Abstract
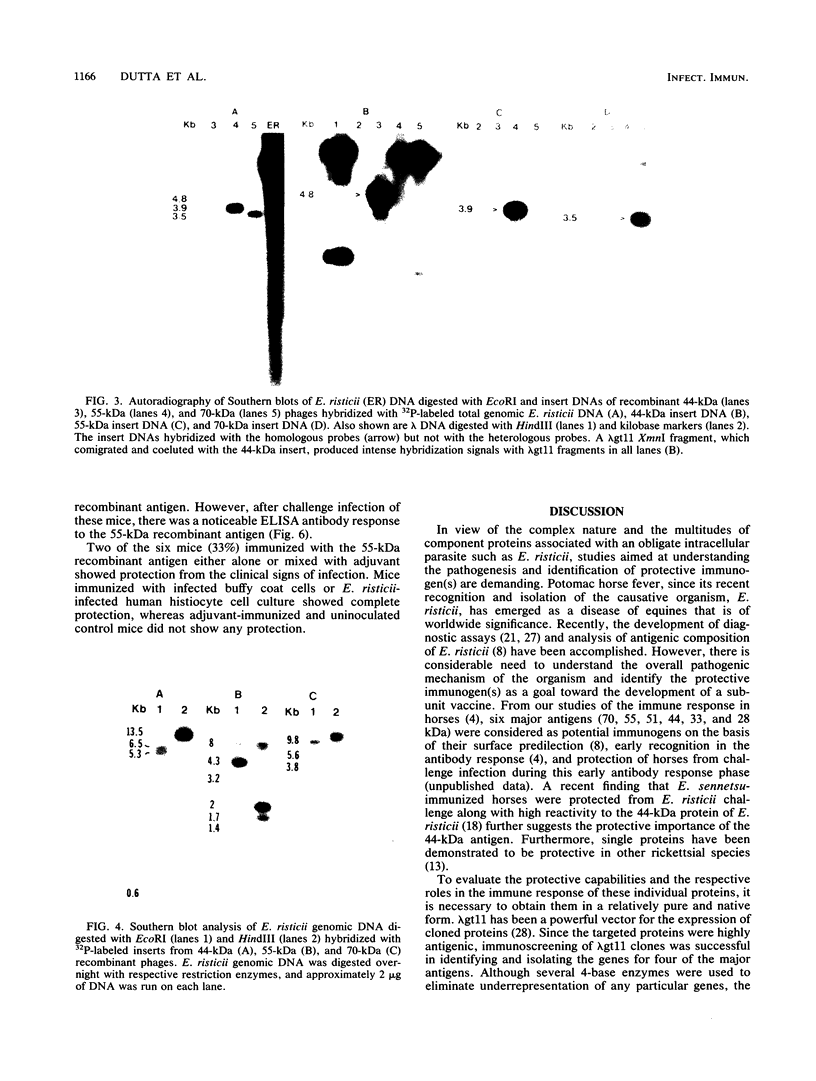
The genome of Ehrlichia risticii, the etiologic agent of Potomac horse fever, was cloned in the lambda gt11 expression vector. The efficiency of recombinant phage production with different restriction fragments of E. risticii DNA was generally between 20 and 95%. The antigen-positive frequency, detected by immunoscreening with E. risticii antibodies, was between 8 and 40 per 10(4) recombinants. Four (70, 55, 51, and 44 kDa) major antigens of E. risticii were identified from the recombinant phages by using recombinant antigen-selected monospecific antibodies. Characterization of three (70, 55, and 44 kDa) of these recombinant antigens indicated that the 70- and 44-kDa polypeptides were beta-galactosidase fusion products that were dependent on isopropylthiogalactoside induction for expression; they contained about 50 and 73%, respectively, of the native polypeptides. The 55-kDa antigen was a nonfusion protein expressed independently of isopropylthiogalactoside induction; it was a complete protein with a molecular weight identical to that of its native counterpart. The cloned E. risticii DNAs from of the recombinants expressing 70-, 55-, and 44-kDa proteins were 3.5, 3.9, and 4.8 kb, respectively, in size, and they were unique. The insert DNAs hybridized to multiple restriction fragments of the genomic DNA, the sum of the sizes of which was much greater than that of the corresponding insert. Mice immunized with the affinity-purified 55-kDa recombinant antigen produced a high titer of antibody in serum as measured by an enzyme-linked immunosorbent assay and gave a monospecific reaction by Western immunoblotting. Challenge infection of these immunized mice showed low protection from clinical infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Yeargan M. R. Use of lambda gt11 and monoclonal antibodies to map the genes for the six major glycoproteins of equine herpesvirus 1. J Virol. 1987 Aug;61(8):2454–2461. doi: 10.1128/jvi.61.8.2454-2461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., McDonald G. A., Jones D. C., Regnery R. L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirala S. S. The nucleotide sequence of the lac operon and phage junction in lambda gt11. Nucleic Acids Res. 1986 Jul 25;14(14):5935–5935. doi: 10.1093/nar/14.14.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Mattingly B. L., Shankarappa B. Antibody response to Ehrlichia risticii and antibody reactivity to the component antigens in horses with induced Potomac horse fever. Infect Immun. 1989 Oct;57(10):2959–2962. doi: 10.1128/iai.57.10.2959-2962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Penney B. E., Myrup A. C., Robl M. G., Rice R. M. Disease features in horses with induced equine monocytic ehrlichiosis (Potomac horse fever). Am J Vet Res. 1988 Oct;49(10):1747–1751. [PubMed] [Google Scholar]
- Dutta S. K., Rice R. M., Hughes T. D., Savage P. K., Myrup A. C. Detection of serum antibodies against Ehrlichia risticii in Potomac horse fever by enzyme-linked immunosorbent assay. Vet Immunol Immunopathol. 1987 Jan;14(1):85–92. doi: 10.1016/0165-2427(87)90077-8. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Shankarappa B., Thaker S. R., Mattingly-Napier B. L. DNA restriction endonuclease cleavage pattern and protein antigen profile of Ehrlichia risticii. Vet Microbiol. 1990 Oct;25(1):29–38. doi: 10.1016/0378-1135(90)90090-i. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Patrick C. C., Hermanstorfer L., McCracken G. H., Jr, Hansen E. J. Conservation of epitopes in the oligosaccharide portion of the lipooligosaccharide of Haemophilus influenzae type b. Infect Immun. 1987 Mar;55(3):513–520. doi: 10.1128/iai.55.3.513-520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald G. A., Anacker R. L., Garjian K. Cloned gene of Rickettsia rickettsii surface antigen: candidate vaccine for Rocky Mountain spotted fever. Science. 1987 Jan 2;235(4784):83–85. doi: 10.1126/science.3099387. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Rice R. M., Kelly D. J., Stover C. K. Antigenic and genetic relatedness of eight Rickettsia tsutsugamushi antigens. Infect Immun. 1989 Oct;57(10):3116–3122. doi: 10.1128/iai.57.10.3116-3122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Stover C. K., Rice R. M. Molecular cloning and expression of Rickettsia tsutsugamushi genes for two major protein antigens in Escherichia coli. Infect Immun. 1987 May;55(5):1156–1162. doi: 10.1128/iai.55.5.1156-1162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankarappa B., Dutta S. K. Production and characterization of monoclonal antibodies to Ehrlichia risticii. Am J Vet Res. 1989 Jul;50(7):1145–1149. [PubMed] [Google Scholar]
- Shankarappa B., Dutta S. K., Sanusi J., Mattingly B. L. Monoclonal antibody-mediated, immunodiagnostic competitive enzyme-linked immunosorbent assay for equine monocytic ehrlichiosis. J Clin Microbiol. 1989 Jan;27(1):24–28. doi: 10.1128/jcm.27.1.24-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., Marana D. P., Carter J. M., Roe B. A., Mardis E., Oaks E. V. The 56-kilodalton major protein antigen of Rickettsia tsutsugamushi: molecular cloning and sequence analysis of the sta56 gene and precise identification of a strain-specific epitope. Infect Immun. 1990 Jul;58(7):2076–2084. doi: 10.1128/iai.58.7.2076-2084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., Vodkin M. H., Oaks E. V. Use of conversion adaptors to clone antigen genes in lambda gt11. Anal Biochem. 1987 Jun;163(2):398–407. doi: 10.1016/0003-2697(87)90241-7. [DOI] [PubMed] [Google Scholar]
- Thaker S. R., Dutta S. K., Adhya S. L., Mattingly-Napier B. L. Molecular cloning of Ehrlichia risticii and development of a gene probe for the diagnosis of Potomac horse fever. J Clin Microbiol. 1990 Sep;28(9):1963–1967. doi: 10.1128/jcm.28.9.1963-1967.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]