Abstract
Background/Purpose
Although ingestion of alkali- and/or hypochlorite-based household cleaners as well as strong acids remain a major cause of esophageal wall injury, little is known about the mechanisms that underlie the injury response to these toxic agents. This study examined the roles of vascular dysfunction and inflammation to the esophageal injury response to different caustic substances in mice.
Methods
The esophageal responses to NaOH (10%, 5% & 2.5%), KOH (10%, 5%, & 2.5%), NaOCl (5.25%), and HCl (10%, pH=2) were evaluated by intravital videomicroscopy, and histopathology. Intravital microscopy was used to monitor changes in the diameter of arterioles and venules, the adhesion and movement of leukocytes in venules, and time of cessation of arteriolar blood flow in mouse esophagus. The esophageal mucosa was exposed to caustic substances for 0–60 minutes prior to evaluation.
Results
The higher concentrations of NaOH and KOH elicited rapid stasis in both arterioles and venules, which was accompanied by arteriolar constriction and thrombosis. An accumulation of adherent leukocytes in venules was not observed with any agent. Histopathologic evaluation revealed marked cellular and interstitial edema in the mucosa with alkali, while HCl and NaOCl decreased the thickness epithelial layer.
Conclusion
These findings suggest that ischemia and thrombosis are dominant processes, while inflammation is less important, in the pathogenesis of acute corrosive injury to the esophageal mucosa.
Index words: esophagus, caustic injury, intravital microscopy, leukocyte adhesion
Introduction
Ingestion of caustic substances remains a common health problem that affects children worldwide and continues to represent the most common cause of esophageal stricture in this age group1. Medical problems caused by caustic ingestion are associated with both acute and chronic complications. Acute complications include local burn and interstititial edema in upper GI passages, and aspiration with subsequent respiratory complications. Chronic complications include esophageal stricture, malnutrition and psychological problems2.
Previous experimental studies of caustic ingestion have largely focused on potential therapeutic agents/interventions that either antagonize the acute effects of the caustic substance or reduce the deposition of fibrous tissue and subsequent stenosis that often follows the injury. While corticosteroids and antibiotics has been advocated to reduce the inflammatory response initiated by caustic ingestion, the clinical benefits of these treatments remain controversial3–17. Most drug therapies for caustic ingestion that have been tested to date have not shown significant clinical efficacy and endoscopic dilatation or surgical resection remain an option for management of patients with severe complications resulting from caustic ingestion2,7,15,16.
The search for effective treatments for esophageal injury that result from caustic ingestion has been hampered by a clear understanding of the nature, time-course, and underlying mechanisms of the tissue responses to caustic agents. Strong acids are believed to cause necrosis through denaturation of proteins (coagulation necrosis), while strong alkalis mediate necrosis via the saponification of fats and solubilization of proteins (liquefactive necrosis)2. However, little else is known about the pathophysiology of caustic injury to the esophagus1. While thrombosis, ischemia and inflammation have all been implicated in the pathogenesis of caustic injury, there is no direct evidence demonstrating the genesis of these responses in the esophageal mucosa after exposure to strong acids or alkali.
Intravital microscopy (IVM) is a widely used method to visualize and study the microcirculation in living tissue. This technology allows for quantification of different physiological variables, including vessel diameter, perfusion rate, pressures, and permeability. The application of specific fluorochromes and fluorescence imaging with IVM also enables the user to quantify the movement of specific blood cell populations within the vessel lumen as well as the adhesive interactions between these cells and vessel wall.18 In the present study, IVM was used to monitor changes in the diameter of arterioles and venules, the adhesion and movement of leukocytes in venules, and time of cessation of arteriolar blood flow in mouse esophagus after exposure to different caustic agents. The overall objective of this study was to determine whether vascular dysfunction and inflammation are early components of the esophageal injury response to commonly ingested caustic substances.
Materials and Methods
Animals
Male C57BL/6J mice (n = 121) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). Animals were maintained on a 12-hour light/12-hour dark cycle under pathogen-free conditions. The mice had ad libitum access to a standard diet and water until reaching the desired age (8–10 weeks) and/or weight (20–25 g). All procedures using animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center and were performed according to the criteria outlined by the National Institutes of Health.
Caustic substances
The following substances were evaluated: sodium hydroxide (NaOH) SigmaUltra® (pellets, minimum concentration 98%) diluted into three concentrations: 10%, 5%, 2.5%, potassium hydroxide (KOH) SigmaUltra® (pellets, KOH ≥85%, K2CO3 ≤2.0%) diluted into three concentrations 10%, 5%, 2.5%, Sodium hypochlorite (NaOCl) 5.25% (commercial Clorox®), hydrochloric acid (HCl) diluted into two concentrations 10% and (pH = 2). Saline was used as the control solution.
Intravital microscopy experiments
Mice (n= 61) were anesthetized subcutaneously with a mixture of Ketamine and Xylazine at doses of 100 and 5 mg/kg, respectively. The right femoral artery and left femoral vein were cannulated with polyethylene tubing (PE-10). A midline abdominal incision was made and the lower part of the esophagus was cannulated using polyethylene tubing (PE-90) just above the gastroesphageal junction. The tubing was secured in place with a silk tie. The neck was opened through an inverted T-incision and the trachea exposed. The left sternomastoid and strap muscles of the neck on both sides were divided by heat cautery to enhance exposure of the trachea, which was cannulated PE90 tubing (PE-90) secured in place by silk suture. Traction was applied to the silk suture to tilt the trachea slightly to the right in order to expose the underlying esophagus. Fascia covering the esophagus was dissected, taking care not to injure the esophageal vascular supply. A PE-90 cannula for delivery of caustic substances was advanced through the mouth to the lower border of the larynx. A thin strip of Saran wrap was placed over the esophagus to prevent dryness.
The mouse esophageal microcirculation was visualized using 40x water immersion lens of an upright Nikon fluorescence microscope (Nikon Inc, Tokyo, Japan) equipped with a 75-watt XBO xenon lamp. A solution of 0.2 ml of rhodamine-6G was injected through the venous cannula and then the mouse was allowed to stabilize for a period of 30 minutes prior to collection of video images of the microvasculature. An arteriole and venule within the wall of the esophagus were selected for study. Digital video recordings of 30-second duration were obtained under control (pre-exposure to caustic agent or saline) conditions, at 2.5 min intervals during the first 10 min of chemical exposure period, and at 5 min intervals thereafter, until the cessation of blood flow or 60 minutes, whichever occurred first. The following variables where measured offline in each of the video recordings: internal diameters of the arteriole and venule, number of leukocytes firmly adhering (stationary) in venules for at least 30 seconds, flux (# per 30 sec) of rolling leukocytes, time to cessation of blood flow in both the arteriole and venule. Blood pressure was also monitored via the femoral artery cannula.
Histopathology
Following intraluminal placement of the caustic solution (0.2 ml) for a period of 10 min, the lumen was rinsed with 1.0 ml of saline. The cervical esophagus was then harvested and immediately fixed in formalin. The tissue samples were cut into sections, stained with hematoxyline and eosin and evaluated histologically. Quantification of the histological changes was performed using a previously described scoring system with a few modifications5. The slides were graded blindly by a pathologist using the following criteria:
*Esophageal viability (0- normal esophagus, 1-only mucosal necrosis, 2- necrosis involving mucosa and superficial muscle layer, 3- necrosis involving all three layers, mucosa, superficial muscular layer and deep muscle with adventitial layer).
* Cornified epithelial cell differentiation, (0- present, 1- not present).
* Epithelial cell nucleoli, (0- normal, 1- nucleoli present only in superficial 1/3 of mucosal cells, 2- nucleoli present only in superficial 2/3 of mucosal cells, 3- nucleoli present in full thickness of the mucosal cells).
* Edema and spongiosis (0- normal, 1- Edema and spongiosis present only in superficial 1/3 of squammous mucosa, 2- Edema and spongiosis present only in superficial 2/3 of squammous mucosa, 3- Edema and spongiosis present in full thickness of the squamous mucosa).
* Thrombosis (0- not present, 1- present).
A total histopathology score, with a maximum potential value of 10, was derived from the sum of the individual scores for tissue viability, cornified epithelial cell differentiation, epithelial cell nucleoli, edema/spongiosis, and thrombosis.
Data analysis
Statistical analyses of the data were performed with StatView 4.5 software (Abacus Concepts Inc, Berkeley, CA) using 1-way ANOVA with Fisher’s (post hoc) test. All values are reported as means ± SEM. Statistical significance was set at P < 0.05.
Results
Intravital microscopy
Blood flows in esophageal arterioles and venules were monitored for a period of 60 min following placement of saline or different caustic solutions in the esophageal lumen. Table 1 summarizes the times required for blood flow cessation in both arterioles and venules after exposure to the different solutions. Saline, as well as HCl (10% and pH 2.0), NaHOCl (5.25%), NaOH (2.5%) and KOH (2.5%) did not result in microvascular flow cessation over the 60 min observation period. However, 10% solutions of either NaOH or KOH lead to a very rapid (<10 min) and complete cessation of arteriolar and venular blood flows. The 5% solutions of the same agents also resulted in blood flow cessation, although a slightly longer time (≥ 15 min) was required to mediate this response.
Table 1.
Effects of different caustic solutions on the time for blood flow cessation in esophageal arterioles and venules.
| Substance and concentration | Time to blood flow cessation (min) |
|---|---|
| Saline | >60 |
| HCl 10% | >60 |
| HCl pH=2 | >60 |
| Na hypochlorite 5.25% | >60 |
| Na hydroxide 10% | 8.25 ± 1.25* |
| Na Hydroxide 5% | 16.4 ± 2.8*, # |
| Na hydroxide 2.5% | >60 |
| K hydroxide 10% | 5.8 ± 0.56* |
| K hydroxide 5% | 15.3 ± 1.63*, # |
| K hydroxide 2.5% | >60 |
P<0.05 vs. saline, sodium hydroxide 2.5%, potassium hydroxide 2.5%
P<0.05 vs. sodium hydroxide 10%, potassium hydroxide 10%
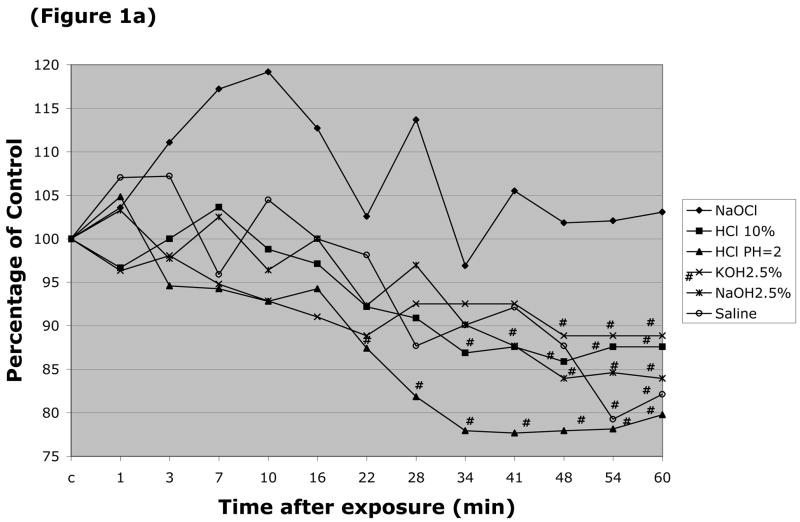
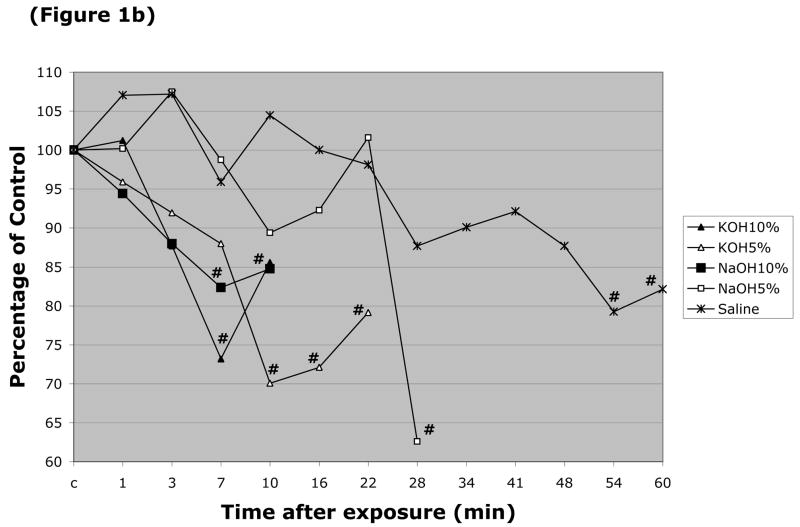
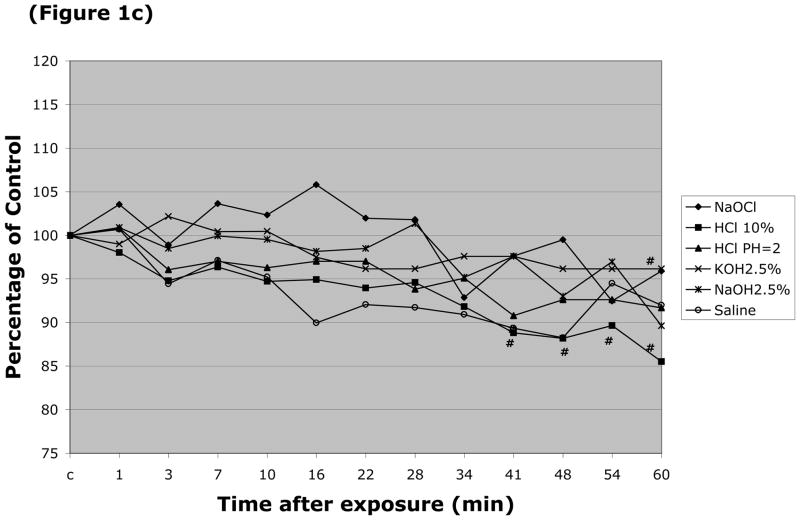
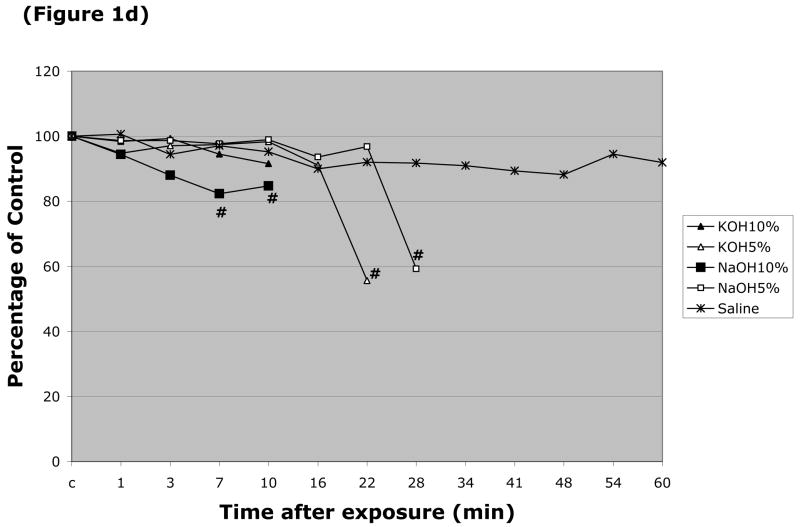
The responses of arteriolar and venular diameter to the different caustic solutions were also monitored. The resting (control) diameter of arterioles in these experiments was 24.2 ± 0.86 μm, while the resting venular diameter was 44.5 ± 1.4 μm. Arteriolar diameter was largely unaffected by luminal perfusion with most the caustic agents, with the exception of HCl (pH=2.0 & 10%) (Figure 1a). More profound changes in arteriolar diameter were noted with 5 & 10% NaOH, and 5% & 10% KOH (Figure 1b), which produced 38%, 18%, 30% and 27% peak reductions in arteriolar diameter, respectively. Only two solutions were noted to significantly alter the diameter of esophageal venules by more than 20% (Figure 1d), i.e., 5% KOH and 5% NaOH, which reduced vessel diameter by 49% and 39%, respectively. 10% NaOH produced an 18% reduction in venular diameter, while an even smaller (11–14% reduction) change was noted with 10% HCl (Figure 1c).
Figure 1.
Changes in esophageal arteriolar diameter over a 60 minute period following intralumenal exposure to (Figure 1a) NaOCl, HCl (pH=2 & 10%), 2.5% KOH, 2.5% KOH, or saline. (Figure 1b) summarizes the changes for 5 & 10% NaOH and 5 & 10% KOH. With the latter solutions, arteriolar diameter was not measured after flow stasis was detected. Changes in the venular diameter where shown in (Figure 1c) for NaOCl, HCl (pH=2 & 10%), 2.5% KOH, 2.5% KOH, or saline, and (Figure 1d) for 5 & 10% NaOH and 5 & 10% KOH. # indicates p < 0.05 compared to corresponding control value.
Under basal conditions, the number of adherent leukocytes in esophageal venules (no leukocyte adherence was noted in arterioles) was 301 ± 58 cells per mm2 vessel area, while the leukocyte flux was 754 ± 82 cells/30 secs per mm2. Neither the number of adherent leukocytes nor leukocyte flux was significantly altered by exposure of the esophagus to saline or any of the caustic solutions, although there was a tendency for a decline in leukocyte recruitment that paralleled the changes in venular blood flow.
Histopathology
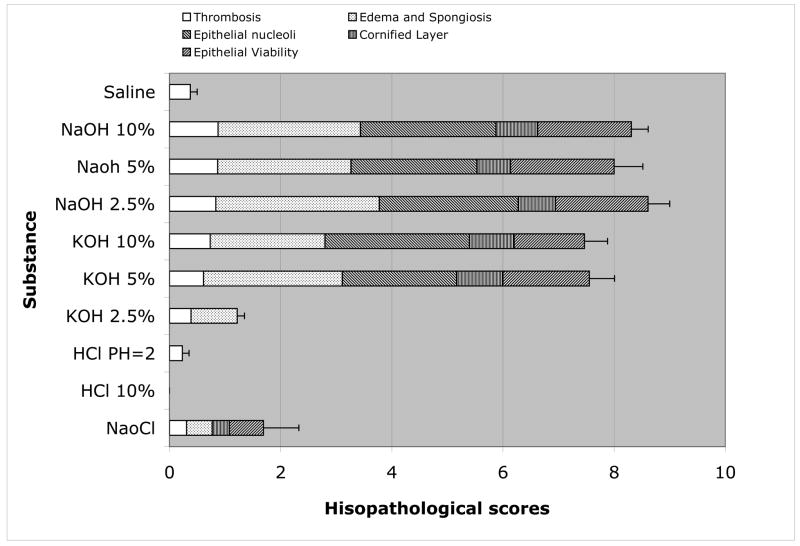
A summary of the scoring of histopathological changes in the esophagus elicited by the different caustic solutions is provided in (Figure 2). The total score (sum of individual indices) and the contributions of tissue viability, cornified epithelial cell differentiation, epithelial cell nucleoli, edema/spongiosis, and thrombosis to the total score are presented for each solution. While no significant tissue pathology was evident after exposure of the mouse esophagus to either saline, HCl (10% & pH=2) or 2.5% KOH, significant histological changes were noted with all other solutions. NaOCl elicited a small (1.7 ± 0.6) but significant increase in histopathology score that reflected changes in epithelial viability, edema/spongiosis, and the presence of a cornified layer as well as thrombosis. The histopathological changes noted with 5 & 10% KOH, and 2.5, 5, and 10% NaOH were substantially larger, with total histopathology scores ranging between ~7.5 (5 & 10% KOH) and 8.0 – 8.6 (2.5, 5 & 10% NaOH). Edema/spongiosis and the appearance of epithelial nucleoli largely accounted for the high total histopathology scores induced by these solutions.
Figure 2.
Histopathology score for murine esophagus exposed to different caustic solutions. The contributions of thrombosis, edema/spongiosis, cornified layer, epithelial viability, and epithelial nucleoli to the total score are also depicted.
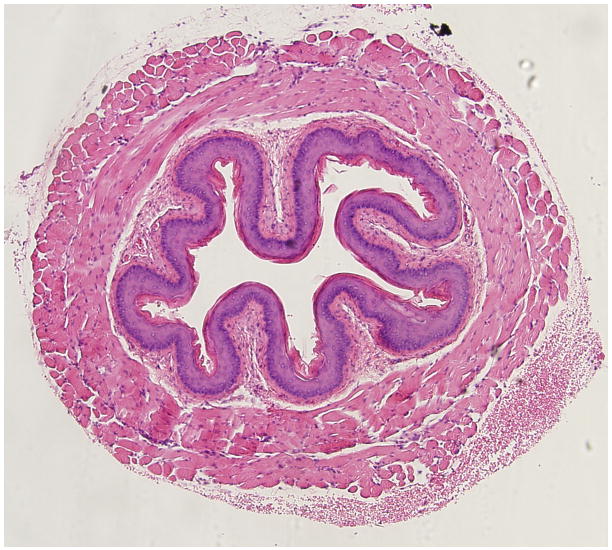
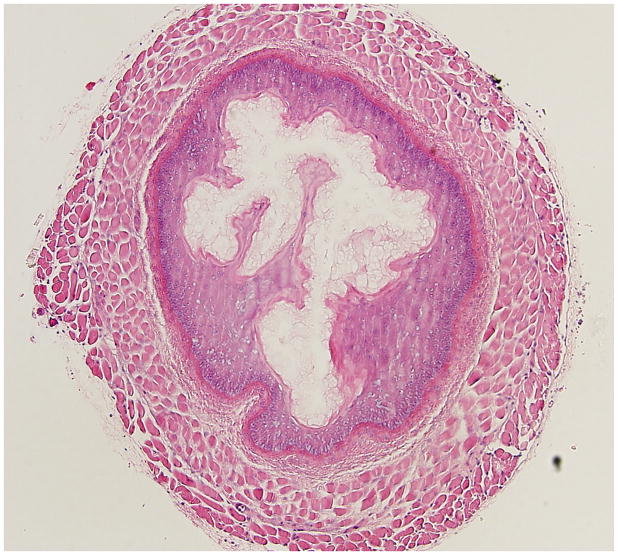
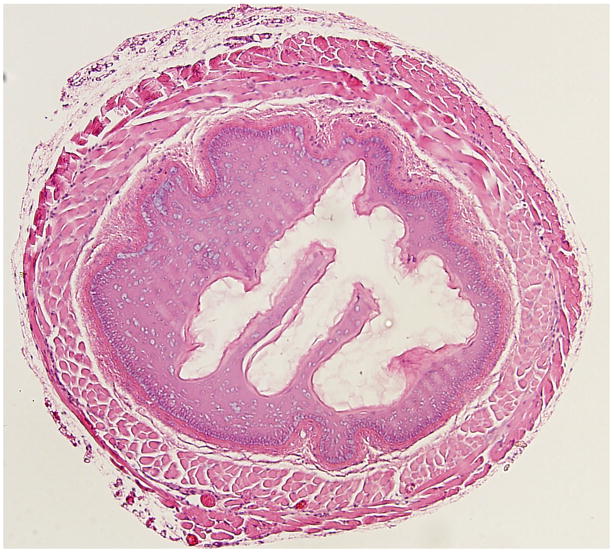
(Figure 3) illustrates representative histopathological changes observed in the murine esophagus exposed to 10% KOH (Figure 3b), or 10% NaOH (Figure 3c), and compares these changes to the saline exposed esophagus (Figure 3a). These changes are typical of the quantitative histopathological data summarized in Figure 2 for the same caustic agents and concentration. (Figure 4) illustrates representative responses of arterioles and venules in the esophageal microcirculation following exposure to KOH 10 %. Figure 4a shows both an arteriole and venule before exposure to the caustic agent. Figure 4 b is an image obtained 4 minutes post-exposure and shows a marked reduction in the arteriolar diameter with almost no change in venular diameter. The number of adherent leukocytes in the venule was largely unaffected by KOH exposure.
Figure 3.
Representative histopathological changes observed in the murine esophagus exposed to 10% KOH (Figure 3b), or 10% NaOH (Figure 3c), and compares these changes to the saline exposed esophagus (Figure 3a).
Figure 4.


illustrates typical changes in an esophageal arteriole (A) and venule (V) following exposure to KOH 10 %. (Figure 4a) shows both an arteriole and venule before the exposure. (Figure 4b) is an image obtained 4 minutes post-exposure.
Discussion
The accidental ingestion of caustic agents remains a leading cause of esophageal injury in children worldwide1. While this has led to an intensive effort to identify therapeutic interventions that protect against chemical burns to the esophagus, no effective treatments for the acute phase of injury have been found to date. Advancements in this field however may be limited by the lack of information on how strong alkali and acids produce rapid tissue necrosis in the esophagus. Although thrombosis, ischemia and inflammation have all been implicated in caustic injury to the esophagus1, there are no reports in the literature that directly address the responses of the esophageal microvasculature to the injury-inducing caustic agents. In this study, intravital videomicroscopy was used to monitor the early responses of the murine esophageal microcirculation to noxious concentrations of alkali and acids in order to determine whether these agents elicit changes that are consistent with thrombosis, ischemia and inflammation. Our findings are consistent with rapid vascular stasis caused by both vessel constriction and thrombosis. Our findings also indicate that the vascular stasis is not accompanied by the recruitment of inflammatory cells in the early period following exposure to the noxious agents.
Previous studies of caustic tissue injury to the esophagus have largely involved the use of rats, rabbits or larger animals12,13,14. The present study represents the first effort to evaluate the toxic effects of strong alkalis and acids in mouse esophagus. The histopathological changes elicited by alkali and acid in mouse esophagus are qualitatively similar to those previously reported for other species and are consistent with the view that strong acids and alkali cause rapid tissue necrosis, edema and thrombosis in this tissue5. Quantitative comparisons of our histopathological changes with those reported in other tissues are difficult since different concentrations of the noxious agents and pathologic scoring systems were used. Nonetheless, the murine model for study of esophageal injury following exposure to caustic agents holds much promise since the animals are readily available and inexpensive. More importantly, a large number and wide variety of mutant mice are available for detailed analysis of molecular mechanisms that contribute to the pathogenesis of esophageal injury. These mutants provide an entirely new strategy for identifying potential therapeutic strategies for treatment of burn injuries in the esophagus.
A striking response of the esophageal circulation to caustic injury was the complete and rapid cessation of blood flow in both arterioles and venules, which supports the view that ischemia is an early manifestation of the injury response to noxious agents. The highest concentrations of alkali (10% KOH & NaOH) studied led to flow cessation within 5–8 minutes, while 5% solutions of the same agents caused flow cessation within 15–16 minutes (Table 1). Our arteriolar diameter and histopathology data suggest that both thrombosis and vasoconstriction may contribute to the rapid stasis in esophageal arterioles and venules. Exposure of the esophagus to the alkali solutions was usually accompanied by thrombosis (Figure 1). Some solutions, such as 5% NaOH and 5% KOH, were also associated with significant peak reductions in arteriolar diameter, ranging between 25 and 40%. Assuming a cylindrical geometry, arteriolar constriction of this magnitude may result in blood reductions ranging between 70 and 90%. The added response of venoconstriction that was noted for 5% KOH may also contribute to the rapid decline in blood flow. It is likely that the combination of vasoconstriction and thrombosis is needed to elicit the stasis that was observed with the alkali solutions. Support for this contention is derived from the observation that HCl (pH =2) was associated with a 23% reduction in arteriolar diameter but less frequently with thrombosis. Similarly, HCl did not lead to flow cessation within 60 min nor did it produce significant histological changes. The absence of histological changes with HCl may reflect the short duration of acid exposure.
Another objective of this study was to determine whether the esophageal microcirculation contributes to the early injury response to caustic agents by mediating the recruitment of inflammatory cells. By monitoring the trafficking of leukocytes in esophageal venules using intravital microscopy we did not observe a significant increase in the recruitment of leukocytes within the first hour after caustic injury. While 60 min may have been too early to expect significant leukocyte recruitment, previous work in other vascular beds of the digestive system have revealed large and highly significant increases in the number of adherent leukocytes when venular blood flow (shear rate) is reduced19,20. This shear rate-dependent recruitment of adherent leukocytes is manifested when blood flow is reduced either locally by vessel occlusion or systemically by graded hemorrhage19,20. The absence of leukocyte recruitment in esophageal venules in the face of profound reductions in blood flow is therefore unexpected, but it may reflect the unique circumstances (such as thrombosis and direct chemical interactions with blood elements) that accompany caustic injury. Our observations in the first 60 min following esophageal exposure to strong alkali and NaOCl do not negate a role for inflammatory cell infiltration and leukocyte-mediated tissue damage at later time points. It is possible that venular blood flow is eventually restored in some esophageal venules after caustic injury, allowing for the delivery of inflammatory cells that have been shown histologically to infiltrate the esophagus days after exposure to the caustic agent 21. Additional work is needed to address how the injured esophageal microvessels can sustain this delivery of inflammatory cells in the face of blood flow stasis.
In conclusion, the results of this study provide novel insights into the early vascular responses that accompany the esophageal injury induced by strong acid and alkali. Our findings indicate that the most toxic noxious agents are alkali which elicit a rapid cessation of blood flow in esophageal microvessels. Vasoconstriction and thrombosis may both contribute to the esophageal ischemia induced by alkali. The changes in esophageal blood flow are not associated with an enhanced recruitment of inflammatory cells that is mediated through adhesive interactions with venular endothelium. Intravital microscopic observation of the murine esophageal microcirculation represents a powerful new approach to defining the mechanisms that underlie the burn injury that is associated with ingestion of caustic agents.
Acknowledgments
Supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK43875)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haller JA, Andrews HG, White JJ, Tamer MA, Cleceland WW. Pathophysiology and management of acute corrosive burns of the esophagus. Results of treatment in 285 children. J Ped Surg. 1971;6:578–584. doi: 10.1016/0022-3468(71)90382-4. [DOI] [PubMed] [Google Scholar]
- 2.Jong AL, Macdonald R, Ein S, Forte V, Turner A. Corrosive esophagitis in children: a 30-year review. Int J Pediatr Otorhinolaryngol. 2001;57:203–211. doi: 10.1016/s0165-5876(00)00440-7. [DOI] [PubMed] [Google Scholar]
- 3.Yukselen V, Karaoglu AO, Yenisey C, Tuncyurek M, Ozutemiz O. Trimetazidine reduces the degree of fibrosis in alkali burns of the esophagus. J Pediatr Surg. 2005;40:505–9. doi: 10.1016/j.jpedsurg.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Basaran UN, Eskiocak S, Altaner S, Ture M, Yapar SB. Inhibition of iNOS with S-methylisothiourea was impaired in wound healing in caustic esophageal burn. Int J Pediatr Otorhinolaryngol. 2005;69:471–7. doi: 10.1016/j.ijporl.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Homan CS, Maitra SR, Lane BP, Thode HC, Sable M. Therapeutic effects of water and milk for acute alkali injury of the esophagus. Ann Emerg Med. 1994;24:14–20. doi: 10.1016/s0196-0644(94)70155-5. [DOI] [PubMed] [Google Scholar]
- 6.Yukselen V, Vardar E, Yukselen O, Karaoglu AO, Yenisey C, Ozutemiz O. Colchicine in experimental alkaline burns of the rat esophagus: an old drug, a new indication? Pediatr Surg Int. 2006;22:363–8. doi: 10.1007/s00383-006-1644-5. [DOI] [PubMed] [Google Scholar]
- 7.Temir ZG, Karkiner A, Karaca I, Ortac R, Ozdamar A. The effectiveness of sucralfate against stricture formation in experimental corrosive esophageal burns. Surg Today. 2005;35:617–22. doi: 10.1007/s00595-004-3005-0. [DOI] [PubMed] [Google Scholar]
- 8.Ocakci A, Coskun O, Tumkaya L, Kanter M, Gurel A, Hosnuter M, Uzun L. Beneficial effects of Ebselen on corrosive esophageal burns of rats. Int J Pediatr Otorhinolaryngol. 2006;70:45–52. doi: 10.1016/j.ijporl.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Ekingen G, Ozden M, Sozubir S, Maral H, Muezzinoglu B, Kahraman H, Guvenc BH. Effect of the prostacyclin derivate iloprost in experimental caustic esophageal burn. Pediatr Surg Int. 2005;21:441–4. doi: 10.1007/s00383-005-1429-2. [DOI] [PubMed] [Google Scholar]
- 10.Yukselen V, Karaoglu AO, Ozutemiz O, Yenisey C, Tuncyurek M. Ketotifen ameliorates development of fibrosis in alkali burns of the esophagus. Pediatr Surg Int. 2004;20:429–33. doi: 10.1007/s00383-004-1170-2. [DOI] [PubMed] [Google Scholar]
- 11.Koltuksuz U, Mutus HM, Kutlu R, Ozyurt H, Cetin S, Karaman A, Gurbuz N, Akyol O, Aydin NE. Effects of caffeic acid phenethyl ester and epidermal growth factor on the development of caustic esophageal stricture in rats. J Pediatr Surg. 2001;36:1504. doi: 10.1053/jpsu.2001.27032. [DOI] [PubMed] [Google Scholar]
- 12.Bingol-Kologlu M, Tanyel FC, Muftuoglu S, Renda N, Cakar N, Buyukpamukcu N, Hicsonmez A. The preventive effect of heparin on stricture formation after caustic esophageal burns. J Pediatr Surg. 1999;34:291–4. doi: 10.1016/s0022-3468(99)90193-8. [DOI] [PubMed] [Google Scholar]
- 13.Bautista A, Tojo R, Varela R, Estevez E, Villanueva A, Cadranel S. Effects of prednisolone and dexamethasone on alkali burns of the esophagus in rabbit. J Pediatr Gastroenterol Nutr. 1996;22:275–83. doi: 10.1097/00005176-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Gehanno P, Guedon C. Inhibition of experimental esophageal lye strictures by penicillamine. Arch Otolaryngol. 1981;107:145–7. doi: 10.1001/archotol.1981.00790390011004. [DOI] [PubMed] [Google Scholar]
- 15.Yagmurlu A, Aksu B, Bingol-Kologlu M, Renda N, Altinok G, Fitoz S, Gokcora IH, Dindar H. A novel approach for preventing esophageal stricture formation: sphingosylphosphorylcholine-enhanced tissue remodeling. Pediatr Surg Int. 2004;20(10):778–82. doi: 10.1007/s00383-004-1145-3. [DOI] [PubMed] [Google Scholar]
- 16.Meyers RL, Glenn L, Orlando RC. Protection against alkali injury to rabbit esophagus by CO2 inhalation. Am J Physiol. 1993;264:G150–6. doi: 10.1152/ajpgi.1993.264.1.G150. [DOI] [PubMed] [Google Scholar]
- 17.Eros G, Kaszaki J, Czobel M, Boros M. Effects of phosphatidylcholine pretreatment during acute experimental biliary reflux. Magy Seb. 2005;58(6):406–14. [PubMed] [Google Scholar]
- 18.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55(5):662–75. [PubMed] [Google Scholar]
- 19.Bienvenu K, Granger DN. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am J Physiol. 1993;264:H1504–8. doi: 10.1152/ajpheart.1993.264.5.H1504. [DOI] [PubMed] [Google Scholar]
- 20.Russell J, Cooper D, Tailor A, Stokes KY, Granger DN. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol Gastrointest Liver Physiol. 2003;284:G123–9. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- 21.Mamede RC, de Mello Filho FV. Ingestion of caustic substances and its complications. Sao Paulo Med J. 2001;119:10–5. doi: 10.1590/S1516-31802001000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]