Abstract
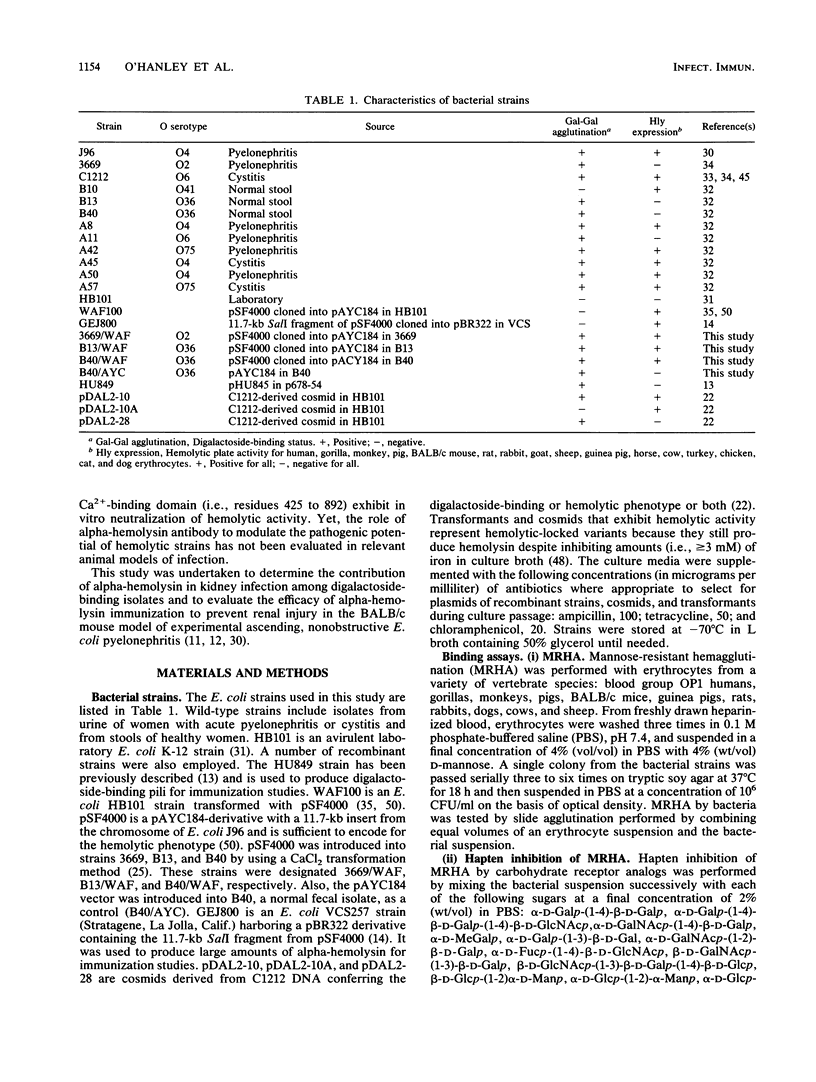
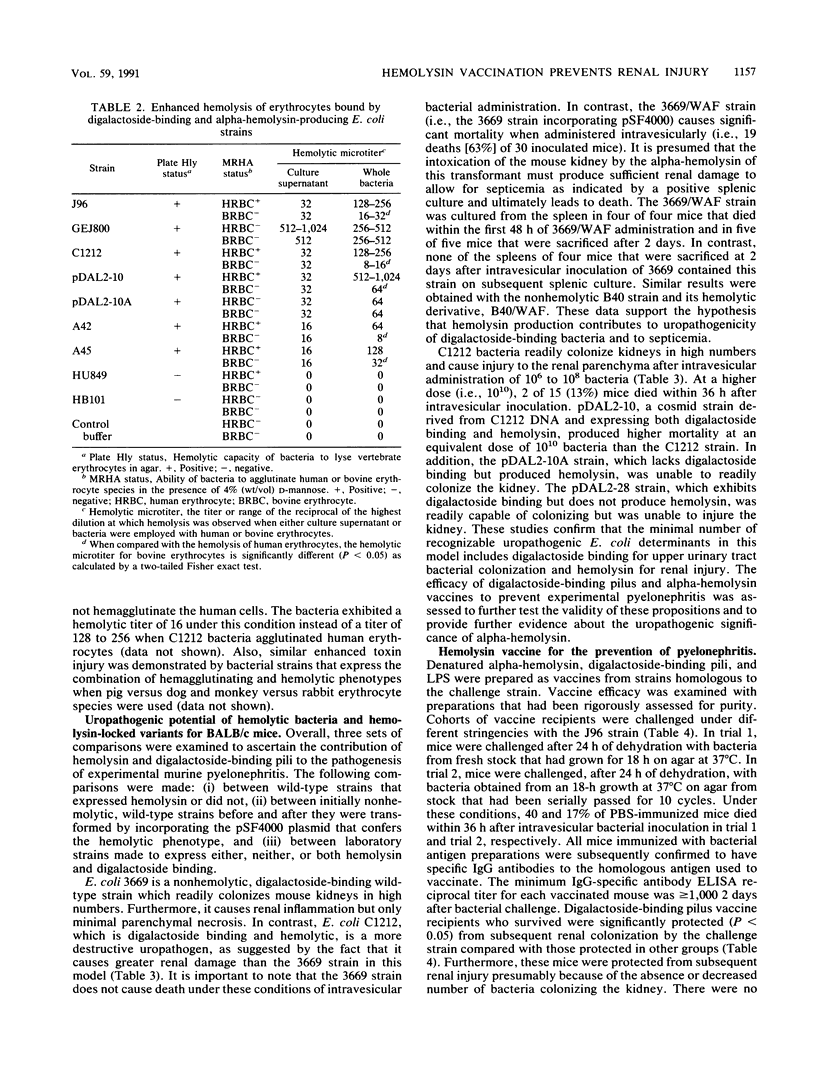
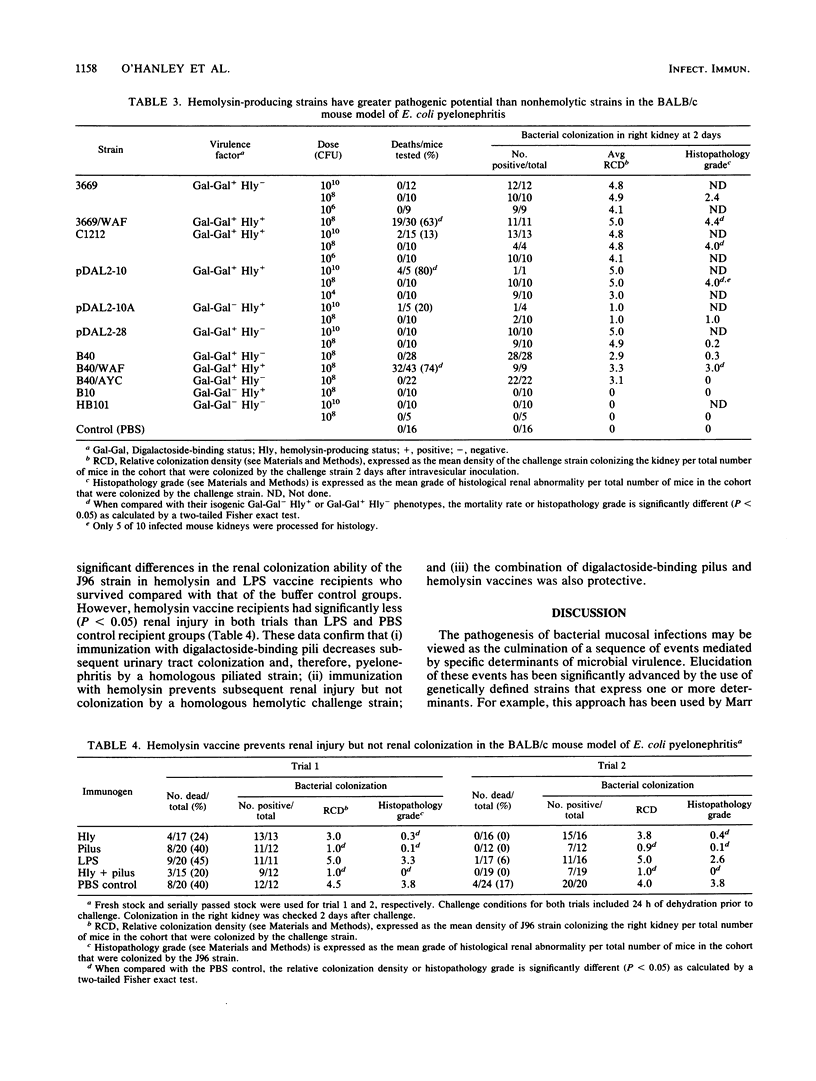
Digalactoside-binding (Gal-Gal) pili and alpha-hemolysin of Escherichia coli have been implicated as important virulence determinants in the pathogenesis of human ascending, nonobstructive pyelonephritis. The pathogenic significance of these determinants was evaluated in vitro and in the BALB/c mouse pyelonephritis model by employing wild-type, avirulent laboratory, and genetically defined cosmids, transformants, and recombinant strains. In vitro data suggest that the cytolytic activity of hemolysin is significantly (P less than 0.05) enhanced among digalactoside-binding strains which agglutinate erythrocytes. The basis of increased hemolysis is related presumably to more efficient delivery of the toxin to target lipid substrate in the host plasma membrane. Intravesicular administration of bacteria that express both digalactoside binding and hemolysin generally resulted in greater mortality and renal parenchymal injury in mice than strains that expressed none or only one of these determinants. Analyses convincingly demonstrate that digalactoside-binding pili are correlated with upper urinary tract colonization and that hemolysin is correlated with septicemia and renal parenchymal damage. These determinants collectively constitute the minimal virulence factors to produce disease in this model. Their efficacy as vaccines for the prevention of pyelonephritis was also assessed. A purified Gal-Gal pilus vaccine prevented (P less than 0.05) subsequent colonization by a challenge wild-type strain that exhibited homologous pili. The hemolysin vaccine did not abrogate subsequent bacterial renal colonization on challenge, but it did protect (P less than 0.05) mice which survived challenge from subsequent renal injury compared with those in the saline control group. The combination of these determinants was also protective. The combination of Gal-Gal pili and hemolysin in a vaccine preparation represents a potentially worthwhile strategy for human immunoprophylaxis against pyelonephritis by interdicting several steps in the pathogenesis of a bacterial mucosal infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Bohach G. A., Snyder I. S. Chemical and immunological analysis of the complex structure of Escherichia coli alpha-hemolysin. J Bacteriol. 1985 Dec;164(3):1071–1080. doi: 10.1128/jb.164.3.1071-1080.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Normark S., Hardy J., O'Hanley P., Lark D., Olsson O., Schoolnik G., Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984 Jan;157(1):330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982 Feb;15(1):11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- Fry T. L., Fried F. A., Goven B. A. Pathogenesis of pyelonephritis. Escherichia coli-induced renal ultrastructural changes. Invest Urol. 1975 Jul;13(1):47–51. [PubMed] [Google Scholar]
- Hacker J., Hof H., Emödy L., Goebel W. Influence of cloned Escherichia coli hemolysin genes, S-fimbriae and serum resistance on pathogenicity in different animal models. Microb Pathog. 1986 Dec;1(6):533–547. doi: 10.1016/0882-4010(86)90039-2. [DOI] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G. E., O'Hanley P. Epitopes of Escherichia coli alpha-hemolysin: identification of monoclonal antibodies that prevent hemolysis. Infect Immun. 1990 Sep;58(9):3029–3035. doi: 10.1128/iai.58.9.3029-3035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. E., Hammer R. F., Wu G. K. Effects of a single hit from the alpha hemolysin produced by Escherichia coli on the morphology of sheep erythrocytes. Infect Immun. 1980 Mar;27(3):988–994. doi: 10.1128/iai.27.3.988-994.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König W., König B., Scheffer J., Hacker J., Goebel W. Role of cloned virulence factors (mannose-resistant haemagglutination, mannose-resistant adhesions) from uropathogenic Escherichia coli strains in the release of inflammatory mediators from neutrophils and mast cells. Immunology. 1989 Jul;67(3):401–407. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Linggood M. A., Ingram P. L. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J Med Microbiol. 1982 Feb;15(1):23–30. doi: 10.1099/00222615-15-1-23. [DOI] [PubMed] [Google Scholar]
- Low D., David V., Lark D., Schoolnik G., Falkow S. Gene clusters governing the production of hemolysin and mannose-resistant hemagglutination are closely linked in Escherichia coli serotype O4 and O6 isolates from urinary tract infections. Infect Immun. 1984 Jan;43(1):353–358. doi: 10.1128/iai.43.1.353-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Normark S. Structure and antigenic properties of the tip-located P pilus proteins of uropathogenic Escherichia coli. J Bacteriol. 1988 Apr;170(4):1887–1894. doi: 10.1128/jb.170.4.1887-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Marre R., Hacker J., Henkel W., Goebel W. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect Immun. 1986 Dec;54(3):761–767. doi: 10.1128/iai.54.3.761-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Normark S., Falkow S., Schoolnik G. K. Mannose-sensitive and Gal-Gal binding Escherichia coli pili from recombinant strains. Chemical, functional, and serological properties. J Exp Med. 1983 Nov 1;158(5):1713–1719. doi: 10.1084/jem.158.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecha B., Low D., O'Hanley P. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model. Single-component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J Clin Invest. 1989 Jun;83(6):2102–2108. doi: 10.1172/JCI114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S., Boehm D. F., Snyder I. S., Rowe G., Welch R. A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990 Mar;58(3):822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. A., Hardaway K., Kaack B., Fussell E. N., Baskin G. Prevention of pyelonephritis by immunization with P-fimbriae. J Urol. 1984 Mar;131(3):602–607. doi: 10.1016/s0022-5347(17)50513-3. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Hanley J., Clubb L., Fanning S. The human antibody response to uropathogenic Escherichia coli: a review. Can J Microbiol. 1988 Mar;34(3):312–318. doi: 10.1139/m88-057. [DOI] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. A., O'Hanley P., Lark D., Schoolnik G. K. Synthetic peptides corresponding to protective epitopes of Escherichia coli digalactoside-binding pilin prevent infection in a murine pyelonephritis model. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1247–1251. doi: 10.1073/pnas.85.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharama S., Cavalieri S. J., Snyder I. S. Immune response to Escherichia coli alpha-hemolysin in patients. J Clin Microbiol. 1988 May;26(5):850–856. doi: 10.1128/jcm.26.5.850-856.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., de Man P. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1987 Dec;1(4):731–750. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventur Y., Scheffer J., Hacker J., Goebel W., König W. Effects of adhesins from mannose-resistant Escherichia coli on mediator release from human lymphocytes, monocytes, and basophils and from polymorphonuclear granulocytes. Infect Immun. 1990 Jun;58(6):1500–1508. doi: 10.1128/iai.58.6.1500-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- van der Bosch J. F., Verboom-Sohmer U., Postma P., de Graaff J., MacLaren D. M. Mannose-sensitive and mannose-resistant adherence to human uroepithelial cells and urinary virulence of Escherichia coli. Infect Immun. 1980 Jul;29(1):226–233. doi: 10.1128/iai.29.1.226-233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



