Abstract
Socialization processes, parents, or peers encouraging play with gender specific toys are thought to be the primary force shaping sex differences in toy preference. A contrast in view is that toy preferences reflect biologically determined preferences for specific activities facilitated by specific toys. Sex differences in juvenile activities, such as rough and tumble play, peer preferences, and infant interest, share similarities in humans and monkeys. Thus if activity preferences shape toy preferences, male and female monkeys may show toy preferences similar to those seen in boys and girls. We compared the interactions of 34 rhesus monkeys, living within a 135 monkey troop, with human wheeled toys and plush toys. Male monkeys, like boys, showed consistent and strong preferences for wheeled toys, while female monkeys, like girls, showed greater variability in preferences. Thus, the magnitude of preference for wheeled over plush toys differed significantly between males and females. The similarities to human findings demonstrate that such preferences can develop without explicit gendered socialization. We offer the hypothesis that toy preferences reflect hormonally influenced behavioral and cognitive biases which are sculpted by social processes into the sex differences seen in monkeys and humans.
Keywords: sex differences, toy preference, gender, hormones, rhesus monkey, children, socialization
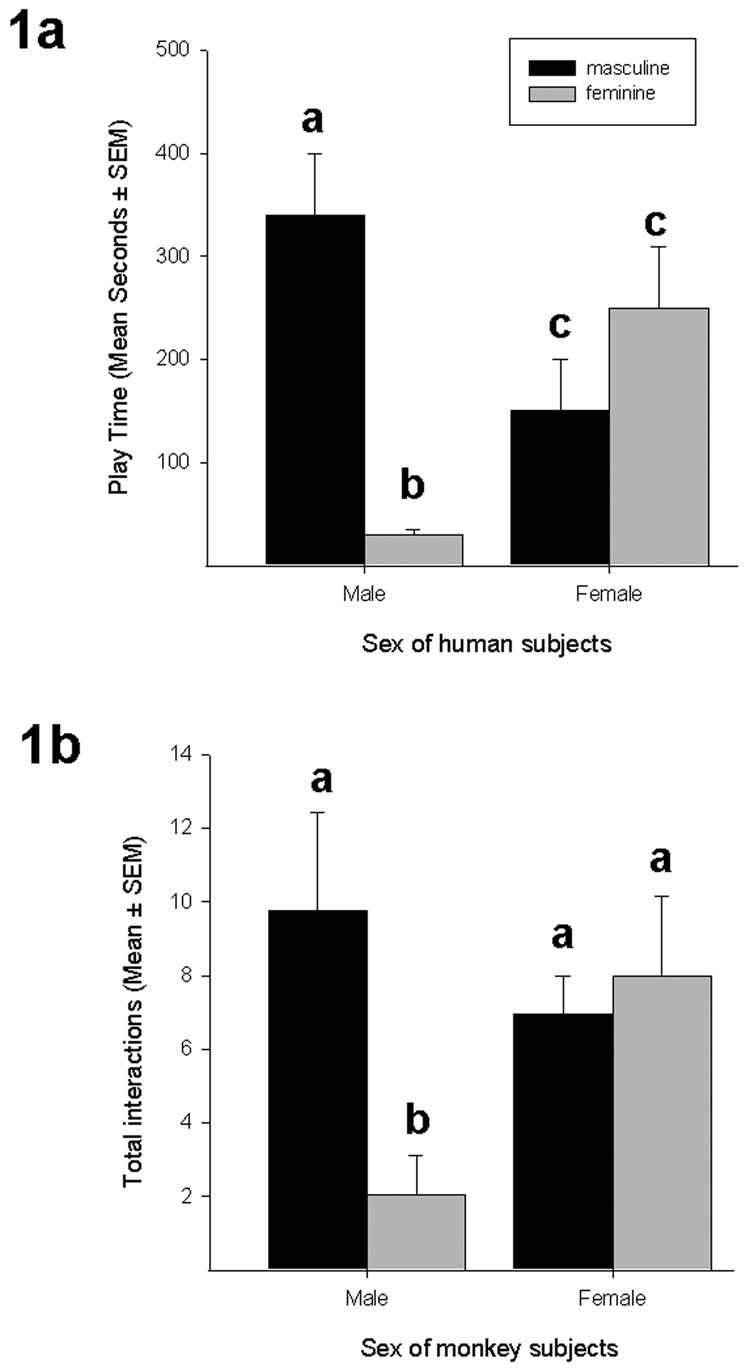
Toy play is one of the most robust human behavioral sex differences, showing moderate to very large effect sizes (Cohen-Bendahan et al., 2005; Collaer and Hines, 1995). As seen in Figure 1a, boys interact more with masculine-type toys than do girls, and girls interact more with feminine-type toys than do boys (Berenbaum and Hines, 1992). Within each sex, boys typically show strong preferences for stereotypically masculine toys, while girls often do not show a statistically greater preference for one toy type over another (Berenbaum and Hines, 1992; Carter and Levy, 1988; Eisenberg and Wolchik, 1985; Frasher et al., 1980; Perry et al., 1984; Sutton-Smith and Rosenberg, 1963; Turner et al., 1993). Thus sex differences in toy preferences are characterized by stronger gender-specific preferences in boys than in girls.
Figure 1.
Figure 1a: Sex difference in play with stereotypical masculine and feminine toys in a choice paradigm. Different superscripts within category or within sex indicate significant differences. (Adapted from Berenbaum and Hines, 1992).
Figure 1b: Sex difference in total frequency of interactions with plush and wheeled toys by rhesus monkeys. Different superscripts within category or within sex indicate significant differences.
Socialization processes have typically been offered as the primary source of the sex differences in human toy preferences. While there are many hypothesized socialization mechanisms (Bandura and Bussey, 2004; Martin and Halverson, 1981; Martin et al., 2002), one view is that societal endorsement of toys as masculine or feminine drive children’s toy preferences to conform to expected masculine and feminine gender roles (Martin and Little, 1990). Some have suggested that a greater preference for gendered toys in boys reflects a greater rejection of opposite-sex behavior in boys than in girls (Bussey and Perry, 1982). Thus, girls are less rigid than boys in their gender-typed beliefs, behaviors, and preferences, including toy preferences (Ruble et al., 2006).
A striking disparity between “masculine” and “feminine” toys is in the kinds of activities with which they are typically associated (Miller, 1987). Possibly, differential attraction to these activities affects children’s toy preferences. In contrast to the socialization perspective this view posits that toy preferences reflect preferences for specific activities, such as active manipulation or cradling, facilitated by specific features of toys and that these activity biases result from the different prenatal hormonal environments of boys and girls. According to this perspective, boys’ and girls’ toy preferences reflect differences in their preference for specific activities and they thus seek out toys that facilitate those preferred activities. The “pink” and “blue” aisles in toy stores thus reflect marked gender preferences for activities and not necessarily societal imposition of gender norms on boys and girls. The socialization and activity bias viewpoints do not resolve the sex differences in the magnitude of the preference for gender specific toys. The more marked preference in boys than girls could reflect either that boys have stronger predispositions to a more limited set of activities, or alternatively that boys’ toy choices are more strongly socially constrained than are girls’ choices (Ruble et al., 2006). One approach to disentangling these potential effects is to look at “toy” preference in a species that shows hormonally biased sexually differentiated juvenile behavior, but where there is no evidence for socialization of specific gendered activities (Wallen, 2005). While demonstration that such nonhuman animals show preferences for toys similar to those seen in children would not eliminate the possibility that children’s toy preferences are primarily socialized, it would lend support to the notion that preferences for specific play objects may reflect underlying preferences for specific activities.
Prenatal hormone exposure is known to influence children’s toy preferences as girls with congenital adrenal hyperplasia (CAH), an inherited enzymatic defect preventing glucocorticoid production that results in elevated prenatal adrenal androgen secretion, show more boy-typical toy preferences than do their unaffected sisters or control girls (Berenbaum and Hines, 1992; Meyer-Bahlburg et al., 2004). This preference is evident in CAH girls who look like and are reared as girls (Berenbaum and Hines, 1992; Meyer-Bahlburg et al., 2004) and despite the fact that most of these girls have typical female gender identity (Meyer-Bahlburg et al., 2004). When parental socialization was explicitly studied, one study found that CAH girls are more strongly encouraged to play with female-typical toys than are unaffected female siblings, yet they still show a masculine toy preference (Pasterski et al., 2005). Thus toy preferences appear sensitive to prenatal androgen exposure and seem unlikely to reflect sex of rearing or gender typical socialization.
There is evidence suggesting that the activities facilitated by a toy determine gendered toy preferences (Campbell et al., 2000; Eisenberg et al., 1982; Miller, 1987; Servin et al., 1999). For example, children tended to explain their toy preferences in terms of what can be done with a toy, 55% of all explanations, and rarely with reference to the gender appropriateness of the toy, less than 1% of all explanations, (Eisenberg et al., 1982). Such findings support the notion that toy preferences might reflect sex differences in activity preferences.
The CAH evidence for hormonal influences on toy preference is striking; however, as long as the research is conducted only in humans, socialization and biological processes are confounded. An alternative approach is to examine nonhuman animal toy preferences, where socialization for specific toys is unlikely to determine preferences. As with boys, juvenile male monkeys engage in more rough-and-tumble play than their female counterparts (Alexander and Hines, 2002; Hines and Kaufman, 1994; Lovejoy and Wallen, 1988; Maccoby, 1998; Wallen, 1996; Wallen, 2005), while girls and juvenile female monkeys show a greater interest in young infants (Herman et al., 2003; Lancaster, 1971; Leveroni and Berenbaum, 1998). These striking behavioral parallels are not reflected in parallel effects of prenatal androgen exposure in monkeys and humans. Although rough and tumble play is strongly influenced by prenatal androgen exposure in monkeys (Goy et al., 1988; Wallen, 1996), it was not increased in CAH girls (Hines & Kaufman, 1994). Similarly, infant interest has been found to be less marked in CAH girls (Leveroni and Berenbaum, 1998), but not in female monkeys treated prenatally with small doses of androgen (Herman, et al., 2003). While these contrasting results from single studies in monkeys and humans may reflect ineffective androgen exposure or inappropriate timing of androgen exposure for the behavioral endpoints, it cannot be ruled out that factors other than androgens influence the development and expression of these behaviors. Nevertheless, if toy preferences stem from activity preferences, behavioral parallels in humans and monkeys predict sex differences in monkey toy preferences.
The one previous study of nonhuman primates’ interactions with human toys did not make subjects choose between masculine and feminine toys simultaneously available and thus could not directly measure preference. Instead they compared the relative proportion of interaction times with singly presented toys as a proxy for preference (Alexander and Hines, 2002). Comparisons between sexes found that the proportion of males’ toy interactions directed to masculine toys was greater than the proportion of females’ interactions directed to masculine toys. A similar, but opposite, difference was found for the proportion of interactions directed towards feminine toys, suggesting clear between-sex differences in preference for masculine and feminine toys similar to that seen in humans. When comparisons were made within sex for the magnitude of the preference, however, the results differed significantly from findings in humans. Unlike boys, male vervets spent comparable percentages of time with both masculine and feminine toys, showing no gendered toy preference. Unlike girls, female vervets spent a significantly greater proportion of time with feminine than with masculine toys. Thus, magnitudes of preferences in vervets were opposite to those seen in children. The authors suggested that the lack of a male vervet preference for masculine toys implied that boys’ strong preferences for masculine toys reflected stronger gendered socialization of boys’ toy preference relative to girls’ toy preference (Alexander and Hines, 2002). This explanation seems unlikely as it would imply that their finding of greater female vervet preference for feminine toys means that vervet monkey females are strongly socialized to prefer female toys, whereas girls’ toy preferences are not socialized. A more parsimonious explanation is that since the vervets were never presented with actual toy choices the results do not accurately reflect preferences, but show substantial cross sex willingness to play with any toy. Thus although there are substantial concordances between human and nonhuman primate gendered social behavior, nonhuman primate data leave unresolved the relative concordance between human and nonhuman primate gendered toy preferences.
We investigated toy preferences in rhesus monkeys living in a 135 member long-term stable outdoor group by presenting the group with multiple trials of simultaneous access to different two toy combinations of multiple toys: one putatively masculine and one putatively feminine. We present here striking evidence of a sex difference in rhesus monkey preference for human gender-stereotyped toys paralleling that reported in humans, suggesting that gender differences in toy choice may reflect evolved sex differences in activity preferences not primarily resulting from socialization processes.
Materials and Methods
Subjects
Subjects were rhesus monkey (Macaca mulatta) members of a multi-male, multi-female social group of 135 animals that had lived together for more than 25 years at the Yerkes National Primate Research Center Field Station. This social group had a species-typical multiple matriline social structure with a full age-range of group members from infants to adults. Fourteen animals were not included in analyses because they had been exposed to varying hormonal treatments prenatally, but there were not enough subjects in any one treatment group to systematically analyze preferences. Additionally, the interactions of 39 newborn (0–3 months) infants, while minimal, were not coded due to difficulty in consistent individual identification. This left 61 females and 21 males as potential subjects. Table 1 displays these animals by rank and age. Subjects were housed with their natal group in 25m × 25m outdoor compounds with attached temperature-controlled indoor quarters. Water was continuously available, and the animals were fed monkey chow twice daily, supplemented once per day with fruits and vegetables. research was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under an environmental enrichment/management protocol of the Yerkes National Primate Research Center approved by Emory’s Institutional Animal Care and Use Committee.
Table 1.
Males and females by rank and age: totals and participation (non-natal animals do not have matrilineal rank)
| Non-natal | Ranks 1–3 | Ranks 4–8 | Ranks 9–13 | Ranks 14–16 | TOTAL | |
|---|---|---|---|---|---|---|
| Males in group | 2 | 1 | 4 | 6 | 8 | 21 |
| Males participating | 1 (50%) | 0 | 3 (75%) | 3 (50%) | 4 (50%) | 11 (52%) |
| Females in group | 0 | 8 | 15 | 17 | 21 | 61 |
| Females participating | 6 (75%) | 6 (40%) | 4 (24%) | 7 (33%) | 23 (38%) | |
| juvenile (1–4) | subadult (5–7) | adult (8–12) | "elderly"(13+) | TOTAL | ||
| Males in group | 12 | 7 | 0 | 2 | 21 | |
| Males participating | 8 (67%) | 2 (29%) | 1 (50%) | 11 (52%) | ||
| Females in group | 23 | 12 | 14 | 12 | 61 | |
| Females participating | 10 (43%) | 5 (42%) | 3 (21%) | 5 (42%) | 23 (38%) | |
Materials
Because we hypothesized that some aspects of sexually differentiated toy preferences reflect activity preferences, we categorized our toys not by traditional gender assignment, but by specific object properties that made our categories comparable, though not exact matches, to stereotypical gender assignments. Thus one set of toys was “wheeled,” most comparable to the masculine vehicle toys and the other was “plush,” most comparable to the feminine doll and stuffed animal toys. The seven plush toys were: Winnie-the-Pooh™, Raggedy-Ann™, a koala bear hand puppet, an armadillo, a teddy bear, Scooby-Doo™, and a turtle. The sizes ranged in length from about 14 cm to 73 cm. The six wheeled toys were: a wagon, a truck, a car, a construction vehicle, a shopping cart, and a dump truck. These ranged in length from 16 to 46 cm. Plush and wheeled toys varied considerably in shape and color as well.
Data Collection
Seven 25min trials were conducted within the large indoor/outdoor enclosure that housed the social group. Prior to each trial, subjects and other social group members were sequestered indoors while one wheeled and one plush toy separated by 10m were placed in the outdoor living area, with left or right placement location counterbalanced across trials. Monkeys were then released into the outdoor area and each toy and any animal interacting with it was videotaped using separate cameras for each toy. In one case, a plush toy was torn into multiple pieces, ending the trial 7min early. After each trial, toys were removed from the outdoor area. The identity of every animal interacting with the toys and specific behaviors (Table 2) directed towards the toys were coded from the videotapes by two observers working together to achieve consensus on both identity and behaviors. Data were entered on Palm Pilots (IIIXE, Palm Inc., Santa Clara, CA) equipped with Handobs (Center for Behavioral Neuroscience, Atlanta, Georgia), a program designed for entering time-stamped behavioral information. Individuals’ social rank and age were included as variables in the analyses. Rank had been assessed for all individuals in the group through extensive behavioral observations documenting the directionality of grooming, dominance, and submission behavior.
Table 2.
Interactions with plush and wheeled objects coded from videotaped trials
| Behavior | Description |
|---|---|
| Extended touch | Placing a hand or foot on toy |
| Hold | Stationary support w/one or more limbs |
| Sit on | Seated on the toy or a part of the toy |
| Carry in hand | Moving w/toy in hand and off the ground |
| Carry in arm | Moving w/ toy in arm and off the ground |
| Carry in mouth | Moving w/toy in mouth and off the ground |
| Drag | Moving the toy along the ground behind the animal |
| Manipulate part | Moving, twisting, or turning a part |
| Turn entire toy | Shifting 3-D orientation of toy |
| Touch | Brief contact using hands or fingers |
| Sniff | Coming very close to the toy with the nose |
| Mouth | Brief oral contact – no biting or pulling |
| Destroy | Using mouth or hands to bite or tear toy |
| Jump away | Approach, then back away from toy with a jumping motion |
| Throw | Project into air with hands |
Data analysis
All instances of any specific behavior were counted to provide frequencies of occurrence. For behaviors that were continuous, onsets and offsets were also recorded to derive durations of those behaviors. Subjects participated in different numbers of trials so raw frequencies and durations for each subject were divided by the number of trials that subject participated in to provide an average frequency or duration of each behavior.. Subjects with fewer than 5 total behaviors (3 males and 14 females) were excluded from analyses, producing a final n of 23 females and 11 males. Males and females did not differ in the proportion of subjects excluded (X2(1)=1.23). Total frequencies and total durations were calculated for each animal by summing the calculated averages for each individual behavior.. Analyses were completed using SPSS for Windows (Version 13, SPSS Inc.) and a Microsoft Excel (Microsoft Corp, Redmond, WA) macro for Heterogeneity G-tests (Sokol and Rohlf, 1995). Cohen’s d, a measure of effect size that compares pairs of means and standard deviations (Cohen, 1992), was calculated separately for contrasts of interest. Prep, a measure of probability of replication based on sample size and effect size (Killeen, 2005), is also reported.
An examination of the distribution of the behavioral variables using the Kolmogorov-Smirnov test revealed positive skew due to a majority of animals showing relatively low frequencies and durations of behaviors with a few individuals showing very high rates of interaction. Focusing analyses on total frequencies and total durations of interaction rather than on individual behaviors reduced but did not eliminate skew. Square root transformations of total frequency data eliminated skew except for total duration data. To make analyses of both types of data as comparable as possible, we conducted ANOVAs on untransformed total frequency and total duration data to allow us to identify interactions. However, when significant interactions were revealed, follow up comparisons used nonparametric tests on the untransformed data. While we found that skew was no particular threat to the validity of our results when using only parametric tests, we felt the combination of parametric ANOVAs with nonparametric tests for other comparisons to be the most conservative approach to analyzing these data.
Results
Table 1 identifies the characteristics of the animals included in the analyses, sorted by sex and rank and by sex and age, and the proportion of the total potential males and females in each age and rank group that participated.
Total frequency showed a significant interaction between toy type and sex, F(1,32)= 4.49, p=.04, and transformed total frequency was also significant. Nonparametric within-sex comparisons revealed that males preferred wheeled over plush toys (Figure 1b; Z=−2.09, p=.04, d=1.14, prep=.95), and that females exhibited no significant preference for plush toys over wheeled toys (Figure 1b; Z=−.55, p=.58, d=.12, prep=.61). For between-sex comparisons, males and females did not differ in their total interactions with wheeled toys (Figure 1b; Z=−.65, p=.52, d=.39, prep=.87), but males interacted significantly less with the plush toys than did females (Z=−2.23, p=.03, d=.76, prep =.98).
Total duration also showed an interaction between toy type and sex, F(1, 32)=4.65, p=.04. This significant interaction is noted with caution, given the violation of the assumption of normality. As a follow up, nonparametric Mann-Whitney U comparisons, reflecting the non-normal distributions, revealed a pattern of within-sex effects similar to that seen for the frequency data: males interacted for a greater total time with wheeled (mean ± SEM: 4.76min ± 2.29) than with plush objects (.53min ± .43; Z=−2.22, p=.03, d=.77, prep=.88), while females did not differ in the duration of interactions with the toy types (wheeled: 1.27 ± .46; plush: 1.49 ± .79; Z=−.82, p=.41, d=.07, prep=.56). Overall comparisons between males and females revealed that they did not differ significantly in the total time spent with wheeled (Z=−1.20, p=.23, d=.33, prep=.87) or with plush (Z=−1.27, p=.21, d=.62, prep=.73) objects.
We compared males and females on the magnitude of preference for sex-typical toys. Difference scores were calculated for males and females in the following way: for males, total frequency wheeled - total frequency plush; for females, total frequency plush - total frequency wheeled. The same calculations of difference scores were also completed for total duration. The duration difference scores were significantly skewed and the skew remained for transformed data. Thus, to provide comparable statistical power, nonparametric Mann-Whitney U tests were used for both the frequency and duration data, even though only the duration data were skewed. A significant sex difference in magnitude of preference was revealed for frequency (Males: 7.71 ± 3.11; Females: 1.00 ± 2.42; Z = −2.45, p = .01, d = .61, prep=.96) and duration (Males: 4.23 ± 2.42; females: .22 ± .85; Z = −2.23, p = .03, d = .63, prep=.96). Thus males exhibited a significantly higher preference for the “masculine” (wheeled) toys than did females for the “feminine” (plush) toys.
As seen in Table 1, participating males and females were comparably distributed across ranks (X2= 3.36, p =.18). In addition, a comparison of mean rank between males (9.3) and females (8.7) revealed no significant differences, t32 =−.77, p=.45. When dominance rank was included as a covariate in frequency data analyses, the interaction between toy type and sex was not significant, F(1,31)=3.90, p=.06, and the interaction between toy type and rank was also not significant F(1,31)=.78, p=.39. When the frequency data were transformed, however, then the interaction between toy type and sex remained significant even with rank as a covariate. For the untransformed duration data, the sex by toy interaction remained significant with rank as a covariate (F(1,31)=4.56, p=.04) and the toy by rank interaction was not significant (F(1,31)=.05, p=.82). We also conducted Spearman’s correlations to determine the relationship between rank and frequency or duration with each toy type. With both sexes combined, rank and total frequency were positively correlated for both the plush toy (rs= .43, p=.01, r2=.18) and the wheeled toy (rs= .38, p=.03, r2=.14), accounting for 18% and 14% of the variance, respectively. For males, plush toy (rs=−.36, p=.27, r2=.18) and wheeled toy (rs=.21, p=.53, r2=.04) total frequencies did not correlate significantly nor did total durations (plush: (rs=−.31, p=.35, r2=.10; wheeled: rs=.005, p=.99, r2<.001). For females, rank correlated positively with total frequency for both plush (rs=.71, p<.001, r2=.50) and wheeled toys (rs=.45, p=.03, r2=.20), and with total duration for plush toys (rs=.55, p=.01, r2=.30), but not for wheeled toys (rs=.34, p=.11, r2=.12). Thus, large percentages of variance, especially for total frequencies of interactions with the plush toy, are explained by rank in females, but not for males, where rank accounts for little if any of the variance in interactions with toys. Thus it is unlikely that social rank determined the sex differences in toy preference reported here.
Overall sample size precluded analysis of individual age groups. However comparing frequencies of interaction, using one-way ANOVAs, by age for juvenile, subadult, adult, and more aged animals did not differ for either the plush object (F(3,30)=.48, p = .70) or the wheeled object (F(3,30)=1.57, p=.22). Similarly, no differences were found in duration of interaction by age for either the plush object (F(3,30)=.62, p=.61) or the wheeled object (F(3,30)=.77, p=.52).
G-tests, which do not require independent observations (Sokol and Rohlf, 1995), were conducted to determine toy preferences in individuals. These results were similar to the group effects: 73% of males significantly preferred wheeled toys, 9% preferred plush toys (G-tests, all p-values<.05), and 18% showed no significant preference. There were no differences in rank or age between males who showed a plush preference, a wheeled preference, or no preference. In comparison, 30% of females significantly preferred plush toys, 39% preferred wheeled toys (G-tests, all p-values<.05), and 30% had no significant preference. Interestingly, there were rank differences among females, but not males, based on their preferences, F(2,20)=4.42, p=.03, such that females with no preference ranked lower than the females with a plush preference, but there were no statistical differences between females who preferred plush and females who preferred wheeled toys (Table 3). There were no age differences according to preferences in the females.
Table 3.
Mean frequencies and durations of interactions with the plush and wheeled toys, sorted by sex
| Frequency |
Duration (minutes) |
||||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||
| Plush | Male | 2.06 | 9.21 | 0.53 | 1.41 |
| Female | 7.97 | 10.48 | 1.49 | 3.81 | |
| Wheeled | Male | 9.77 | 8.86 | 4.76 | 7.59 |
| Female | 6.96 | 4.92 | 1.27 | 2.2 | |
Discussion
Mirroring the marked sex difference in infant interactions and children’s toy preferences, male monkeys interacted significantly less with plush toys than did female monkeys. By contrast, males and females interacted with wheeled toys comparably, displaying no reliable sex differences. As is the case with sex differences in children’s toy preferences, only male monkeys showed a significant preference for one toy type over the other, preferring wheeled over plush toys. Unlike male monkeys and like girls, female monkeys did not show any reliable preference for either toy type.
Social rank appeared to play a role in interactions with the toys, but only for the females as rank was unrelated to toy interactions in males. High ranking females had higher frequencies and durations of interaction with the plush toy as well as higher frequencies of interaction with the wheeled toy. While rank affected overall toy interactions in females, it did not appear to be a factor in the sex differences in toy preference. Our results suggest that these sex differences cannot be accounted for by the effects of age and social rank, but instead, as has been suggested for children, reflect the more rigid preferences of males compared to the more varied and flexible preferences of females. Like young boys, who express strong preferences for stereotypically masculine toys, male rhesus monkeys showed strong preferences for wheeled toys. Like young girls, who show moderate preferences for stereotypically feminine toys, female rhesus monkeys demonstrated a nonsignificant preference for plush toys.
Our findings stand in contrast to the findings in vervet monkeys’ interactions with human toys, which were less similar to findings in children than are our results in rhesus monkeys (Alexander and Hines, 2002). While Alexander and Hines (2002) reported that male vervets interacted with masculine toys more than did female vervets, their males interacted with all toys at higher frequencies making this putative sex difference hard to interpret as it may simply reflect a bias in males to interact at higher rates with any object. More germane to the issues raised here is whether male and female vervets showed a preference for one toy type over the other. Alexander and Hines (2002) did not directly measure preference, but created a proxy for preference by calculating the proportion of interactions with a specific toy type to correct for the males’ overall higher interactions with all toys. This “preference” measure revealed a sexually differentiated pattern contrary to that generally seen in human children. Unlike girls, female vervets showed a strong “preference” for feminine toys, whereas male vervets, unlike boys, showed no toy “preference” (Alexander and Hines, 2002). The difference in findings between our study and those in vervets may reflect species differences, the exemplars of toy categories chosen, or that we used an explicit preference test more comparable to those used in human studies. Using methods more comparable to human studies, and even though we used group rather than individual preference testing, we obtained results strikingly similar to those in humans, suggesting that differences between our study and Alexander and Hines’ (2002) likely reflect methodological and not species differences.
It is apparent from both Alexander and Hines’ (2002) study and our results, however, that monkey toy preferences, no matter their direction and magnitude are unlikely to result from specific adult socialization or from the formation of gender schemas. Monkeys live in a socially complex world with substantial maternal support, but differential maternal treatment of males and females is limited to maternal retrieval in response to infant distress and physical inspection of their infant’s genitals (Wallen, 2005). Sex differences in maternal treatment do not include preventing their male or female offspring from engaging in opposite-sex typed behavior or in encouraging them to interact with specific objects (Wallen, 2005). While social context certainly affects the developmental environment of males and females, it is unlikely that it determines the basic predisposition to engage in specific patterns of sexually differentiated behavior such as interest in infants or rough and tumble play. In the case of rough play, it is likely that females voluntarily limit their participation, not because males exclude them, but because females don’t find this style of play particularly attractive. Evidence in support of this view comes from female rhesus monkeys prenatally exposed to elevated androgens late in gestation and who look completely anatomically female. Even though they cannot be physically distinguished from females and do not look like juvenile males, they still show male-like levels of rough and tumble play compared to control females (Goy et al., 1988) suggesting that the sexual differentiation of play reflects sex differences in activity preferences and not social constraints on play. Thus we think it unlikely that monkey toy preferences reflect socialization processes, maternal or otherwise. That sex differences in toy preference have been found in two nonhuman primate species, albeit differing in direction and magnitude, demonstrates that such preferences can occur without the necessity of positing any specific socializing influence,, a principle that may also apply to the development of children’s toy preferences.
Previous research has demonstrated that prenatal androgens influence postnatal sex differences in activity preferences (Wallen, 2005). We offer the hypothesis that there are hormonally organized preferences for specific activities that shape preference for toys that facilitate these activities. Human toys capitalize on sex differences in preferred activities, creating a gendered toy market. Thus, in addition to adults socializing children’s toy preferences, children may socialize adults to provide toys facilitating their preferred activities. In this view biologically based sex differences in activity preferences significantly influence sex differences in childhood object choice.
This proposed interaction between the child’s preferences and adult socialization is not inconsequential. Traditionally, socialization pressures are conceptualized as the primary determinants of preference. There can be little doubt that boys and girls learn that some activities are socially more appropriate for males or for females and this is likely reflected in the sex-stereotyped toys they choose. However, girls are less likely to receive negative information about boys’ toys and activities than are boys about girls’ activities and toys (Kane, 2006). Thus, girls’ toys and activities are often stigmatized for boys, but boys’ toys and activities not as stigmatized for girls (Martin, 1990). One could view such stigmatization as devaluing female-typical toys for boys without comparably devaluing male-typical toys for girls. Such differential devaluation might produce the markedly greater preference difference between toy types seen in boys contrasting with the lack of preference seen in girls. Because we chose toys based on object properties and not on previously established sex-typed categorizations, our wheeled and plush toys are not entirely analogous to the more stereotypical categories used in the human studies or to toys typically marketed as for boys and girls. Our findings suggest that sex differences in toy preferences in humans and nonhuman primates rely to some extent on physical object properties, but that social characteristics likely also influence preference, and some of these may be unique to humans. For example, a toy such as a plastic shopping cart, one of our wheeled toys, might appeal to boys or rhesus monkey males for its physical properties, but the same shopping cart also has symbolic properties related to imaginative play, and in humans may be socially stigmatized for boys. Because the shopping cart relates to a specific human activity, the toy facilitates different activities for humans than for rhesus monkeys. However, our finding that male monkeys show a preference of comparable magnitude to those seen in boys makes a cultural devaluation explanation unlikely.
An alternative, not necessarily mutually exclusive, explanation is that boys and girls prefer different physical activities with different types of behaviors and different levels of energy expenditure. It is these activity preferences which cause boys and girls to seek different experiences and it is these experiences, in turn, which are reflected in their preferences for specific objects that facilitate expression of their activity preferences. Possibly, as they move into adulthood, these divergent activity preferences and the experiences they engender become reflected in adult preferences for different lifestyles and careers (Maccoby, 1998). Preference and experience thus interact with each other such that biologically-determined and socialized effects are inseparable. We suspect that such interaction reflects a more general principle in which pre-existing preferences shape the developmental environment, which in turn shapes subsequent experience. In this manner both biological predispositions and socialization processes are necessary for the full development and differentiation of behavior.
Acknowledgements
Jessica Raper and Anne Graff assisted with data collection. Research was supported in part by Howard Hughes Medical Institute Grant 52003071, by the STC Program, the Center for Behavioral Neuroscience, of the NSF under agreement No. IBN-9876754, and NIH grants R01-MH50268 and K02-MH01062 (K.W.), and NCRR grant RR-00165 to the Yerkes Regional Primate Research Center, which is fully accredited with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Hines M. Sex differences in response to children's toys in nonhuman primates (Cercopithecus aethiops sabaeus) Evolution and Human Behavior. 2002;23:467–479. [Google Scholar]
- Bandura A, Bussey K. On broadening the cognitive, motivational, and sociostructural scope of theorizing about gender development and functioning: comment on Martin, Ruble, and Szkrybalo (2002) Psychol Bull. 2004;130:691–701. doi: 10.1037/0033-2909.130.5.691. [DOI] [PubMed] [Google Scholar]
- Benenson JF, et al. Propulsion: A behavioural expression of masculinity. British Journal of Developmental Psychology. 1997;15:37–50. [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychological Science. 1992;3:203–206. [Google Scholar]
- Bussey K, Perry DG. Same-sex imitation: The avoidance of cross-sex models or the acceptance of same-sex models? Sex Roles. 1982;8:773–784. [Google Scholar]
- Campbell A, et al. Infants' visual preference for sex-congruent babies, children, toys and activities: A longitudinal study. British Journal of Developmental Psychology. 2000;18:479–498. [Google Scholar]
- Carter DB, Levy GD. Cognitive Aspects of Early Sex-Role Development: The Influence of Gender Schemas on Preschoolers' Memories and Preferences for Sex-Typed Toys and Activities. Child Development. 1988;59:782–792. [Google Scholar]
- Cohen-Bendahan CC, et al. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. A Power Primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychological Bulletin. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Connor JM, Serbin LA. Behaviorally based masculine and feminine activity preference scales for preschoolers -correlates with other classroom behaviors and cognitive tests. Child Development. 1977;48:1411–1416. [Google Scholar]
- Eisenberg N, et al. Childrens reasoning regarding sex-typed toy choices. Child Development. 1982;53:81–86. [Google Scholar]
- Eisenberg N, Wolchik SA. Parental socialization of young children's play: a short-term longitudinal study. Child Development. 1985;56:1506–1513. [Google Scholar]
- Frasher RS, et al. Children's toy preferences revisited: implications for early childhood education. Child Care Quarterly. 1980;9:26–31. [Google Scholar]
- Goy RW, et al. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Hormones and Behavior. 1988;22:552–571. doi: 10.1016/0018-506x(88)90058-x. [DOI] [PubMed] [Google Scholar]
- Herman RA, et al. Sex differences in interest in infants in juvenile rhesus monkeys: relationship to prenatal androgen. Hormones and Behavior. 2003;43:573–583. doi: 10.1016/s0018-506x(03)00067-9. [DOI] [PubMed] [Google Scholar]
- Hines M, Kaufman FR. Androgen and the development of human sex-typical behavior - rough-and-tumble play and sex of preferred playmates in children with congenital adrenal-hyperplasia (CAH) Child Development. 1994;65:1042–1053. [PubMed] [Google Scholar]
- Kane EW. No way my boys are going to be like that! Gender and Society. 2006;20:149–176. [Google Scholar]
- Killeen PR. An alternative to null-hypothesis significance tests. Psychol Sci. 2005;16:345–353. doi: 10.1111/j.0956-7976.2005.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JB. Play-mothering: the relations between juvenile females and young infants among free-ranging vervet monkeys (Cercopithecus aethiops) Folia Primatologica. 1971;15:163–182. [PubMed] [Google Scholar]
- Leveroni CL, Berenbaum SA. Early androgen effects on interest in infants: Evidence from children with congenital adrenal hyperplasia. Developmental Neuropsychology. 1998;14:321–340. [Google Scholar]
- Lovejoy J, Wallen K. Sexually dimorphic behavior in group-housed rhesus-monkeys (Macaca-Mulatta) at 1 year of age. Psychobiology. 1988;16:348–356. [Google Scholar]
- Maccoby EE. The two sexes: growing up apart, coming together. Cambridge: Harvard University Press; 1998. [Google Scholar]
- Martin CL. Attitudes and Expectations About Children with Nontraditional and Traditional Gender-Roles. Sex Roles. 1990;22:151–165. [Google Scholar]
- Martin CL, Halverson CF. A schematic processing model of sex typing and stereotyping in children. Child Development. 1981;52:1119–1134. [Google Scholar]
- Martin CL, Little JK. The relation of gender understanding to children's sex-type preferences and gender stereotypes. Child Development. 1990;61:1427–1439. [PubMed] [Google Scholar]
- Martin CL, et al. Cognitive theories of early gender development. Psychol Bull. 2002;128:903–933. doi: 10.1037/0033-2909.128.6.903. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, et al. Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Archives of Sexual Behavior. 2004;33:97–104. doi: 10.1023/b:aseb.0000014324.25718.51. [DOI] [PubMed] [Google Scholar]
- Miller CL. Qualitative differences among gender-stereotyped toys - implications for cognitive and social-development in girls and boys. Sex Roles. 1987;16:473–487. [Google Scholar]
- O'Brien M, et al. Sex-typed play of toddlers in a day care center. Journal of Applied Developmental Psychology. 1983;4:1–9. [Google Scholar]
- Pasterski VL, et al. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development. 2005;76:264–278. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Perry DG, et al. Does early sex typing result from children's attempts to match their behavior to sex role stereotypes? Child Development. 1984;55:2114–2121. [Google Scholar]
- Ruble DN, et al. Gender Development. In: Damon W, Lerner RM, editors. Handbook of Child Psychology. Hoboken New Jersey: John Wiley & Sons; 2006. [Google Scholar]
- Serbin LA, et al. Gender stereotyping in infancy: Visual preferences for and knowledge of gender-stereotyped toys in the second year. International Journal of Behavioral Development. 2001;25:7–15. [Google Scholar]
- Servin A, et al. Sex differences in 1-, 3-, and 5-year-olds' toy-choice in a structured play-session. Scandinavian Journal of Psychology. 1999;40:43–48. doi: 10.1111/1467-9450.00096. [DOI] [PubMed] [Google Scholar]
- Sokol R, Rohlf F. Biometry. New York, USA: Freeman WH; 1995. [Google Scholar]
- Sutton-Smith B, Rosenberg BG. Development of sex differences in play choices during preadolescence. Child Development. 1963;34:119–126. doi: 10.1111/j.1467-8624.1963.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Turner PJ, et al. Gender-typing in young children: Preferences, behavior, and cultural differences. British Journal of Developmental Psychology. 1993;11:323–342. [Google Scholar]
- Wallen K. Nature needs nurture: The interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys. Hormones and Behavior. 1996;30:364–378. doi: 10.1006/hbeh.1996.0042. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Weinraub M, et al. The development of sex-role stereotypes in the 3rd year - relationships to gender labeling, gender identity, sex-typed toy preference, and family characteristics. Child Development. 1984;55:1493–1503. [PubMed] [Google Scholar]