Abstract
We evaluated the validity of anti-D2-40 and anti-LYVE-1 (antibodies against lymphatic endothelium) for IHC diagnosis and semiquantification of lymphatic vessels in the dura mater of the intraorbital portion of the human optic nerve (ON). Fourteen specimens were analyzed using light microscopy within 12 hr postmortem. We found in all specimens that both D2-40 and LYVE-1 stained lymphatic vessels as well as venules and arterioles. Our findings show lymphatic vessels in the meninges of the intraorbital portion of the human ON. Anti-D2-40 and anti-LYVE-1 antibodies, however, are not found to be exclusively specific to the endothelial layer of lymphatics because they also stain the endothelial layer of venules and arterioles. For the unequivocal identification of lymphatics, additional morphological criteria are necessary. Nevertheless, D2-40 and LYVE-1 staining allows rapid identification of endothelial layers. (J Histochem Cytochem 56:1087–1092, 2008)
Keywords: LYVE-1, D2-40, optic nerve, lymphatics
Lymphatic vessels were not recognized as structures of the optic nerve (ON) until 1999. In fact, it was once thought that lymphatics did not exist within or around the nerve (Hayreh 1984). Nevertheless, lymphatics in the dura of the human ON have now been shown by both light and transmission electron microscopy (TEM) (Gausas et al. 1999; Killer et al. 1999,2003). The ON is enveloped into the dura mater and surrounded by cerebrospinal fluid (CSF) throughout its entire length. The function of lymphatics in the dura of the ON is not yet fully understood but may be related to a CSF outflow system that is designed to maintain homogeneous pressure and homogeneous biochemical milieu (Rubenstein 1998; Silverberg et al. 2003; Killer et al. 2006). The correlation of a non-physiological CSF composition and fluid dynamics has been recently shown in patients with degenerative neurological disorders (Rubenstein 1998; Silverberg et al. 2003). In addition to CSF resorption in arachnoid villi, the concept of CSF drainage through the lymphatics has been shown in animal experiments (Boulton et al. 1998a,b,1999; Johnston 2000,2003; Johnston and Papaiconomou 2002; Zakharov et al. 2003; Lüdemann et al. 2005).
Several antibodies are reported to be specific for the diagnosis of lymphatics (e.g., podoplanin, VEGFR-3, Prox-1) (Breiteneder-Geleff et al. 1997; Wigle and Oliver 1999; Mäkinen et al. 2001; Petrova et al. 2002). The validity of IHC for the diagnosis of lymphatics as an isolated method, however, has been questioned in the past (Sleeman et al. 2001). In this study, we evaluated the validity of anti-D2-40 and anti-LYVE-1—antibodies against lymphatic endothelium (Banerji et al. 1999; Jackson et al. 2001; Prevo et al. 2001; Jackson 2003) and D2-40 (Kahn et al. 2002; Gausas et al. 2007)—for IHC diagnosis and semiquantification of lymphatic vessels in the dura mater of the intraorbital portion of the human ON.
Materials and Methods
Materials
The orbital portions of both ONs were obtained postmortem within 12 hr after death from seven adult men (mean age, 62.4 ± 15.0 years) without known ophthalmological or neurological disease and were immediately fixed in paraformaldehyde (4%). In three specimens, India ink was injected into the subarachnoid space (SAS) surrounding the nerve. The causes of death in the seven patients were heart failure (n=5), aortic aneurysm (n=1), and metastasis of small-cell carcinoma (n=1). The sample collection followed the tenets of the Helsinki declaration.
Tissue Preparation
All ONs (including those injected with India ink) were divided into three sections, the intraorbital anterior portion (IOAP), intraorbital middle portion (IOMP), and intraorbital posterior portion (IOPP), and were embedded in paraffin. Serial cross- and longitudinal sections of 4-μm thickness were obtained with a microtome.
IHC
Sections were mounted on gelatin-chromalum–coated glass slides and deparaffinized. IHC staining was performed with the Elite ABC Kit (Vector Laboratories; Burlingame, CA) according to the manufacturer's protocol. After pretreatment in a microwave oven (98C, 60 min in citrate buffer 10 mM, pH 6.0), the sections were reacted with primary antibody over 16 hr at 4C. For D2-40 (Dako; Glostrup, Denmark), the optimum concentration of the primary antibody was previously determined to be 1:20; for LYVE-1 (DCS; Hamburg, Germany), it was 1:10. After several washes with PBS, the sections were incubated with biotinylated secondary antibodies. The antigen was visualized using the peroxidase substrate 3-amino-9-ethylcarbazole (AEC). Small gut tissue served as a positive control for both antibodies.
Light Microscopy
All sections were assessed by three observers using light microscopy for localization and intensity of specific immunoreactivity on a semiquantitative scale of 1–3. In case of disagreement, consent was achieved by discussion. In addition, various morphological criteria were used to identify of various vascular structures (i.e., lymphatic, venule, arteriole): (a) endothelial cell-lined vessel; (b) vessel with thin endothelial cells, the nuclei of which protrude into the lumen; (c) thin vessel wall in a collapsed or semicollapsed state with poorly developed basal lamina; and (d) no or only a few red blood cells in the lumen of the vessel.
Results
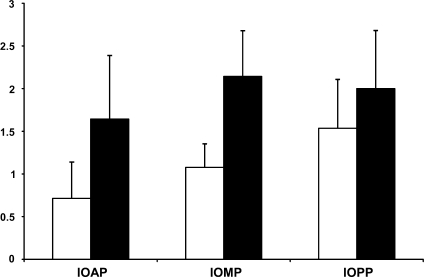
In all specimens, endothelial staining (LYVE-1 or D2-40) was observed (Figures 1 and 2); however, neither stain was exclusively specific for lymphatic endothelial cells because the endothelial layers of small arterioles and small venules (using morphological criteria) were stained as well (Figures 1A and 1B). In some specimens, the lumen of the vessels connected directly to the SAS of the ON (Figure 2D). In addition, in some specimens, discrete staining for D2-40 was observed in the arachnoid and pia layers (Figure 2B). Ink was noted to be present in some vessels in the vicinity of the SAS (Figure 1C). Some of these ink-containing vessels met the criteria for lymphatics (Figure 1D). The semiquantitative evaluation showed a slightly increased number of stained lymphatic vessels toward the IOPP of the ON and a difference in the sensitivity of the staining methods, with D2-40 staining less vessels than LYVE-1 (Figure 3)—IOAP: D2-40 staining, 0.714 ± 0.426 SD; LYVE-1 staining, 1.643 ± 0.745 SD; IOMP: D2-40 staining, 1.077 ± 0.277 SD; LYVE-1 staining, 2.143 ± 0.535 SD; IOPP: D2-40 staining, 1.536 ± 0.571 SD; LYVE-1 staining, 2.000 ± 0.679 SD (Figure 3).
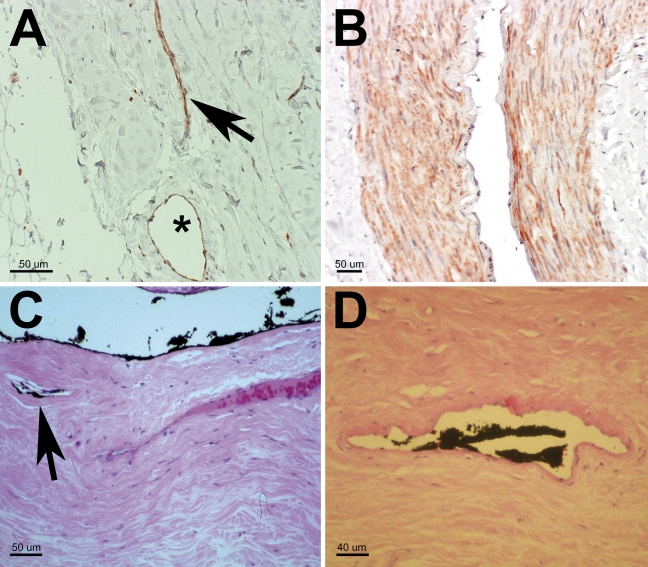
Figure 1.
(A) IHC (anti-LYVE-1 antibody staining) showing a vessel with a collapsed, narrow lumen, meeting the criteria for lymphatics (arrow) next to a vessel with a large lumen, suggestively surrounded by a smooth muscle cell layer (asterisk). (B) LYVE-1 staining of the arteriole muscular layer is shown. (C) Specimen with ink injection into the subarachnoid space (SAS) of the optic nerve before tissue preparation. Ink can be seen on the arachnoid layer, in the SAS, and in small vessels (meeting criteria for lymphatics) close to the SAS (arrow) (hematoxylin-eosin stain). (D) Ink in the lumen of a small vessel. The lack of a pronounced muscular layer is suggestive for a lymphatic vessel (hematoxylin-eosin stain).
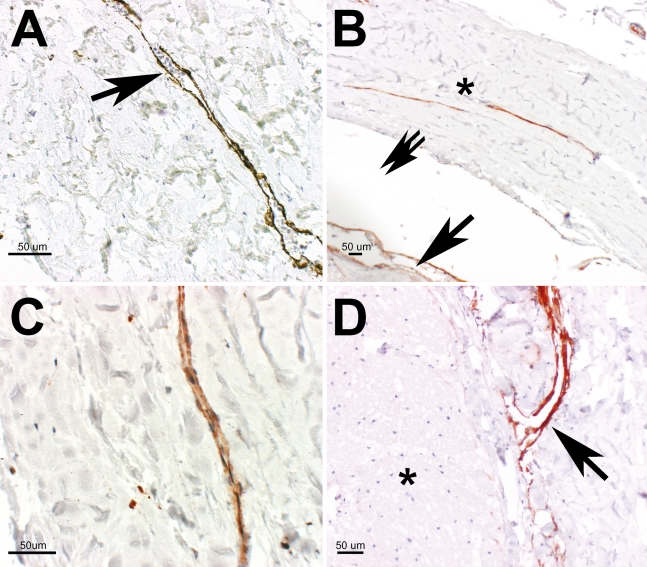
Figure 2.
(A) IHC (anti-LYVE-1 antibody staining) showing a collapsed vessel with intraluminal particles, erythrocytes (arrow). (B) Anti-D2-40 antibody staining: longitudinal sliced vessels in the dura mater (asterisk) of the optic nerve (probably meeting criteria for lymphatics) and staining of the artificially detached arachnoid layer and the pia mater (arrow), some artificial empty space caused by tissue preparation (double arrow). (C) Anti-D2-40 antibody staining: small lymphatic vessel in the dura mater of the optic nerve. (D) Anti-D2-40 antibody staining: small vessel, probably a lymphatic with direct access to the subarachnoid space of the optic nerve (arrow). Optic nerve (ON) fibers (asterisk).
Figure 3.
Semiquantitative assessment of the number of stained lymphatic vessels in the different parts of the ON with regard to staining method (D2-40, blank bar; LYVE-1, dark bar). Grading: 0–0.5, only weak endothelial staining; 0.5–1.5, one to three lymphatic vessels per section; 1.5–2.5, five to seven lymphatic vessels per section; 2.5–3, more than seven lymphatic vessels per section. Intraorbital anterior part (IOAP): D2-40, 0.714 ± 0.426 SD; LYVE-1, 1.643 ± 0.745 SD. Intraorbital middle part (IOMP): D2-40, 1.077 ± 0.277 SD; LYVE-1, 2.143 ± 0.535 SD. Intraorbital posterior part (IOPP): D2-40, 1.536 ± 0.571 SD; LYVE-1, 2.000 ± 0.679 SD.
Discussion
This postmortem study of the orbital portion of the human ON and its sheath, using anti-LYVE-1 and anti-D2-40 antibodies, showed endothelial staining of lymphatics, but also of venules and arterioles, in all specimens examined. None of the patients in this study had known ocular or neurological disease. The distribution of lymphatic vessels was (more or less) homogenous in all portions. For detailed analysis of the stained vessels, additional morphological criteria were applied.
The bulk of the dura of the human ON is made up of dense connective tissue that is rich in elastic fibers (Andres 1967; Klika 1967; Zenker et al. 1994). The function of the ON sheath is to provide protection against mechanical damage during eye movements and to form the SAS that contains the CSF that both nourishes the axons and glial cells of the nerve and helps maintain a constant metabolic microenvironment. If the CSF pressure rises, axoplasmic flow is compromised, leading to papilledema (Hayreh 1968). Stasis of local CSF in the SAS of the ON may lead to loss of visual function, probably triggered by accumulation of metabolically active CSF components. Such stasis can be shown with contrast-aided cisternography and by assessing the concentration CSF-specific molecules, such as lipocalin-like prostaglandin D synthase (L-PGDS) (Killer et al. 2006,2007).
Lymphatics in the dura of the ON sheath were first described in 1999 by Gausas et al. (1999) and Killer et al. (1999). Their function is not yet fully understood, but they probably contribute, at least to some degree, to CSF outflow from the SAS into the dura, thereby helping to maintain a constant local CSF pressure and thereby protecting the ON from pressure spikes during eye movements. This assumption is supported by the presence of intradural lymphatics with direct access to the SAS (Figure 2D). In addition, lymphatic vessels may help prevent accumulation of biologically active CSF components. India ink injected into the SAS of the ON appears in dural lymphatics on TEM (Killer et al. 1999), similar to what occurs in some animals (e.g., sheep) when India ink is injected in the SAS of the brain and the lymphatics in the olfactory region are observed (Boulton et al. 1998a).
Information about the distribution and the number of lymphatic vessels in the ON sheath may help shed light on their physiological function. Although TEM does not provide quantitative data regarding the distribution of lymphatics in the ON sheath, IHC offers an easy way to obtain this information. In particular, IHC staining using LYVE-1 and D2-40 enables us to distinguish lymphatics from other vessels, but only when additional morphological criteria are used, such as the presence of a muscular layer surrounding a stained endothelium (Figure 1A), cellular components of blood within a stained vessel lumen (Figure 2A), or an unusual size of a stained vessel, because it is clear from our study that LYVE-1 and D2-40 are not specific for lymphatic endothelium cells but also stain the endothelium of venules and arterioles. These findings are in contrast to those from other studies in which a high specificity for lymphatics was reported (Prevo et al. 2001; Kahn et al. 2002).
IHC staining using anti-D2-40 antibodies for lymphatics in the ON sheath was recently reported by Gausas et al. (2007). The eyes studied were sampled from 12 patients with retinal or orbital disease and patients who underwent enucleation of eyes with end-stage open-angle glaucoma. In 8 of 10 enucleated glaucomatous eyes, D2-40 staining for lymphatics was not present, in contrast to eyes without glaucoma (Gausas et al. 2007; Gausas RE, unpublished data). Although this study did not focus on lymphatics in relationship to certain diseases, it is of interest that lymphatics were shown in only 2 of 10 glaucomatous ONs. This is in contrast to the results of this study that showed lymphatics in all specimens (although in eyes without known ocular disease) and may be a hint to a possible function of lymphatic vessels in the ON sheath. What is the chicken and what is the egg (i.e., is the absence of lymphatics part of the pathogenesis of ON disease or is advanced ON disease the cause for disappearance of lymphatic vessels)?
The morphological criteria to establish the structure of lymphatics used in this study were (a) an endothelial cell-lined vessel, (b) thin endothelial cells with nuclei protruding in lumen, (c) thin vessel wall in a collapsed or semicollapsed state with poorly developed basal lamina, and (d) no or only few red blood cells in the lumen of the vessel. Using these criteria, we showed D2-40–positive staining in vessels whose walls contain muscle fibers and thus are not lymphatics. We thus believe that IHC alone is insufficient to identify lymphatic vessels. Instead, we believe that it is necessary to perform both IHC staining and morphological criteria using light microscopy to obtain reliable information concerning the number and location of vessels in the dura of the ON, only some of which meet the criteria for lymphatics.
Acknowledgments
The authors thank Prof. Peter Groscurth, Head of Institute of Anatomy, University of Zurich, Zurich, Switzerland, for important and helpful support, the laboratory staff of the Eye Institute of the University of Basel, Basel, Switzerland, for the specimen preparation, and Prof. Dr. Stamm, PD Dr. Laeng, and Verena Jaggi, Department of Pathology, Kantonsspital Aarau, Aarau, Switzerland, for reliable assistance obtaining the tissue samples.
References
- Andres KH (1967) On the fine structure of the arachnoidea and dura mater of mammals. Z Zellforsch Mikrosk Anat 79:272–295 [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, et al. (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M (1998a) Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol 275:R889–896 [DOI] [PubMed] [Google Scholar]
- Boulton M, Flessner M, Armstrong D, Hay J, Johnston M (1998b) Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol 274:R88–96 [DOI] [PubMed] [Google Scholar]
- Boulton M, Flessner M, Armstrong D, Mohamed R, Hay J, Johnston M (1999) Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol 276:R818–823 [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, et al. (1997) Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 151:1141–1152 [PMC free article] [PubMed] [Google Scholar]
- Gausas RE, Daly T, Fogt F (2007) D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthal Plast Reconstr Surg 23:32–36 [DOI] [PubMed] [Google Scholar]
- Gausas RE, Gonnering RS, Lemke BN, Dortzbach RK, Sherman DD (1999) Identification of human orbital lymphatics. Ophthal Plast Reconstr Surg 15:252–259 [DOI] [PubMed] [Google Scholar]
- Hayreh SS (1968) Pathogenesis of oedema of the optic disc. Doc Ophthalmol 24:289–411 [DOI] [PubMed] [Google Scholar]
- Hayreh SS (1984) The sheath of the optic nerve. Ophthalmologica 189:54–63 [DOI] [PubMed] [Google Scholar]
- Jackson DG (2003) The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med 13:1–7 [DOI] [PubMed] [Google Scholar]
- Jackson DG, Prevo R, Clasper S, Banerji S (2001) LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol 22:317–321 [DOI] [PubMed] [Google Scholar]
- Johnston M (2000) Relationship between cerebrospinal fluid and extracranial lymph. Lymphology 33:1–3 [PubMed] [Google Scholar]
- Johnston M (2003) The importance of lymphatics in cerebrospinal fluid transport. Lymphat Res Biol 1:41–44 [DOI] [PubMed] [Google Scholar]
- Johnston M, Papaiconomou C (2002) Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci 17:227–230 [DOI] [PubMed] [Google Scholar]
- Kahn HJ, Bailey D, Marks A (2002) Monoclonal antibody D2–40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 15:434–440 [DOI] [PubMed] [Google Scholar]
- Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR (2006) The optic nerve: A new window into cerebrospinal fluid composition? Brain 129:1027–1030 [DOI] [PubMed] [Google Scholar]
- Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A (2007) Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain 130:514–520 [DOI] [PubMed] [Google Scholar]
- Killer HE, Laeng HR, Flammer J, Groscurth P (2003) Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol 87:777–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killer HE, Laeng HR, Groscurth P (1999) Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol 19:222–228 [PubMed] [Google Scholar]
- Klika E (1967) The ultrastructure of meninges in vertebrates. Acta Univ Carol [Med] (Praha) 13:53–71 [PubMed] [Google Scholar]
- Lüdemann W, Berens von Rautenfeld D, Samii M, Brinker T (2005) Ultrastructure of the cerebrospinal fluid outflow along the optic nerve into the lymphatic system. Childs Nerv Syst 21:96–103 [DOI] [PubMed] [Google Scholar]
- Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, et al. (2001) Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 20:4762–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, et al. (2002) Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21:4593–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 276:19420–19430 [DOI] [PubMed] [Google Scholar]
- Rubenstein E (1998) Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet 351:283–285 [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D (2003) Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol 2:506–551 [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Krishnan J, Kirkin V, Baumann P (2001) Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech 55:61–69 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98:769–778 [DOI] [PubMed] [Google Scholar]
- Zakharov A, Papaiconomou C, Djenic J, Midha R, Johnston M (2003) Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of microfil. Neuropathol Appl Neurobiol 29:563–573 [DOI] [PubMed] [Google Scholar]
- Zenker W, Bankoul S, Braun JS (1994) Morphological indications for considerable diffuse reabsorption of cerebrospinal fluid in spinal meninges particularly in the areas of meningeal funnels. An electronmicroscopical study including tracing experiments in rats. Anat Embryol (Berl) 189:243–258 [DOI] [PubMed] [Google Scholar]