronic infections. There is also evidence that monomeric and polymeric IgA have different biological functions. Serum was taken from patients with juvenile periodontitis before and at intervals during and after initiation of therapy. IgA subclass distribution was determined against a sonic extracts of A. actinomycetemcomitans ATCC 2952a (serotype b) by using monoclonal anti-subclass reagents in an enzyme-linked immunosorbent assay. To determine the molecular form of the antibodies, sera were separated by high-performance liquid chromatography on a size-exclusion column. Fractions were assayed for antibody activity by the enzyme-linked immunosorbent assay, and described above. The results of the subclass analysis of the sera indicated that while both IgA1 and IgA2 antibodies to A. actinomycetemcomitans sonic extract are often found before, during, and after treatment, IgA1 antibodies dominated the response. There was a predominance of monomeric IgA1 antibodies to A. actinomycetemcomitans sonic extracts in most samples before, during, and after treatment. The monomeric form is consistent with what is seen in other chronic infections. The predominance of IgA1 antibodies implies that any protective effects of the IgA response to A. actinomycetemcomitans could be compromised by microbial IgA1 proteases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomeusz R. C., Forrest B. D., Labrooy J. T., Ey P. L., Pyle D., Shearman D. J., Rowley D. The serum polymeric IgA antibody response to typhoid vaccination; its relationship to the intestinal IgA response. Immunology. 1990 Feb;69(2):190–194. [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Clements M. L., Murphy B. R., Radl J., Haaijman J. J., Mestecky J. Molecular form and subclass distribution of IgA antibodies after immunization with live and inactivated influenza A vaccines. Adv Exp Med Biol. 1987;216B:1691–1700. [PubMed] [Google Scholar]
- Brown T. A., Mestecky J. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect Immun. 1985 Aug;49(2):459–462. doi: 10.1128/iai.49.2.459-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Murphy B. R., Radl J., Haaijman J. J., Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985 Aug;22(2):259–264. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Hodgson H. J., McPherson A., Dive C., Vaerman J. P. Selective transport of polymeric immunoglobulin A in bile. Quantitative relationships of monomeric and polymeric immunoglobulin A, immunoglobulin M, and other proteins in serum, bile, and saliva. J Clin Invest. 1982 Aug;70(2):230–241. doi: 10.1172/JCI110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J., Genco R. J., Frey D. E. Human immune responses to oral micro-organisms. I. Association of localized juvenile periodontitis (LJP) with serum antibody responses to Actinobacillus actinomycetemcomitans. Clin Exp Immunol. 1982 Jan;47(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J., Haffajee A. D. Effect of subgingival scaling on systemic antibody responses to oral microorganisms. Infect Immun. 1985 May;48(2):534–539. doi: 10.1128/iai.48.2.534-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascart-Lemone F., Carlsson B., Jalil F., Hahn-Zoric M., Duchateau J., Hanson L. A. Polymeric and monomeric IgA response in serum and milk after parenteral cholera and oral typhoid vaccination. Scand J Immunol. 1988 Oct;28(4):443–448. doi: 10.1111/j.1365-3083.1988.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Mascart-Lemone F., Duchateau J., Conley M. E., Delacroix D. L. A polymeric IgA response in serum can be produced by parenteral immunization. Immunology. 1987 Aug;61(4):409–413. [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Kilian M. Immunoglobulin A (IgA). Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Russell M. W., Jackson S., Brown T. A. The human IgA system: a reassessment. Clin Immunol Immunopathol. 1986 Jul;40(1):105–114. doi: 10.1016/0090-1229(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Moldoveanu Z., Brown T. A., Ventura M. T., Michalek S. M., McGhee J. R., Mestecky J. IgA subclass responses to lipopolysaccharide in humans. Adv Exp Med Biol. 1987;216B:1199–1205. [PubMed] [Google Scholar]
- Negro Ponzi A., Merlino C., Angeretti A., Penna R. Virus-specific polymeric immunoglobulin A antibodies in serum from patients with rubella, measles, varicella, and herpes zoster virus infections. J Clin Microbiol. 1985 Oct;22(4):505–509. doi: 10.1128/jcm.22.4.505-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Douchin M., Tokuda A., Sato F., Ito S., Kohli Y., Fujiki N. Clinical significance of immunoglobulin A antibody to hepatitis B core antigen of polymeric and monomeric forms in chronic type B liver disease with acute exacerbation. Am J Gastroenterol. 1988 Dec;83(12):1390–1394. [PubMed] [Google Scholar]
- Outlaw M. C., Dimmock N. J. Mechanisms of neutralization of influenza virus on mouse tracheal epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the viral haemagglutinin. J Gen Virol. 1990 Jan;71(Pt 1):69–76. doi: 10.1099/0022-1317-71-1-69. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Lue C., Moldoveanu Z., Kiyono H., McGhee J. R., Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J Immunol. 1990 May 15;144(10):3770–3778. [PubMed] [Google Scholar]
- Taylor H. P., Dimmock N. J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985 Jan 1;161(1):198–209. doi: 10.1084/jem.161.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. E., Horoszewicz H. U., Cianciola L. J., Genco R. J. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980 Jan;27(1):124–132. doi: 10.1128/iai.27.1.124-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



