Abstract
T cell receptors (TCRs) exhibit genetic and structural diversity similar to antibodies, but they have binding affinities that are several orders of magnitude lower. It has been suggested that TCRs undergo selection in vivo to maintain lower affinities. Here, we show that there is not an inherent genetic or structural limitation on higher affinity. Higher-affinity TCR variants were generated in the absence of in vivo selective pressures by using yeast display and selection from a library of Vα CDR3 mutants. Selected mutants had greater than 100-fold higher affinity (KD ≈ 9 nM) for the peptide/MHC ligand while retaining a high degree of peptide specificity. Among the high-affinity TCR mutants, a strong preference was found for CDR3α that contained Pro or Gly residues. Finally, unlike the wild-type TCR, a soluble monomeric form of a high-affinity TCR was capable of directly detecting peptide/MHC complexes on antigen-presenting cells. These findings prove that affinity maturation of TCRs is possible and suggest a strategy for engineering TCRs that can be used in targeting specific peptide/MHC complexes for diagnostic and therapeutic purposes.
T cells recognize a foreign peptide bound to the MHC product through the αβ heterodimeric receptor. The T cell receptor (TCR) repertoire has extensive diversity created by the same gene rearrangement mechanisms used in antibody heavy- and light-chain genes (1). Most of the diversity is generated at the junctions of V and J (or diversity, D) regions that encode the complementarity-determining region three (CDR3) of the α and β chains (2). However, TCRs do not undergo somatic point mutations as do antibodies, and perhaps not coincidentally, TCRs also do not undergo the same extent of affinity maturation as antibodies. TCRs appear to have affinities that range from 105 to 107 M−1 whereas antibodies have affinities that range from 105 to 1010 M−1 (3, 4).
Whereas the absence of somatic mutation in TCRs may be associated with lower affinities, it has also been argued that there is not a selective advantage for a TCR to have higher affinity (5–7). In fact, the serial-triggering (6) and kinetic proofreading (7) models of T cell activation both suggest that very slow off-rates (associated with higher affinity) would be detrimental to the signaling process. On the other hand, the fastest off-rates that have been measurable have been associated with altered pMHC that exhibits antagonist activity (8–11). Whereas the narrow range of natural TCR affinities has provided some evidence for the relationships between off-rates and agonist/antagonist activity, there are also examples that appear to be inconsistent with these hypotheses (12, 13).
There are other possible explanations for why the T cell system maintains relatively low TCR:pMHC affinities in vivo. Peptides bound within the MHC groove display limited accessible surface (14), which may in turn limit the amount of free energy that can be generated in the interaction. On the other hand, raising the affinity of a TCR by directing the free energy toward the MHC helices would presumably lead to thymic deletion during negative selection (15). Even if such higher-affinity TCR could escape thymic deletion, they would likely not maintain the peptide specificity required for T cell responses.
It has not been possible to directly test these possibilities because the generation of TCRs with affinities above 107 M−1 has not been accomplished. In addition to allowing a kinetic basis of T cell triggering, high-affinity TCRs could be used to more easily explore the role of peptide in pMHC recognition, and as quantitative probes for the expression of pMHC on various target cells. Because in vivo selection schemes have not yielded TCRs with the intrinsic binding affinities of affinity-matured antibodies, in this report, we have used an in vitro method for the directed evolution of high-affinity TCRs. The method relies on the expression of a library of mutant single-chain (Vβ-linker-Vα) TCRs on the surface of yeast, as a fusion to the surface protein Aga-2 (16, 17). Our previous studies have shown that the yeast display system could be used to engineer variants of the 2C single-chain TCR (scTCR) that were more thermally stable and secreted at higher levels (17, 18). The stability mutants were isolated by subjecting the entire TCR gene to random mutagenesis and selecting for increased surface levels with anti-TCR antibodies (17). The mutations that increased stability resided at the Vα:Vβ interface or on the outside surface of Vβ in a region not involved in pMHC binding. To isolate TCR with higher affinity for pMHC, in the present study, we mutated only the CDR3α loop, which is at the center of the pMHC-binding site (19). Our efforts were guided by previous findings that this region contributed minimal binding free energy to the interaction of the 2C TCR with the pMHC ligand QL9/Ld (20), suggesting that productive interactions might be improved by focusing on this region. Remarkably, selection from a relatively small library (105 mutants) yielded many different TCRs with up to 100-fold increased affinity for QL9/Ld. The high-affinity TCRs retained a high degree of peptide specificity although there was some variation in fine specificity among the mutants. These findings suggest that the in vitro evolution process described here can be used to isolate TCRs with specificities that one defines by selection with appropriate pMHC ligands.
The high-affinity receptors in this study were derived by variation at the VJ junction, the same process that operates very effectively in vivo through gene rearrangements in T cells (2). The fact that we could readily isolate a diverse set of high-affinity TCR in vitro indicates that there is not a genetic or structural limitation to high-affinity receptors. This supports the view that inherently low affinities of TCRs found in vivo are caused by a lack of selection for higher affinity and perhaps a selection for lower affinity (5–7). Finally, the high-affinity TCR were used in monomeric form to detect pMHC on the surface of target cells, indicating that soluble forms of the TCR selected with the yeast display system can serve as probes for tumor-associated pMHC or other T cell-specific ligands.
Materials and Methods
Library Construction.
The 2C single-chain TCR (scTCR) used as the scaffold for directed evolution (T7) contained six mutations (βG17E, βG42E, βL81S, αL43P, αW82R, and αI118N) that have been shown to increase the stability of the TCR but still allow pMHC binding (E.V.S., K.D.W., and D.M.K., unpublished results; and ref. 18). Mutagenic PCR of the T7 scTCR VαCDR3 was performed by using an AGA-2-specific upstream primer and a degenerate downstream primer 5′-CTTTTGTGCCGGATC-CAAATGTCAG(SNN)5GCTCACAGCACAGAAGTACACG-GCCGAGTCGCTC-3′. Underlined bases indicate the positions of silent mutations introducing unique BamHI and EagI restriction sites. The purified PCR product was digested with NdeI and BamHI and ligated to NdeI–BamHI-digested T7/pCT302 (16–18). The ligation mixture was transformed into DH10B electrocompetent Escherichia coli (GIBCO/BRL), and transformants were pooled into 250-ml LB supplemented with ampicillin at 100 μg/ml and grown overnight at 37°C. Plasmid DNA was transformed into the yeast strain EBY100 by the method of Gietz and Schiestl (21).
Cell Sorting.
The yeast library (22) was grown in 2% dextrose/0.67% yeast nitrogen base/1% Casamino acids (Difco) at 30°C to an OD600 = 4.0. To induce surface scTCR expression, yeast were pelleted by centrifugation, resuspended to an OD600 = 1.0 in 2% galactose/0.67% yeast nitrogen base/1% Casamino acids, and incubated at 20°C for ≈24 h. In general, ≈107 cells per tube were incubated on ice for 1 h with 50 μl of QL9/Ld/IgG dimers (23) diluted in PBS (pH 7.4) supplemented with 0.5 mg/ml BSA. After incubation, cells were washed and labeled for 30 min with FITC-conjugated goat anti-mouse IgG F(ab′)2 (Kirkegaard & Perry) in PBS (pH 7.4) supplemented with 0.5 mg/ml BSA. Yeast were then washed and resuspended in PBS (pH 7.4) supplemented with 0.5 mg/ml BSA immediately before sorting. Cells exhibiting the highest fluorescence were isolated by using a Coulter 753 bench fluorescence-activated cell sorter. After isolation, sorted cells were expanded in 2% dextrose/0.67% yeast nitrogen base/1% Casamino acids and induced in 2% galactose/0.67% yeast nitrogen base/1% Casamino acids for subsequent rounds of selection. A total of four sequential sorts were performed. The concentrations of QL9/Ld/IgG dimers used for staining were 50 μg/ml for sorts one to three and 0.5 μg/ml for the final sort. The percentages of total cells isolated from each sort were 5.55, 2.68, 2.56, and 0.58%, respectively. Aliquots of sorts three and four were plated on 2% dextrose/0.67% yeast nitrogen base/1% Casamino acids to isolate individual clones which were analyzed by flow cytometry by using a Coulter Epics XL instrument.
Soluble scTCR Production.
The T7 and m6 scTCR genes were excised from pCT302 NheI–XhoI and ligated into NheI–XhoI digested pRSGALT, a yeast expression plasmid (18). Ligations were transformed into DH10B electrocompetent E. coli (GIBCO/BRL). Plasmid DNA was isolated from bacterial cultures and transformed into the Saccharomyces cerevisiae strain, BJ5464 (α ura3–52 trp1 leu2Δ1 his3Δ200 pep4∷HIS3 prb1Δ1.6R can1 GAL) (18). Yeast clones were grown in 1 liter of 2% dextrose/0.67% yeast nitrogen base/1% Casamino acids/20 mg/liter Trp for 48 h at 30°C. To induce scTCR secretion, cells were pelleted by centrifugation at 4,000 × g, resuspended in 1 liter of 2% galactose/0.67% yeast nitrogen base/1% Casamino acids/20 mg/l Trp supplemented with 1 mg/ml BSA, and incubated for 72 h at 20°C. Culture supernatants were harvested by centrifugation at 4,000 × g, concentrated to ≈50 ml, and dialyzed against PBS (pH 8.0). The six His-tagged scTCRs were purified by native nickel affinity chromatography [Ni-NTA Superflow, Qiagen (Chatsworth, CA); 5 mM and 20 mM imidazole (pH 8.0) wash; 250 mM imidazole elution] (18).
Cell-Binding Assays.
The binding of soluble scTCRs to QL9/Ld was monitored in a competition format as described (20, 24). Peptide-loaded T2-Ld cells (3 × 105 per well) were incubated for 1 h on ice in the presence of 125I-labeled anti-Ld Fabs (30–5-7) and various concentrations of scTCRs. Bound and unbound [125I] 30–5-7 Fabs were separated by centrifugation through olive oil/dibutyl phthalate. Inhibition curves were constructed to determine inhibitor concentrations yielding 50% maximal inhibition. Dissociation constants were calculated by using the formula of Cheng and Prusoff (25). To monitor direct binding of scTCRs to cell-bound pMHC, peptide-loaded T2-Ld cells (5 × 105 per tube) were incubated for 40 min on ice with biotinylated soluble scTCRs followed by staining for 30 min with streptavidin-phycoerythrin (PharMingen). Cellular fluorescence was detected by flow cytometry.
Results and Discussion
To examine if it is possible to generate higher-affinity TCR that would retain peptide specificity, we subjected a TCR to a process of directed in vitro evolution. Phage display (26) has not yet proven successful in the engineering of single-chain TCRs (scTCRs, Vβ-linker-Vα) despite the extensive structural similarity between antibody and TCR V regions. However, we recently showed that a scTCR could be displayed on the surface of yeast (17) in a system that has proven successful in antibody engineering (16, 27). A temperature-stabilized variant (T7) (18) of the scTCR from the cytotoxic T lymphocyte clone 2C was used in the present study. Cytotoxic T lymphocyte clone 2C recognizes the alloantigen Ld with a bound octamer peptide called p2Ca, derived from the enzyme 2-oxoglutarate dehydrogenase (28). The nonameric variant QL9 is also recognized by cytotoxic T lymphocyte 2C, but with 10-fold higher affinity by the 2C TCR (29). Alanine scanning mutagenesis showed that the CDR3α loop contributed minimal free energy to the binding interaction (20), even though structural studies have shown that CDR3α of the 2C TCR is near the peptide and it undergoes a conformational change to accommodate the pMHC complex (19). Thus, we focused our mutagenesis efforts on five residues that form the CDR3α loop.
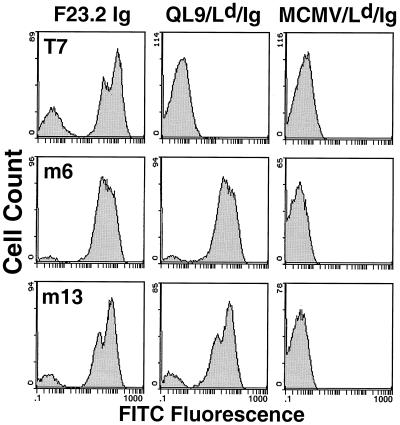
A library of 105 independent TCR-CDR3α yeast mutants was subjected to selection by flow cytometry with a fluorescently labeled QL9/Ld ligand (23). After four rounds of sorting and growth, 15 different yeast colonies were examined for their ability to bind the ligand, in comparison to the scTCR variant T7, which bears the wild-type CDR3α sequence (Fig. 1 and data not shown). The anti-Vβ8.2 antibody F23.2 which recognizes residues in the CDR1 and CDR2 was used as a control to show that wild-type scTCR-T7 and scTCR mutants (m6 and m13, in Fig. 1, and others, data not shown) each had approximately equivalent surface levels of the scTCR (Fig. 1). In contrast, the soluble QL9/Ld ligand bound very well to each mutant yeast clone but not to wild-type scTCR-T7. The MCMV/Ld complex, which is not recognized by cytotoxic T lymphocyte clone 2C, did not bind to the scTCR mutants or wild-type scTCR-T7, indicating that the scTCR mutants retained peptide specificity.
Figure 1.
Flow cytometric analysis of yeast cells that express wild-type and mutant 2C TCR on their surface. Yeast cells displaying wild-type (T7) and mutant (m6 and m13) scTCR were stained with anti-Vβ8 antibody F23.2 (120 nM), the specific alloantigenic peptide-MHC, QL9/Ld/Ig (40 nM), or a null peptide MCMV/Ld/Ig (40 nM). The peptides used in this study were QL9 (QLSPFPFDL), MCMV (YPHFMPTNL), and p2Ca (LSPFPFDL). Binding was detected by FITC-conjugated goat anti-mouse IgG F(ab′)2 and analyzed by flow cytometry. The negative population (e.g., seen with F23.2 staining) has been observed for all yeast-displayed proteins and is thought to be caused by cells at a stage of growth or induction that are incapable of expressing surface fusion protein (16, 17, 27).
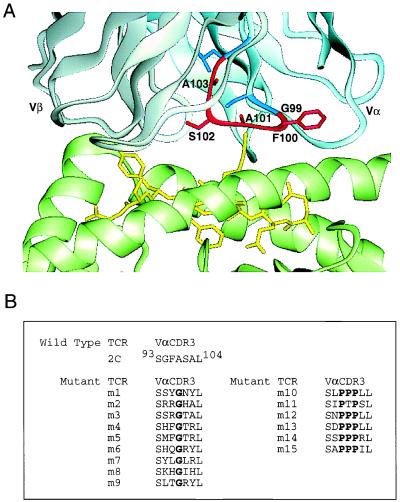
The CDR3α sequences of the 15 mutants all differed from the 2C TCR (Fig. 2). It was readily apparent (and confirmed by a blast alignment algorithm) that the sequences could be aligned into two motifs. One motif contained Gly in the middle of the five residue stretch whereas the other motif contained three tandem Pro. Evidence that all three Pro are important in generating the highest affinity site is suggested by results with mutant m11. Mutant m11 contained only two of the three Pro and exhibited reduced binding compared with the triple-Pro mutants (data not shown). The Gly-containing mutants appeared to have preferences for positive-charged residues among the two residues to the carboxyl side (7/9) and aromatic and/or positive-charged residues among the two residues to the amino side (4/9 and 5/9). The selection for a glycine residue at position 102 in the motif may indicate that the CDR3α loop required conformational flexibility around this residue to achieve increased affinity. This is consistent with the large (6-Å) conformational difference observed between the CDR3α loops of the liganded and unliganded 2C TCR (19). It is also interesting that Gly is the most common residue at the V(D)J junctions of antibodies and the presence of a Gly has recently been associated with increased affinity in the response to the (4-hydroxy-3-nitrophenyl) acetyl hapten (30).
Figure 2.
Structure and sequences of the 2C TCR CDR3α. (A) X-ray crystallographic structure of the 2C/dEV8/Kb complex with CDR3α aa highlighted. Five residues of the 2C VαCDR3 that were randomized by PCR with a degenerate primer are shown in red. The adjacent CDR3 residues, Ser-93 and Leu-104 shown in blue, were retained in the yeast display library because they have been shown to be important in pMHC binding (17, 18, 20). (B) Alignment of aa sequences of mutant scTCRs isolated by yeast display and selection with QL9/Ld. Display plasmids were isolated from yeast clones after selection and sequenced to determine CDR3α sequences. Mutants m1, m2, m3, m4, m10, and m11 were isolated after the third round of sorting. All other mutants were isolated after the fourth round of sorting.
In contrast to the isolates that contain Gly, the selection for a Pro-rich sequence at the tip of the CDR3α loop may suggest that these TCR exhibit a more rigid conformation that confers higher affinity. The x-ray crystallographic structures of a germ-line antibody of low affinity compared with its affinity-matured derivative showed that the high-affinity state was associated with stabilization of the antibody in a configuration that accommodated the hapten (31). Similarly, the NMR solution structure of a scTCR that may be analogous to the germ-line antibody showed that the CDR3α and β loops both exhibited significant mobility (32). Recent thermodynamic studies of TCR:pMHC interactions have also suggested the importance of conformational changes in binding (33, 34). Structural and thermodynamic studies of the TCR mutants that we report here should allow us to examine if the two CDR3α motifs (Gly- versus Pro-rich) might differ in the mechanism by which they confer higher affinity.
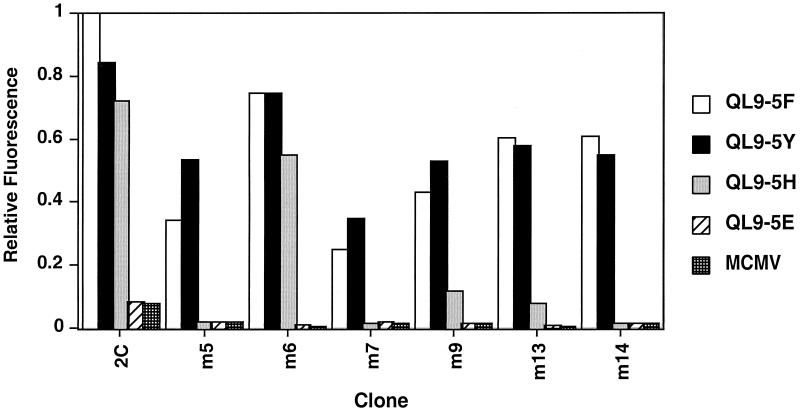
Although the scTCR mutants did not bind the null peptide/Ld complex MCMV/Ld, it remained possible that the increase in affinity might be accompanied by a change in fine specificity. To examine this issue, we used QL9 position 5 (Phe) peptide variants that have been shown previously to exhibit significant differences in their binding affinity for the wild-type 2C TCR (35). The binding of these pMHC to various TCR mutants on the yeast surface and clone 2C were measured by flow cytometry. As shown in Fig. 3, the native TCR on 2C is capable of binding QL9 variants that contain either Tyr or His at position five but not Glu. Each of the higher-affinity TCR mutants retained their ability to recognize the conserved Tyr-substituted peptide and they were likewise incapable of recognizing the Glu-substituted peptide. However, several of the TCR mutants (m6, m9, and m13) bound to the His-substituted peptide (albeit to different extents) whereas other mutants (m5, m7, and m14) did not bind the peptide. Thus, the CDR3α loop can influence the peptide fine specificity of recognition but it is not the only region of the TCR involved. The effect on peptide specificity could be through direct interaction of CDR3α residues with the variant peptide, as suggested from earlier studies involving CDR3-directed selections (36, 37). Alternatively, binding free energy may be directed at peptide-induced changes in the Ld molecule itself. The latter possibility is perhaps more likely in the case of the 2C TCR:QL9/Ld interaction, because position five of QL9 has been predicted to point toward the Ld groove (35, 38). The fine-specificity analysis also shows that it is possible to engineer TCRs with increased, or at least altered, specificity for cognate peptides. Thus, directed evolution of only a short region (CDR3α) of a single TCR allows the design of TCR variants with altered peptide-binding specificities.
Figure 3.
Fine specificity analysis of mutant scTCR binding to different QL9 variant peptides bound to Ld. The original T cell clone 2C and various yeast clones were analyzed by flow cytometry for binding to Ld/Ig dimers loaded with wild-type QL9 (QL9–5F), position 5 variants of QL9 (QL9–5Y, QL9–5H, and QL9–5E) or MCMV. Binding was detected with FITC-labeled goat anti-mouse IgG. Relative fluorescence was measured by comparison with mean fluorescence values of 2C cells or yeast cells stained with anti-Vβ8 antibody F23.2.
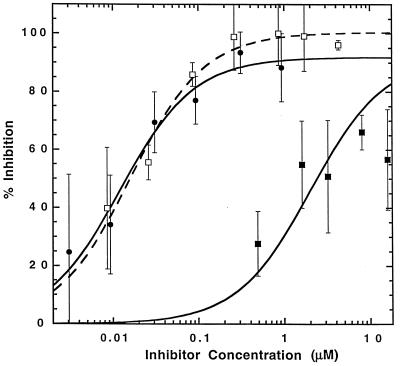
To determine the magnitude of the affinity increases associated with a selected CDR3α mutant, the wild-type T7 scTCR and the m6 scTCR were expressed as soluble forms in a yeast secretion system. Purified scTCR preparations were compared for their ability to block the binding of a 125I-labeled anti-Ld Fab fragments to QL9 or MCMV loaded onto Ld on the surface of T2-Ld cells. As expected, neither T7 nor m6 scTCR were capable of inhibiting the binding of 125I-Fab fragments to T2-Ld cells up-regulated with the MCMV peptide (data not shown). However, both T7 and m6 were capable of inhibiting the binding of anti-Ld Fab fragments to QL9/Ld (Fig. 4). The m6 scTCR variant was as effective as unlabeled Fab fragments in inhibiting binding, whereas the T7 scTCR was 160-fold less effective (average of 140-fold difference among four independent titrations). The KD values of the scTCR for QL9/Ld were calculated from the inhibition curves to be 1.5 μM for T7 and 9.0 nM for m6. The value for T7 is in close agreement with the 3.2 μM KD reported for the 2C scTCR (39). These findings show that the yeast system, combined with CDR3α-directed mutagenesis, is capable of selecting mutants with 100-fold higher intrinsic binding affinities for a pMHC ligand.
Figure 4.
QL9/Ld binding by soluble scTCRs. T2-Ld cells loaded with QL9 were incubated with 125I-labeled anti-Ld Fab fragments (30–5-7) and various concentrations of unlabeled Fab (□), scTCR-T7 (■), or mutant scTCR-m6 (●). Bound and unbound [125I]30–5-7 Fab fragments were separated by centrifugation through olive oil/dibutyl phthalate. Binding of 125I-labeled anti-Ld Fab fragments to T2-Ld cells loaded with the control peptide MCMV was not inhibited even at the highest concentrations of scTCRs (data not shown).
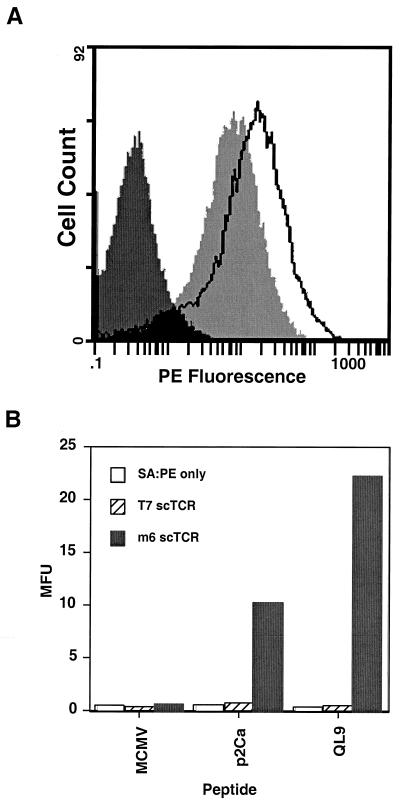
If the soluble scTCR has a high affinity for its pMHC ligand, then it may be useful, like antibodies, as a specific probe for cell surface-bound antigen. To test this possibility, the soluble T7 and m6 scTCR were biotinylated and the labeled scTCR were incubated with T2-Ld cells loaded with QL9, p2Ca, or MCMV. The m6 scTCR, but not the T7 scTCR, yielded easily detectable staining of the T2 cells that had been incubated with QL9 or p2Ca (Fig. 5 A and B). It is significant that p2Ca-up-regulated cells were also readily detected by m6 scTCR, because p2Ca is the naturally processed form of the peptide recognized by the alloreactive clone 2C and it has an even lower affinity than the QL9/Ld complex for the 2C TCR (29). However, it remains to be determined if the levels of pMHC derived from endogenous antigen processing are sufficient to allow detection by using soluble TCR as probes. It is reasonable to predict that, in some cases, the level will be too low to distinguish from background by using standard flow cytometry procedures.
Figure 5.
Flow cytometric analysis of the binding of scTCR/biotin to cell surface peptide/MHC. Peptide-loaded T2-Ld cells were incubated with biotinylated m6 scTCR (≈0.3 μM) or T7 scTCR (≈1.6 μM) scTCR followed by streptavidin-PE and analyzed by flow cytometry. (A) Flow cytometry histograms of T2-Ld cells loaded with QL9 (unshaded), p2Ca (light shade), or MCMV (dark shade) and stained with m6 scTCR/biotin. (B) Mean fluorescent units (MFU) of T2-Ld cells loaded with QL9, p2Ca, or MCMV and stained with either secondary SA-PE only, T7 scTCR/biotin + SA-PE, or m6 scTCR/biotin + SA-PE.
The high-affinity receptors described in our study were derived by variation at the VJ junction, the same process that operates very effectively in vivo through gene rearrangements in T cells (2). The fact that we could readily isolate a diverse set of high-affinity TCR in vitro indicates that there is not a genetic or structural limitation to high-affinity receptors. This supports the view that inherently low affinities of TCRs found in vivo are caused by a lack of selection for higher affinity and perhaps a selection for lower affinity (5–7). In this respect, the higher-affinity TCRs now provide the reagents for directly testing hypotheses about the effects of affinity on T cell responses (4–7). It is interesting to note that similar arguments have been used to suggest that the kinetic properties of antibodies may also set an in vivo “affinity ceiling,” above which there may not be a selective advantage to B cells (40).
In addition to their utility for testing T cell responses, high-affinity TCRs can be engineered like antibodies to yield high-affinity, antigen-specific probes. Soluble versions of the high-affinity receptor can directly detect specific peptide/MHC complexes on cells (Fig. 5). Thus, these engineered proteins have potential, for example, as tumor cell diagnostics, or on conjugation with cytotoxins, potential agents for cancer therapy.
Acknowledgments
We thank Michele Kieke for providing 2C scTCR genes cloned in the yeast display vector and Gary Durack of the University of Illinois Biotechnology Center Flow Cytometry Facility for assistance and advice. We also thank Jeff Bluestone and Ian Wilson for comments and discussion on the manuscript and results. This work was supported by National Institutes of Health Grant RO1 GM55767.
Abbreviations
- TCR
T cell receptor
- scTCR
single-chain T cell receptor
- CDR3
complementarity-determining regions three
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080078297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080078297
References
- 1.Tonegawa S. Biosci Rep. 1988;8:3–26. doi: 10.1007/BF01128968. [DOI] [PubMed] [Google Scholar]
- 2.Davis M M, Bjorkman P J. Nature (London) 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 3.Eisen H N, Sykulev Y, Tsomides T J. Adv Protein Chem. 1996;49:1–56. doi: 10.1016/s0065-3233(08)60487-8. [DOI] [PubMed] [Google Scholar]
- 4.Davis M M, Boniface J J, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 5.Sykulev Y, Cohen R J, Eisen H N. Proc Natl Acad Sci USA. 1995;92:11990–11992. doi: 10.1073/pnas.92.26.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Nature (London) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 7.Rabinowitz J D, Beeson C, Lyons D S, Davis M M, McConnell H M. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinowitz J D, Beeson C, Wulfing C, Tate K, Allen P M, Davis M M, McConnnell H M. Immunity. 1996;5:125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 9.Sloan-Lancaster J, Allen P M. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Alam S M, Travers P J, Wung J L, Nasholds W, Redpath S, Jameson S C, Gascoigne R J. Nature (London) 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 11.Kersh G J, Kersh E N, Fremont D H, Allen P M. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ramadi B K, Jelonek M T, Boyd L F, Margulies D H, Bothwell A L M. J Immunol. 1995;155:662–673. [PubMed] [Google Scholar]
- 13.Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh H L, Eisen H N. Immunity. 1998;9:475–483. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- 14.Bjorkman P J. Cell. 1997;89:167–170. doi: 10.1016/s0092-8674(00)80195-6. [DOI] [PubMed] [Google Scholar]
- 15.Bevan M J. Immunity. 1997;7:175–178. doi: 10.1016/s1074-7613(00)80520-8. [DOI] [PubMed] [Google Scholar]
- 16.Boder E T, Wittrup K D. Nat Biotechnol. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- 17.Kieke M C, Shusta E V, Boder E T, Teyton L, Wittrup K D, Kranz D M. Proc Natl Acad Sci USA. 1999;96:5651–5656. doi: 10.1073/pnas.96.10.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shusta E V, Kieke M C, Parke E, Kranz D M, Wittrup K D. J Mol Biol. 1999;292:949–956. doi: 10.1006/jmbi.1999.3130. [DOI] [PubMed] [Google Scholar]
- 19.Garcia K C, Degano M, Pease L R, Huang M, Peterson P, Teyton L, Wilson I A. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 20.Manning T C, Schlueter C J, Brodnicki T C, Parke E A, Speir J A, Garcia K C, Teyton L, Wilson I A, Kranz D M. Immunity. 1998;8:413–425. doi: 10.1016/s1074-7613(00)80547-6. [DOI] [PubMed] [Google Scholar]
- 21.Gietz R D, Schiestl R H, Willems A, Woods R A. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 22.Shusta E V, VanAntwerp J, Wittrup K D. Curr Opin Biotechnol. 1999;10:117–122. doi: 10.1016/s0958-1669(99)80020-2. [DOI] [PubMed] [Google Scholar]
- 23.Dal Porto J, Johansen T E, Catipovic B, Parfiit D J, Tuveson D, Gether U, Kozlowski S, Fearon D T, Schneck J P. Proc Natl Acad Sci USA. 1993;90:6671–6675. doi: 10.1073/pnas.90.14.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sykulev Y, Brunmark A, Jackson M, Cohen R J, Peterson P A, Eisen H N. Immunity. 1994;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y C, Prusoff W H. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 26.Clackson T, Hoogenboom H R, Griffiths A D, Winter G. Nature (London) 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 27.Kieke M C, Cho B K, Boder E T, Kranz D M, Wittrup K D. Protein Eng. 1997;10:1303–1310. doi: 10.1093/protein/10.11.1303. [DOI] [PubMed] [Google Scholar]
- 28.Udaka K, Tsomides T J, Walden P P, Fukusen N, Eisen H N. Proc Natl Acad Sci USA. 1993;90:11272–11276. doi: 10.1073/pnas.90.23.11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sykulev Y, Brunmark A, Tsomides T J, Kageyama S, Jackson M, Peterson P A, Eisen H N. Proc Natl Acad Sci USA. 1994;91:11487–11491. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukawa K, Akasako-Furukawa A, Shirai H, Nakamura H, Azuma T. Immunity. 1999;11:329–338. doi: 10.1016/s1074-7613(00)80108-9. [DOI] [PubMed] [Google Scholar]
- 31.Wedemayer G J, Patten P A, Wang L H, Schultz P G, Stevens R C. Science. 1997;276:1665–1669. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]
- 32.Hare B J, Wyss D F, Osburne M S, Kern P S, Reinherz E L, Wagner G. Nat Struct Biol. 1999;6:574–581. doi: 10.1038/9359. [DOI] [PubMed] [Google Scholar]
- 33.Willcox B E, Gao G F, Wyer J R, Ladbury J E, Bell J I, Jakobsen B K, Anton van der Merwe P. Immunity. 1999;10:357–365. doi: 10.1016/s1074-7613(00)80035-7. [DOI] [PubMed] [Google Scholar]
- 34.Boniface J J, Reich Z, Lyons D S, Davis M M. Proc Natl Acad Sci USA. 1999;96:11446–11451. doi: 10.1073/pnas.96.20.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlueter C J, Manning T C, Schodin B A, Kranz D M. J Immunol. 1996;157:4478–4485. [PubMed] [Google Scholar]
- 36.Sant'Angelo D B, Waterbury G, Preston-Hurburt P, Yoon S T, Medzhitov R, Hong S, Janeway C A. Immunity. 1996;4:367–376. doi: 10.1016/s1074-7613(00)80250-2. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen J L, Esser U, de St. Groth B F, Reay P A, Davis M M. Nature (London) 1992;355:224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- 38.Speir J A, Garcia K C, Brunmark A, Degano M, Peterson P A, Teyton L, Wilson I A. Immunity. 1998;8:553–562. doi: 10.1016/s1074-7613(00)80560-9. [DOI] [PubMed] [Google Scholar]
- 39.Manning T C, Parke E A, Teyton L, Kranz D M. J Exp Med. 1999;189:461–470. doi: 10.1084/jem.189.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foote J, Eisen H N. Proc Natl Acad Sci USA. 1995;92:1254–1256. doi: 10.1073/pnas.92.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]