Abstract
Exposure to heavy-ion radiation is considered a potential health risk in long-term space travel. In the central nervous system (CNS), loss of critical cellular components may lead to performance decrements that could ultimately compromise mission goals and long-term quality of life. Hippocampal-dependent cognitive impairments occur after exposure to ionizing radiation, and while the pathogenesis of this effect is not yet clear, it may involve the production of newly born neurons (neurogenesis) in the hippocampal dentate gyrus. We irradiated mice with 0.5–4 Gy of 56Fe ions and 2 months later quantified neurogenesis and numbers of activated microglia as a measure of neuroinflammation in the dentate gyrus. Results showed that there were few changes after 0.5 Gy, but that there was a dose-related decrease in hippocampal neurogenesis and a dose-related increase in numbers of newly born activated microglia from 0.5–4.0 Gy. While those findings were similar to what was reported after X irradiation, there were also some differences, particularly in the response of newly born glia. Overall, this study showed that hippocampal neurogenesis was sensitive to relatively low doses of 56Fe particles, and that those effects were associated with neuroinflammation. Whether these changes will result in functional impairments or if/how they can be managed are topics for further investigation.
Introduction
Exposure to heavy-ion radiation is considered a potential health risk in long-term space travel. A unique feature of the space radiation environment is the presence of high-energy charged particles (e.g. 56Fe), which could constitute a significant hazard to space flight crews who may be unavoidably exposed in the course of in-flight activities. It has been estimated that during a 3-year deep space exploration, the risk of a traversal of a cell nucleus by charged heavy particles would be 0.6 and 0.03 for carbon and iron ions, respectively (1). Given the high linear energy transfer (LET) of these energetic particles, the probability of survival of affected cells would be fairly small. Loss of critical cellular components in a highly organized structure like the central nervous system (CNS) may lead to performance decrements that ultimately could compromise mission goals and/or long-term quality of life. One of the possible debilitating effects of such exposure could be a cognitive decline similar to that seen in some patients exposed to low-LET radiation during cancer treatment. Such cognitive changes include deficits in hippocampal-dependent functions (2–7), and in fact, experimental data exist suggesting that 56Fe-particle radiation induces significant deficits in spatial learning and memory (8, 9) as well as other behavioral end points (10, 11) in animals.
The underlying pathogenesis of radiation-induced cognitive impairments is not well understood, but recent studies suggest that a number of factors may be involved, including alterations in hippocampal neurogenesis (12–17), apolipoprotein E (18) and glutamate receptor composition (19). A number of studies have addressed the acute response of neurogenic cells in the subgranular zone (SGZ) of the hippocampal dentate gyrus after exposure to low-LET radiation; they showed that proliferating SGZ precursor cells and their progeny, immature neurons, undergo apoptosis shortly after irradiation (20–24). Furthermore, reductions in the generation of new neurons (i.e. neurogenesis) are observed months after exposure (20, 25, 26) and appear to be persistent (15). These effects on neurogenesis are associated with hippocampal-dependent cognitive impairments (12–17, 27, 28).
Considerable data exist showing that X radiation induces microenvironmental changes (e.g. inflammation, oxidative stress) in and around the dentate SGZ and that such changes are associated with altered neurogenesis (15, 20, 25, 26, 29). There are also recent data showing that after irradiation with 1–3 Gy of 56Fe ions, proliferating neural precursor cells and immature neurons exhibit dose-dependent reductions in cell numbers (30, 31) and that such changes may involve indications of neuroinflammation (30, 31). While these latter data give some important insight into the impact of high-LET radiation on cell numbers in neurogenic sites, they do not specifically address the ability of surviving cells to differentiate and express markers of mature cell phenotypes. The present investigation was designed to assess the survival and phenotypic fate of newly born cells in the dentate SGZ and to determine if such findings were associated with changes in numbers of activated microglia, a marker of neuroinflammation.
Materials And Methods
Animals
Twenty-seven male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), 2 months old, were used in these experiments. Mice were housed and cared for in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and institutional IACUCs. Animals were shipped to Brookhaven National Laboratory and allowed to acclimatize for 3–5 days prior to irradiation. After irradiation, the animals were returned to the BNL animal facility and housed for 1–2 days prior to shipment to Loma Linda University by courier. All animals were perfused 2 months after irradiation (see below).
Irradiation
Up to four mice were exposed simultaneously to brain-only radiation using a collimated beam of 600 MeV/nucleon 56Fe particles produced by the AGS Booster Accelerator at Brookhaven National Laboratory (BNL) and transferred to the experimental beam line in the NASA Space Radiation Laboratory (NSRL) facility. The delivered beam was restricted to 1-cm-diameter circular apertures that covered the brain areas of the mice. The collimator design was modeled and fabricated by Inland Technical Services (San Bernardino, CA), and the modeling calculations were done using a modified version of BRYNTRN (32). The collimator consisted of a 10-g/cm2 (8.40-cm) layer of polymethylmethacrylate, followed by a 20-g/cm2 (7.41-cm) layer of aluminum, and finally an 8.5 g/cm2 (8.95 cm) layer of high-density polyethylene. The upstream acrylic layer slowed down the primary ions with low-Z material to minimize fragmentation, followed by the aluminum layer that attenuated the primary particles. The polyethylene layer attenuated low-Z fragments (e.g. protons, neutrons and α particles) produced upstream. The four 1-cm-diameter holes penetrated the material stack. For calibration, an NIST-traceable 1-cm3 Far West thimble chamber with an air-filled bulb and tissue-equivalent walls was placed at the target position behind the collimator apertures. Dose delivery and beam cut-off were controlled by three parallel-plate ion chambers positioned along the beam line upstream and downstream of the target plane and referenced to the calibration chamber. The 56Fe26+ ion beam was extracted at 600 MeV/nucleon, and had an energy at the target surface of 585.1 MeV/nucleon, a residual range of 12.3 cm in water, and an LET of 175.2 keV/μm. The beam was delivered as twenty 300-ms pulses per minute for an average dose rate of ∼5 Gy/min. Delivered doses were ±0.5% of the requested value. The reference number for the NASA-sponsored experimental campaign was NSRL-7. A description of dose composition and fragmentation behind various target materials for similar iron ion beams (1087 MeV/nucleon and 555 MeV/nucleon) produced at the BNL AGS accelerator is given by Zeitlin et al. (33), and a full description of the NSRL facility can be found at www.bnl.gov/medical/NASA/NSRL_description.asp.
Mice were anesthetized with 4% isoflurane and placed in custom bite-bar cradles to stabilize the head position. The cradles were then placed in a clear acrylic anesthesia box prealigned with the collimator and the beam line. Sedation was maintained with 2.5% isoflurane administered throughout the irradiation procedure. A single fraction of 0.5, 1, 2 or 4 Gy was delivered to the brain of each animal. For the entire irradiation procedure, animals were under isoflurane anesthesia for an average of 10 min. Nonirradiated control mice were treated identically without being exposed to radiation.
Neurogenesis
To determine the effects of radiation on the survival and fate of newly born cells in the dentate SGZ, groups (n = 5–6) of mice received a single i.p. injection (50 mg/kg) of 5-bromo-2′deoxyuridine (BrdU, Sigma, St. Louis, MO) daily for 7 days starting 30 days after irradiation. Three weeks after the last BrdU injection, mice were anesthetized with i.p. Nembutal (100 mg/kg; Ovation Pharmaceuticals, Deerfield, IL) and perfused with ice-cold saline followed by freshly prepared, ice-cold 4% para-formaldehyde. The brain was removed, processed, and sectioned using a sliding microtome (20). Fifty-micrometer-thick sections were stored at 4°C in cryoprotectant solution until needed. Free-floating sections were immunostained as described (15, 20) using the following primary antibodies and working concentrations: rat anti-BrdU (1:10; Oxford Biotechnology, Kidlington, Oxford, UK); mouse anti-NeuN (1:200; Chemicon, Temecula, CA); rabbit anti-NG2 (1:200; Chemicon); goat anti-GFAP (1:100, Santa Cruz Biotechnology, Santa Cruz, CA); rat anti-CD68 (1:20; Serotec, Inc. Raleigh, NC). Nuclei were counterstained with DAPI (Molecular Probes, Eugene, OR).
To calculate the numbers of BrdU-positive (BrdU+) cells in the dentate gyrus, at least 12 sections of a one-in-six series were scored per animal (26). All counts were limited to the dentate granule cell layer and a 50-μm border along the hilar margin that included the dentate SGZ. Total numbers were obtained by multiplying the measured value by 6. BrdU+ cells displaying the various lineage-specific phenotypes were counted in representative sections from each animal using confocal microscopy. When possible, ∼100 BrdU-positive cells that co-expressed each lineage-specific phenotype were scored for each marker per animal. For each phenotype, the percentage of BrdU+ cells expressing that marker within the section was determined. Total numbers of lineage-specific BrdU+ cells were then calculated by multiplying this percentage by the total number of BrdU+ cells in the dentate gyrus. Confocal microscopy was performed using a Nikon C-1 confocal microscope (Melville, NY), using techniques described previously (15, 20, 26). Appropriate gain and black-level settings were obtained on control tissues stained with secondary antibodies alone. Upper and lower thresholds were always set using a range indicator function to minimize data loss due to saturation. Each cell was examined manually in its full “z” dimension with use of split panel analysis, and only those cells for which the BrdU+ nucleus was unambiguously associated with the lineage-specific marker were scored as positive.
Total Activated Microglia
The impact of 56Fe-particle radiation on total numbers of activated microglia in the SGZ was determined using free-floating brain sections from groups of mice (n = 5–6). After three washes with Tris-buffered saline (TBS) and quenching of endogenous peroxidase activity in TBS with 3% H2O2 for 15 min, sections were incubated in TSA blocking buffer (PerkinElmer Life Sciences, Emeryville, CA) for 30 min, followed by application of the polyclonal rabbit anti-CD68 antibody (1:20; Serotec, Inc.). After 12 h at 4°C, sections were incubated for 1 h at room temperature with a secondary anti-rabbit biotinylated antibody (Vector, Burlingame, CA), followed by incubation with an Avidin+Biotin amplification system (Vector) for 45 min. Cell staining was visualized using the TSA fluorescence system CY3 (PerkinElmer Life Sciences); nuclear counterstaining was with Sytox-Green (Molecular Probes). No staining was detected in the absence of the primary or secondary antibodies.
Total numbers of activated microglia were counted in each of four coronal sections of the dentate gyrus and hilar regions at the level of the dorsal hippocampus. Images were collected as described previously (34), and mosaics were reconstructed with a Zeiss AxioImager Apotome microscope using a 20× objective; parameters were kept constant across sections. Regions of interest containing the dentate gyrus and hilus were selected using AxioImager imaging software (Zeiss), and the numbers of positive cells were counted within the selected area. To avoid classification errors, we carefully verified that the staining belonged to the cell of interest and not to a dendrite or the cell body of an adjacent cell. The final numbers of activated microglia were expressed as numbers of cells per mm2.
Statistics
For each histology end point, values for all animals in a given treatment group were averaged and the SEM (standard error of mean) was calculated. A t test was then used to compare each radiation treatment to unirradiated controls. Because variability tended to be somewhat higher with larger values, i.e. cell counts, and because a parametric test requires equal variability across treatment groups, we used a log transform of the data to stabilize variability. In the rare case where there were cell values of zero (e.g. NG2), a numerical value of 1 was used. The overall statistical significance level was set at P = 0.05, and adjustment for multiple comparisons was made using the Hochberg procedure (35). With this procedure, if only a subset of the comparisons have P < 0.05, a lower P value is required to declare statistical significance to prevent declaring statistical significance by chance. For example, if there were three comparisons, and one had P > 0.05, then the other two are required to have P < 0.025 to be declared statistically significant. The P values in the text represent the nominal values before the adjustment for multiple comparisons.
Results
The radiation treatments and BrdU injection procedures were well tolerated by all animals; no mouse was lost over the 2-month follow-up period.
BrdU+ cells observed 3 weeks after the last BrdU injection represent the long-term survival of newly born cells in the dentate SGZ. In unirradiated controls, the total number of BrdU+ cells in the dentate gyrus averaged 5,393 ± 526. There appeared to be a dose-related decrease in cell number from 0–2 Gy and a relative increase after 4 Gy (Fig. 1A), but none of the changes were statistically significant.
Fig. 1.
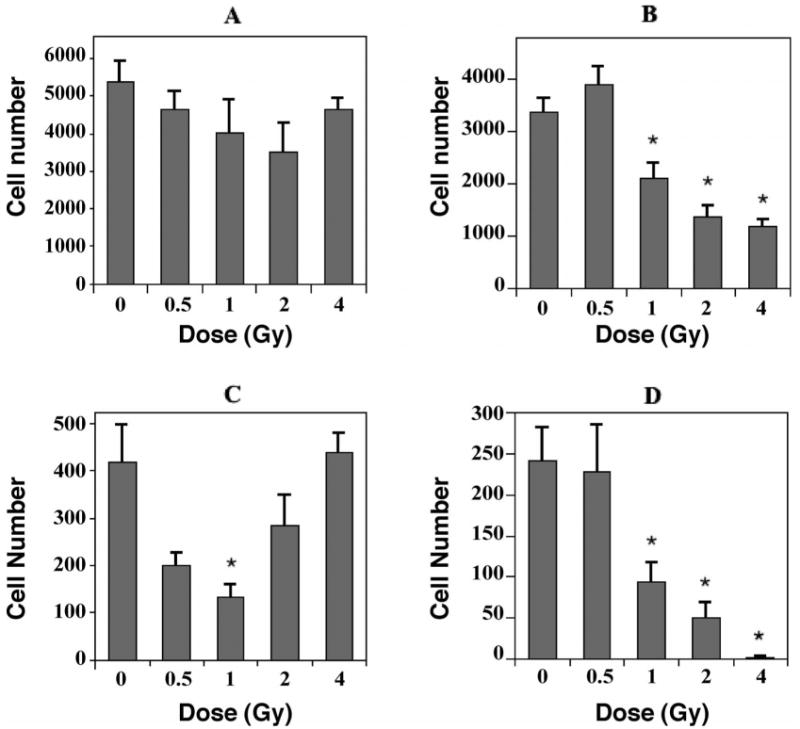
The effects of 56Fe-particle radiation on newly born cells in the dentate subgranular zone of mice. One month after irradiation mice received a single daily injection of 5-bromo-2′deoxyuridine (BrdU) for 7 consecutive days. Three weeks later, tissues were collected and immunohistochemistry and confocal microscopic analyses were done to quantify the numbers of cells co-labeled with BrdU and cell-specific antibodies. Cell numbers represented an estimate of the total number of positively labeled cells in the subgranular zone in both hemispheres. Values for irradiated mice were compared to those for nonirradiated controls. Panel A: Total numbers of BrdU-positive cells decreased slightly over the dose range of 0–2 Gy; the increase after 4 Gy largely represents newly born activated microglia (see Fig. 2). Panel B: Newly born neurons, which were labeled with an antibody against NeuN, showed significant decreases at all doses greater than 0.5 Gy. Panel C: Newly born astrocytes, labeled with an antibody against GFAP, showed substantial decreases after 0.5 and 1.0 Gy. The relative increases seen from 2–4 Gy likely represent a reactive gliosis but could also involve, in part, a regenerative response in GFAP-producing stem cells. Panel D: Newly born oligodendrocytes, which were labeled with an antibody against NG2, showed significant decreases after doses greater than 0.5 Gy. Each bar represents the mean for five or six mice; error bars are standard errors of the means. *P < 0.05 after adjustment for multiple comparisons.
The fate of newly born cells was determined by quantifying cells that were co-labeled with BrdU and cell-specific antibodies: NeuN for neurons, GFAP for astrocytes, NG2 for oligodendrocyte precursors, CD68 for activated microglia. There was an average of 3,381 ± 257 newly born neurons (BrdU+/NeuN+) in unirradiated controls and a small but nonsignificant (P = 0.28) increase after 0.5 Gy (Fig. 1B). In contrast, there were significant decreases in numbers of newly born neurons after 1 (P < 0.03), 2 (P < 0.002) and 4 Gy (P < 0.001) (Fig. 1B). With respect to newly born astrocytes (BrdU+/GFAP+), there was an average of 419 ± 80 cells in unirradiated animals. Relative to controls, newly born astrocytes were reduced by about 50% after 0.5 Gy (P = 0.031) and by about 70% after 1 Gy (P < 0.01); after 2 and 4 Gy, the numbers of newly born astrocytes increased (Fig. 1C). While the decreases in numbers of newly born astrocytes appeared substantial, after adjusting for multiple comparisons only the value at 1 Gy constituted statistical significance. The numbers of oligodendrocyte precursors (BrdU+/NG2+) averaged 241 ± 40 in controls, and there was no change after 0.5 Gy (Fig. 1D). Relative to controls, there were significant differences in numbers of newly born oligodendrocytes after 1, 2 and 4 Gy (all P < 0.001, Fig. 1D). Newly born activated microglia (BrdU+/CD68+) averaged 273 ± 71 in controls and 249 ± 33 after 0.5 Gy (P = 0.97). After irradiation with 1, 2 or 4 Gy, there were highly significant increases (P < 0.001), with an almost 17-fold increase after 4 Gy (Fig. 2).
Fig. 2.
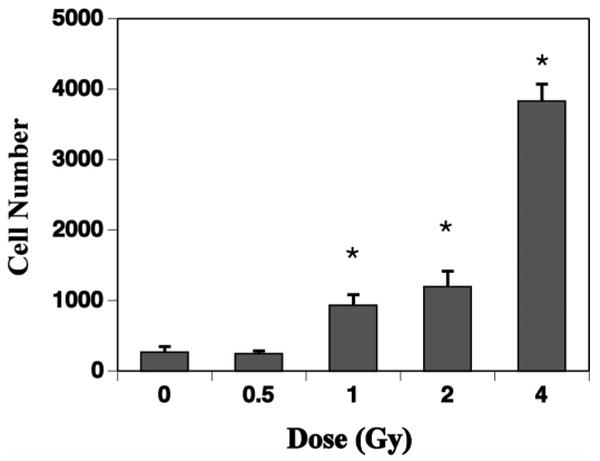
The effects of 56Fe-particle radiation on newly born activated microglia in the dentate subgranular zone of mice. One month after irradiation, mice received a single daily injection of 5-bromo-2′deoxyuridine (BrdU) for 7 consecutive days. Three weeks later, tissues were collected and immunohistochemistry and confocal microscopic analyses were done to quantify the numbers of cells co-labeled with BrdU and CD68. Cell numbers represented an estimate of the total number of positively labeled cells in the dentate subgranular zone in both hemispheres. Compared to nonirradiated controls, there were significant dose-related increases in the numbers of double-labeled cells at all doses greater than 0.5 Gy. Each bar represents the mean for five or six mice; error bars are standard errors of the means. *P < 0.05 after adjustment for multiple comparisons.
Numbers of total activated microglia (CD68 only) were detected throughout the dentate gyrus of all mice and averaged 85.7 ± 14.1 cells/mm2 in controls (Fig. 3). There were minor but nonsignificant increases in total numbers of activated microglia after 0.5, 1 and 4 Gy. There was a much more substantial increase after 2 Gy (Fig. 3), but given the limitations associated with multiple comparison analyses, this increase was not statistically significant.
Fig. 3.
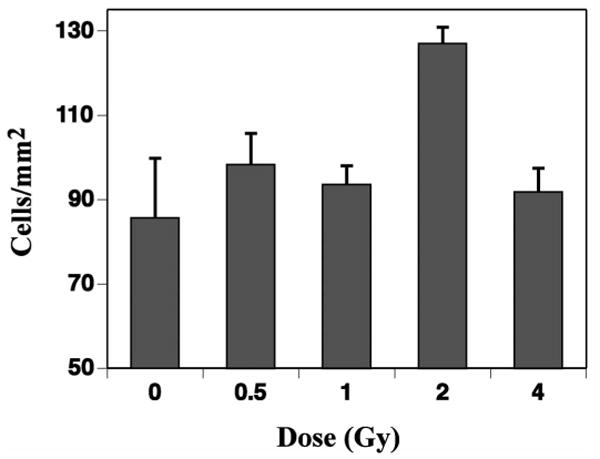
The effects of 56Fe-particle radiation on total numbers of activated microglia in the dentate gyrus of mice. Cell numbers/unit area (mm2) were determined from four representative coronal sections of the dentate gyrus at the level of the dorsal hippocampus. While there tended to be more activated microglia in all the irradiated groups, none of the changes were significant. Each bar represents the mean for five or six mice, and error bars are standard errors of the means.
Discussion
Neurogenesis describes a series of developmental steps that progress from the division of a stem/precursor cell to the development of a mature cell (36). This process can be quantified by giving repeated administrations of the thymidine analog BrdU and 3–4 weeks later counting newly born BrdU+ cells in the dentate SGZ that express mature cell markers. In this study, 1 month after irradiation, BrdU was given for 7 consecutive days and tissues were collected 3 weeks later. The immunohistochemistry/confocal analyses used here facilitated the assessment of survival and phenotypic fate of newly born SGZ cells. Using this approach, we saw a general trend toward decreasing numbers of newly born cells in the SGZ after 0.5–2.0 Gy, but even at the lowest point (i.e. 2 Gy) the total loss amounted to only about 35% (Fig. 1A). The importance of this finding is difficult to assess given that there are actually increased numbers of BrdU+ activated microglia and astrocytes, particularly after the higher doses used here (Fig. 2). What is likely to be more relevant is the phenotypic characterization of newly born cells and their response after irradiation. The cells most sensitive to 56Fe-particle radiation were newly generated neurons and oligodendrocyte precursors, both of which showed significant dose-related decreases after doses higher than 0.5 Gy (Fig. 1). While the neuronal response was qualitatively similar to what was seen after X irradiation (20), i.e. a decrease with dose, the oligodendrocyte precursor response was quite different. After exposure to X rays there is no apparent decrease in oligodendrocyte precursor cells with dose (20), but in the present study there was a clear decrease with 56Fe-particle dose with virtually no co-labeled cells after 4 Gy (Fig. 1D). These precursor cells, which are labeled with an antibody against NG2 chondroitin sulfate proteoglycan, constitute a major dividing population throughout the mammalian CNS (37). While the physiological role of NG2+ cells remains to be determined, they have generally been considered committed to the oligodendrocyte lineage (37), although that has been questioned (38). Given the proliferative capacity of these cells, assessment of treatment effects with BrdU as used here can be complicated by dilution effects, which could lead to loss of some BrdU+ cells with time (38). However, even if there is label dilution to some extent, there still are significant numbers of BrdU+/NG2+ cells in unirradiated controls 4 weeks after BrdU treatment, and 56Fe-particle irradiation significantly alters that pattern (Fig. 1D). Relative to what is seen after photon irradiation (20), whether this latter observation represents an intrinsic sensitivity of young oligodendrocytes or some sort of aberrant differentiation after exposure to high-LET radiation is not clear. The astrocytic response seen after 56Fe-particle irradiation was also different from that seen after low-LET irradiation, where, after X irradiation, there was little effect after doses of 2–10 Gy (15, 20). In contrast, after 56Fe-particle irradiation, there were significant decreases after 0.5 and 1.0 Gy (Fig. 1C). The subsequent increases in the numbers of newly generated astrocytes may represent astrogliosis such as that seen after low-LET radiation (39), although we have no specific data here to confirm that. However, in a previous study of 56Fe-particle radiation, we reported a diffuse astrogliosis in the dentate gyrus, particularly after a dose of 3 Gy (30). On the other hand, given that the putative stem cell in the SGZ expresses GFAP (40, 41) and that astrocytes play an active role in regulating neurogenesis (42), increasing numbers of GFAP-producing cells may represent, in part, increased stem cell activity and perhaps a regenerative response. Future studies are needed to address this idea and to determine whether there are any functional consequences of the apparent increase in newly born GFAP-producing cells.
While the appearance of neuroinflammation can be multi-faceted, one of the principal elements is the activation of microglia, the resident inflammatory cells in the CNS. Activated microglia have generally been considered to be a hostile cell population whose presence can negatively affect cell survival (43) and neurogenesis (15, 20, 25, 29). It has also been suggested, however, that activated microglia can play a protective role and that the protective and deleterious effects may change as a function of the type of activation (44), the environmental context within which the activated cells exist (45), or their state of proliferation (46). Additionally, microglia cross-talk with CNS-specific autoimmune T cells has functional consequences on specific processes like neurogenesis (47). It is of particular interest that a recent study of radiation effects in mice deficient in extracellular superoxide dismutase suggests that in the presence of persistent oxidative stress, the activation of microglia may have a beneficial effect in terms of neurogenesis (26).
In the present study, neuroinflammation was assessed by counting total activated microglia (CD68+ only) and numbers of newly born activated microglia (BrdU+/CD68+). The latter were quantified using the same stereologic approach used for quantifying neurons and glia (Fig. 1) (26). That analysis showed a clear dose-related increase in newly born activated microglia that was qualitatively similar to what was seen after low-LET irradiation (20). The overall increase in newly born activated microglia (Fig. 2) was associated with a general decrease in the production of newly born neurons (Fig. 1B); this also was similar to what was reported after X irradiation (15, 20, 48), suggesting a common cellular response in the SGZ after exposure to different types of ionizing radiation. The potential importance of these findings is highlighted by a recent study showing that anti-inflammatory treatment restores neurogenesis after irradiation (29). Whether such an approach could ultimately be useful in preventing or mitigating radiation-induced changes in neurogenesis and behavioral changes after high-LET radiation is a topic for further investigation.
Newly born activated microglia, as described above, constituted only a small fraction (∼1%) of the total population of activated microglia (data not shown). The larger numbers of total activated microglia in the brain after irradiation therefore necessitated a different quantitative approach, which has been recently described in detail (26). Thus, while the absolute values shown in Fig. 3 are smaller than those in Fig. 2, they are shown in the context of area and represent a much larger population of cells. The dose–response relationship for total activated microglia was different from that seen for newly born activated microglia, i.e., no major changes in total numbers of activated microglia as a function of dose, except perhaps after 2 Gy, and that value did not reach significance in the context of multiple comparisons. The apparent relative decrease from 2 to 4 Gy suggests that whatever is responsible for activating the cells may be inhibited or suppressed after a relatively high dose of 56Fe particles; however, that contention is tentative. Our results on total and newly born activated microglia, along with the neurogenesis data shown in Figs. 1B and 2, suggest that proliferating or newly born activated microglia may be the most critical inflammatory cell within the context of neurogenesis. This idea agrees with data from a recent study using an ischemic brain injury model (46), although the time course and end points of that study were quite different from those used here.
In conclusion, 56Fe-particle radiation has significant effects on SGZ neurogenesis, but with the exception of newly born astrocytes, only at doses higher than 0.5 Gy. While this study represents only one time after irradiation, it appears that whole-brain doses of 0.5 Gy or less are tolerated, at least in the context of hippocampal neurogenesis. Whether that same finding will hold after whole-body exposures, or at lower dose rates, more likely scenarios in the space environment, remains to be determined. While many of the findings seen here are similar to what has been reported after low-LET radiation, there are some striking differences, most apparent in the response of glial cell populations. Whether this has functional consequences will need to be determined in future experiments. Finally, the reductions in new neuron production seem to be associated with neuroinflammation (newly born activated microglia). Whether neuroinflammation constitutes a target for protective or mitigating strategies for the radiation space environment remains to be seen.
Acknowledgments
Support for this work was provided by in part by NASCOR grant NNJ04HC90G and NIH grant R01 NS46051. The authors would like to thank Drs. M. Pecaut and C. Favre and Ms. H. Milliken for technical assistance. We would also like to thank M. Vasquez, P. Guida and A. Rusek and the support staff at Brookhaven National Laboratory.
References
- 1.Curtis SB, Letaw JR. Galactic cosmic rays and cell-hit frequencies outside the magnetosphere. Adv Space Res. 1989;9:293–298. doi: 10.1016/0273-1177(89)90452-3. [DOI] [PubMed] [Google Scholar]
- 2.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 3.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Lee PW, Hung BK, Woo EK, Tai PT, Choi DT. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52:488–492. doi: 10.1136/jnnp.52.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 6.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 7.Surma-aho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, Paetau A, Kallio M, Pyykkonen J, Jaaskelainen J. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56:1285–1290. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 8.Denisova NA, Shukitt-Hale B, Rabin BM, Joseph JA. Brain signaling and behavioral responses induced by exposure to 56Fe-particle radiation. Radiat Res. 2002;158:725–734. doi: 10.1667/0033-7587(2002)158[0725:bsabri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Shukitt-Hale B, Casadesus G, McEwen JJ, Rabin BM, Joseph JA. Spatial learning and memory deficits induced by exposure to iron-56-particle radiation. Radiat Res. 2000;154:28–33. doi: 10.1667/0033-7587(2000)154[0028:slamdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Rabin BM, Joseph JA, Shukitt-Hale B, McEwen J. Effects of exposure to heavy particles on a behavior mediated by the dopaminergic system. Adv Space Res. 2000;25:2065–2074. doi: 10.1016/s0273-1177(99)01014-5. [DOI] [PubMed] [Google Scholar]
- 11.Rabin BM, Shukitt-Hale B, Joseph JA, Carrihill-Knoll KL, Carey AN, Cheng V. Relative effectiveness of different particles and energies in disrupting behavioral performance. Radiat Environ Biophys. 2007;46:173–177. doi: 10.1007/s00411-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 12.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 13.Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–389. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 14.Raber J, Rola R, LeFevour A, Morhardt DR, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 15.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 19.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Brunso-Bechtold JK. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 20.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 21.Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 22.Nagai R, Tsunoda S, Hori Y, Asada H. Selective vulnerability to radiation in the hippocampal dentate granule cells. Surg Neurol. 2000;53:503–506. doi: 10.1016/s0090-3019(00)00214-7. [DOI] [PubMed] [Google Scholar]
- 23.Peissner W, Kocher M, Treuer H, Gillardon F. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71:61–68. doi: 10.1016/s0169-328x(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki R, Matsumoto A, Itoh K, Kawabe T, Ota Y, Yamada K, Maruta T, Soejima T, Sugimura K. Target cells of apoptosis in the adult murine dentate gyrus and O4 immunoreactivity after ionizing radiation. Neurosci Lett. 2000;279:57–60. doi: 10.1016/s0304-3940(99)00910-6. [DOI] [PubMed] [Google Scholar]
- 25.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 26.Rola R, Zou Z, Huang TT, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. Lack of EC-SOD in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42:1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 28.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 29.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 30.Rola R, Otsuka S, Obenaus A, Nelson GA, Limoli CL, VandenBerg SR, Fike JR. Indicators of hippocampal neurogenesis are altered by 56Fe-particle irradiation in a dose-dependent manner. Radiat Res. 2004;162:442–446. doi: 10.1667/rr3234. [DOI] [PubMed] [Google Scholar]
- 31.Rola R, Sarkissian V, Obenaus A, Nelson GA, Otsuka S, Limoli CL, Fike JR. High-LET irradiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat Res. 2005;164:556–560. doi: 10.1667/rr3412.1. [DOI] [PubMed] [Google Scholar]
- 32.Wilson JW, Townsend LW, Nealy JE, Chun SY, Hong BS, Buck WW, Lamkin SL, Ganapol BD, Khan F, Cucinotta FA. BRYNTRN: A Baryon Transport Model. Technical Paper 2887. NASA; Washington, DC: 1989. [Google Scholar]
- 33.Zeitlin C, Heilbronn L, Miller J. Detailed characterization of the 1087 MeV/nucleon iron-56 beam used for radiobiology at the alternating gradient synchrotron. Radiat Res. 1998;149:560–569. [PubMed] [Google Scholar]
- 34.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 36.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 37.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 38.Thallmair M, Ray J, Stallcup WB, Gage FH. Functional and morphological effects of NG2 proteoglycan deletion on hippocampal neurogenesis. Exp Neurol. 2006;202:167–178. doi: 10.1016/j.expneurol.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 40.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 41.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 43.Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 44.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendroge-nesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 48.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18:115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]


