Abstract
Dental pulp elaborates both bone and dentin under pathological conditions such as tooth replantation/transplantation. This study aims to clarify the capability of dental pulp to elaborate bone tissue in addition to dentin by allogenic tooth transplantation using immunohistochemistry and histochemistry. After extraction of the molars of 3-week-old mice, the roots and pulp floor were resected and immediately allografted into the sublingual region in a littermate. In addition, we studied the contribution of donor and host cells to the regenerated pulp tissue using a combination of allogenic tooth transplantation and lacZ transgenic ROSA26 mice. On Days 5–7, tubular dentin formation started next to the preexisting dentin at the pulp horn where nestin-positive odontoblast-like cells were arranged. Until Day 14, bone-like tissue formation occurred in the pulp chamber, where intense tartrate-resistant acid phosphatase–positive cells appeared. Furthermore, allogenic transplantation using ROSA26 mice clearly showed that both donor and host cells differentiated into osteoblast-like cells with the assistance of osteoclast-lineage cells, whereas newly differentiated odontoblasts were exclusively derived from donor cells. These results suggest that the odontoblast and osteoblast lineage cells reside in the dental pulp and that both donor and host cells contribute to bone-like tissue formation in the regenerated pulp tissue. (J Histochem Cytochem 56:1075–1086, 2008)
Keywords: allograft, bone development, dental pulp, tooth transplantation, mouse [Crlj:CD1;ICR, B6.129S7-Gt(ROSA)26Sor/J]
A unique feature of dental pulp is that it is a non-mineralized neural crest–derived mesenchymal tissue surrounded by dentin, a mineralized tissue, and that it communicates with the periodontal tissue through apical foramens. The dentin–pulp complex is capable of repair after tooth injuries (Nanci 2008). Tooth injuries such as cavity preparation and tooth replantation induce destructive changes in the odontoblasts at the affected site and an acute inflammatory reaction (Ohshima 1990; Nakakura-Ohshima et al. 2003; Ohshima et al. 2003). If the odontoblasts do not survive, pulpal mesenchymal cells replace the degenerated cells to differentiate into odontoblast-like cells (Smith 2002). Recent studies have shown that human dental pulp from adult teeth and exfoliated deciduous teeth contains dental pulp stem cells (DPSCs) (Gronthos et al. 2000,2002; Batouli et al. 2003; Miura et al. 2003) characterized by multipotent differentiation and the expression of mesenchymal stem cell markers such as Stro-1 and CD146. DPSCs might contain heterogeneous populations of cells from the pulp tissue (Gronthos et al. 2000,2002; Liu et al 2006). Recent studies using transgenic mice carrying a green fluorescent protein (GFP) reporter gene have also shown that DPSCs from mouse incisors can give rise to odontoblasts, osteoblasts, and chondrocytes and functionally produce matrix when transplanted into kidney capsules (Braut et al. 2002,2003).
Tooth replantation induces at least two types of healing patterns in the regenerated dental pulp: tertiary dentin and bone tissue formation in the regenerated pulp tissue (Kvinnsland et al. 1991; Byers et al. 1992; Rungvechvuttivittaya et al. 1998; Shimizu et al. 2000; Ohshima et al. 2001; Tsukamoto-Tanaka et al. 2006; Hasegawa et al. 2007). Two possibilities are proposed regarding the derivation of bone-forming cells in the replanted pulp: cells migrating from the periodontal tissue and/or resident pulpal mesenchymal cells (Shimizu et al. 2000). To exclude the possibility that the periodontal tissue contributes to this event, we established the animal model of autogenic tooth transplantation of the coronal portion into the sublingual region. In this experiment, both tubular dentin and bone tissue depositions were always induced in the pulp chamber despite the absence of periodontal tissue, suggesting that dental pulp contains two types of competent progenitor cells capable of differentiating into either odontoblast- or osteoblast-like cells (Ogawa et al. 2006). The determination of the healing pattern after tooth replantation may be directly linked to the death or survival of odontoblast lineage cells such as fully differentiated odontoblasts and/or odontoblastic progenitor cells. The proper oxygenated medium is probably decisive for the survival of odontoblast lineage cells. Intentionally prolonged time operating for tooth replantation induces the total death of odontoblast lineage cells before osteoblast lineage cells (Hasegawa et al. 2007). Certain cell populations in the pulp tissue might have the ability to differentiate into bone-forming cells, and their differentiation might take place under pathological conditions in which certain regulatory mechanisms for suppressing bone formation are disturbed. The existence of both odontoblast and osteoblast lineage cells may be supported by the notion that dental pulp is composed of various cell populations, including resident mesoderm-derived and cranial neural crest (CNC)-derived cells (Goldberg and Smith 2004).
Previous studies have been unable to exclude the possibility of the host tissue contributing to bone tissue formation in the transplants (Zussman 1966; Luostarinen and Ronning 1977; Yamamura 1985; Takei et al. 1988; Inoue and Shimono 1992; Laino et al. 2005). Isolated pulp tissue implanted in a variety of sites gives rise to an osteo-typical matrix but not to tubular dentin. In our previous study using autogenic tooth transplantation, the presence of surviving pulp tissue was suggested to be necessary for the induction of bone-like tissue formation in transplants with the assistance of osteoclast lineage cells (Ogawa et al. 2006). This notion is supported by the findings that the allograft of teeth without pulp fails to induce hard tissue formation in the transplants (Ogawa et al. 2006). However, we still cannot exclude the possibility that the progenitor cells from the surrounding lingual tissue migrate into the pulp chamber and proliferate to give rise to hard tissue-forming cells. Actually, numerous tartrate-resistant acid phosphatase (TRAP)-positive cells appear from the circulatory system of the host tissue in both the auto- and allograft transplants. Allograft transplantation experiments using GFP or ROSA26 reporter mice are necessary to exclude this possibility. This study aims to clarify the capability of dental pulp to elaborate bone tissue in addition to tubular dentin by allogenic tooth transplantation of the coronal portion into the sublingual region using IHC for 5-bromo-2′-deoxyuridine (BrdU) as a cell proliferation assay, nestin as an odontoblastic marker (Terling et al. 1995; About et al. 2000b; Ogawa et al. 2006; Hasegawa et al. 2007), osteopontin (OPN) as a marker for osteoblast lineage cells (Haylock and Nilsson 2006), and histochemistry for TRAP as a marker for osteoclast lineage cells (Bonucci and Nanci 2001). Furthermore, we studied the contribution of donor and host cells to the regenerated pulp tissue using a combination of allogenic tooth transplantation and lacZ transgenic ROSA26 mice.
Materials and Methods
Allogenic Tooth Transplantation Into the Sublingual Region
All experiments were reviewed by the Committee on the Guidelines for Animal Experimentation of Niigata University and performed according to the recommendations or under the conditions proposed by the Review Committee. Crlj:CD1 (ICR) mice, 3 weeks old, were used in this study. The upper right first molar was extracted with a pair of dental forceps with modification under anesthesia by an intraperitoneal injection of chloral hydrate (350 mg/kg), and the roots and pulp floor were resected with a surgical knife. The coronal portion of the resected samples without the periodontal tissue was immediately transplanted into the sublingual region after cutting the ventral side of the tongue of the littermates, and the section was sutured with a nylon suture. Furthermore, we performed allogenic tooth transplantation between lacZ transgenic and wild-type ROSA26 mice.
LacZ Transgenic and Wild-type ROSA26 Mice
The strain of the lacZ transgenic ROSA 26 mice was B6.129S7-Gt(ROSA)26Sor/J. These transgenic mice were purchased from Jackson Laboratory (Bar Harbor, ME). Cells of these lacZ transgenic ROSA 26 mice can be stained by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) solution, because the endogenous promoter of the lacZ gene is ubiquitously activated. In contrast, cells of wild-type ROSA26 mice cannot be stained by X-gal solution. In our study, 3-week-old lacZ transgenic and wild-type ROSA26 mice were used as the donor and host, respectively, and vice versa. Our preliminary experiment using lacZ transgenic ROSA26 mice clearly showed that the combination of allogenic tooth transplantation and lacZ transgenic ROSA26 mice can be used to study the contribution of donor and host cells to the regenerated pulp tissue (Kim et al. 2006).
Histological Procedure
Materials were collected in groups of five animals at intervals of 1, 3, 5, 7, and 14 days after transplantation (n=25) in addition to 10 animals at 14 days after allogenic tooth transplantation into the sublingual region between lacZ ROSA26 transgenic teeth and wild-type teeth (n=10). At each stage, the animals were intraperitoneally injected with BrdU (150 mg/kg) and subsequently perfused with physiological saline transcardially followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) 2 hr after BrdU injection under deep anesthesia. BrdU labeling was omitted in the lacZ transgenic and wild-type ROSA26 mice. The tongues including the transplanted teeth were removed en bloc and immersed in the same fixative for an additional 6 hr. After decalcification in a 10% EDTA disodium salt (EDTA-2Na) solution for 2 weeks at 4C, the specimens were embedded in paraffin, and sagittal sections of transplants with surrounding lingual tissues were cut at 5 μm. The paraffin sections were mounted on Matsunami adhesive silane (MAS)-coated glass (Matsunami Glass; Osaka, Japan) slides and stained with hematoxylin and eosin (H&E).
IHC Analysis
For the immuno-peroxidase procedure, the sections were processed for the Calbiochem BrdU IHC system (EMD Biosciences; Darmstadt, Germany), avidin-biotin peroxidase complex (ABC) method using a rabbit anti-OPN polyclonal antibody diluted to 1:5000 (Cosmo Bio Co.; Tokyo, Japan), and Nichirei Histofine Simple Stain Mouse MAX-PO (Nichirei Biosciences; Tokyo, Japan) using a mouse anti-nestin monoclonal antibody diluted to 1:50 (Chemicon International; Temecula, CA). The sections were counterstained with hematoxylin or 0.05% methylene blue. The brown in BrdU-labeled cells was changed to red and graphically emphasized by using graphic software (Adobe Photoshop CS2 for Windows; Adobe Systems, San Jose, CA). IHC controls were performed by (a) replacing the primary antibodies with non-immune serum or PBS and (b) omitting the streptavidin-peroxidase or the MAX-PO solution. These immunostained sections contained no specific immunoreaction.
Histochemical Analysis
For the histochemical demonstration of TRAP activity, the azo-dye method was used with slight modifications (Tsukamoto-Tanaka et al. 2006). The frozen sections were incubated for 15 min at room temperature in a medium comprising 0.01% naphthol AS-BI phosphatase (Na salt; Sigma Chemical, St. Louis, MO), 0.06% fast red violet LB salt (Sigma Chemical), and 50 mM l-(+)-tartaric acid in 0.2 M acetate buffer (pH 5.3). The sections were counterstained with 0.5% methyl green or 0.05% methylene blue.
Identification of the lacZ Transgenic Cell in the Tissue Section
After decalcification in a 10% EDTA-2Na solution for 2 weeks at 4C, the fixed tongues including the transplanted teeth were washed with 2 mM MgCl2 in PBS for 5 min, rinsed three times with a rinse buffer (2 mM MgCl2, 0.02% NP-40, 0.01% sodium deoxycholate in PBS) for 20 min at room temperature, stained with β-gal staining solution (1 mg/ml of X-gal, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide), and incubated at 37C for 1 hr. After X-gal staining, the samples were washed again with PBS for 10 min. The specimens were embedded in paraffin, and sagittal sections of transplants with surrounding lingual tissues were cut at 5 μm. The paraffin sections were mounted on MAS-coated glass (Matsunami Glass) slides and counterstained with nuclear fast red.
Statistical Analysis of Cell Proliferation
The number of BrdU-positive cells in the pulp areas including the pulp horn and chamber of each specimen was calculated. Quantitative analysis was performed in three areas for each sample. The data were obtained from the samples of 20 animals (5 animals per group; the final number of samples was 60 areas), and the grid (211 × 169 μm2) was selected at random in each area (pulp horn and chamber). All data were presented as the means and SD of each group. Furthermore, the number of cells in the pulp horn and chamber among different times after transplantation was compared using Bonferroni's test (one-way ANOVA) by using statistical software (SPSS 14.0J for Windows; SPSS Japan, Tokyo, Japan).
Results
Histological Analysis
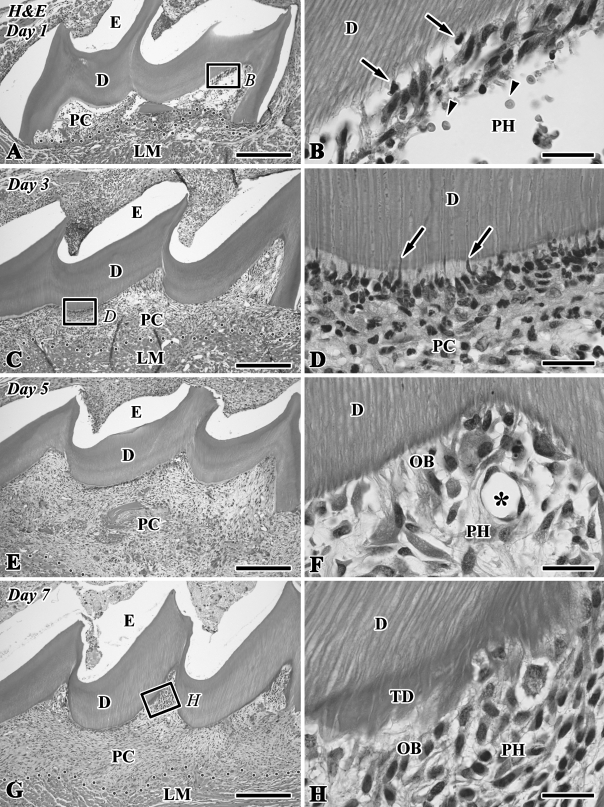
The H&E-stained paraffin sections clearly showed the two types of hard tissue formation (i.e., tubular dentin and bone-like tissue) in the dental pulp chamber of the transplant during the healing process (Figures 1, 2E, and 2F). On Day 1, the pulp chamber was mainly occupied by inflammatory lesions including numerous neutrophils, red blood cells, and fibrin networks. Neutrophils migrated into the degenerating odontoblast layer in the pulp horn (Figures 1A and 1B). On Day 3, a different type of cells appeared along the pulp–dentin border and extended their cellular processes into the dentinal tubules (Figures 1C and 1D). The odontoblast-like cells came to be arranged along the pulp–dentin border and deposited tubular dentin next to the preexisting dentin at the pulp horn on Days 5–7 (Figures 1E–1H). On Day 14, bone-like tissue formation occurred in the pulp chamber independently of the tubular dentin (Figures 2E, 2F, 3A, 3C, and 3D). The distinction between dentin and bone-like tissue was determined by the existence of dentinal tubules in the case of dentin and cell inclusion in the case of bone-like tissue (Figures 1H and 2F) in combination with the immunoreactivity for nestin and OPN (Figures 2B, 2G, 3B, and 3C), although occasionally the mixed features of dentin and bone appeared in the regenerated tissue.
Figure 1.
Hematoxylin and eosin (H&E)-stained sections of the transplanted teeth at 1 (A,B), 3 (C,D), 5 (E,F), and 7 (G,H) days after operation (D, dentin; E, enamel space; LM, lingual muscle; OB, odontoblast-like cells; PC, pulp chamber; PH, pulp horn). (A) The pulp chamber is mainly occupied by inflammatory lesions including numerous neutrophils and fibrin networks. (C,E,G) The pulp tissue is gradually increased in cell density and volume, concomitant with the reduced number of inflammatory cells. (B) Higher magnification of boxed area labeled by B in A. Neutrophils migrate into the degenerating odontoblast layer in the pulp horn (arrows), and red blood cells are also recognized in the extravascular space (arrowheads). (D) Higher magnification of boxed area labeled by D in C. Cells with an irregular shape (arrows) appear along the pulp–dentin border in addition to the neutrophils. (F) Higher magnified view of the pulp horn. The odontoblast-like cells are arranged along the pulp–dentin border. Note the regenerated blood vessels (asterisk). (H) Higher magnification of boxed area labeled by H in G. Odontoblast-like cells with columnar-shaped deposit tubular dentin (TD) next to the preexisting dentin at the cusped area. Dotted lines show the boundary between the pulp chamber and surrounding lingual muscle tissue. Bars: A,C,E,G = 250 μm; B,D,F,H = 25 μm.
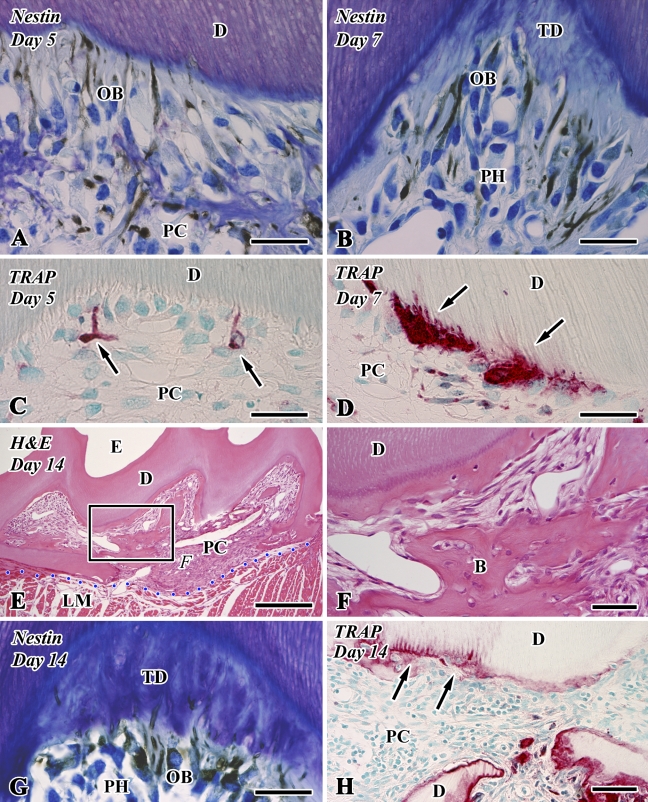
Figure 2.
Nestin immunoreactivity (A,B,G), tartrate-resistant acid phosphatase (TRAP) reactions (C,D,H), and H&E staining (E,F) in sections of the transplanted teeth at 5 (A,C), 7 (B,D), and 14 (E–H) days after operation (B, bone-like tissue; D, dentin; E, enamel space; LM, lingual muscle; PC, pulp chamber; PH, pulp horn; TD, tubular dentin). (A,B,G) Newly differentiated odontoblast-like cells (OB) in the pulp horn show intense immunoreactivity for nestin in their cytoplasm. (C,D) Intense TRAP-positive cells appear in the pulp chamber, where they are occasionally situated at the pulp–dentin border and elongate their cellular processes into the dentinal tubules (arrows). (E) Bone-like tissue is formed in the pulp chamber. (F) Higher magnification of boxed area labeled by F in E. The distinction between dentin and bone-like tissue is determined by the absence of dentinal tubules and the existence of cell inclusion in the case of bone-like tissue. (H) TRAP-positive cells remain around the bone matrix. Note the TRAP-positive reactions along the pulp–dentin border (arrows). Blue dotted line shows the boundary between the pulp chamber and surrounding lingual muscle tissue. Bars: E = 250 μm; F,H = 50 μm; A–D,G = 25 μm.
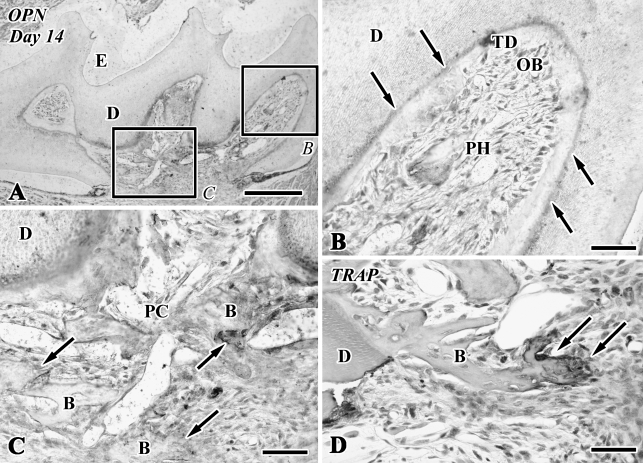
Figure 3.
Osteopontin (OPN) immunoreactivity (A–C) and TRAP reactions (D) in sections of the transplanted teeth at 14 days after surgery (B, bone-like tissue; D, dentin; E, enamel space; PC, pulp chamber; PH, pulp horn). (A,B) Newly differentiated odontoblast-like cells in the pulp horn lack an OPN-immunopositive reaction. Note the boundary between the preexisting and tertiary dentin (TD) showing an OPN-positive reaction (arrows). (B) Higher magnification of boxed area labeled by B in A. (C) Higher magnification of boxed area labeled by C in A. The osteoblast-like cells beneath the bone-like tissue matrix show an OPN-positive reaction (arrows). (D) TRAP-positive cells remain around the bone matrix (arrows). Bars: A = 250 μm; B–D = 50 μm.
Nestin and OPN Analyses
On Days 1–3, although an intense nestin-positive reaction was not recognizable in the pulp chamber (data not shown), the newly differentiated odontoblast-like cells in the pulp horn showed intense immunoreactivity for nestin in their cytoplasm (Figures 2A, 2B, and 2G) and lacked an OPN-immunopositive reaction (Figure 3B) after Days 5–14. The boundary between the preexisting and tertiary dentin always represented an OPN-positive reaction (Figures 3A and 3B). In contrast, the osteoblast-like cells beneath the bone-like tissue matrix showed OPN-positive (Figures 3A and 3C) and nestin-negative reactions (data not shown).
TRAP Analysis
Tooth transplantation caused the appearance of intense TRAP-positive reactions in the pulp chamber after Day 5 (Figure 2C). TRAP-positive cells were occasionally situated at the pulp–dentin border, elongated their cellular processes into the dentinal tubules (Figure 2D), and remained around the bone matrix until Day 14 (Figures 2H and 3D).
Cell Proliferation Assay by BrdU Labeling
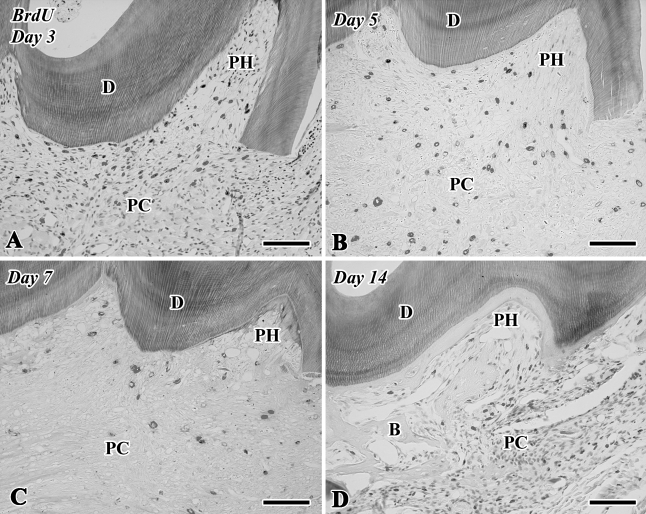
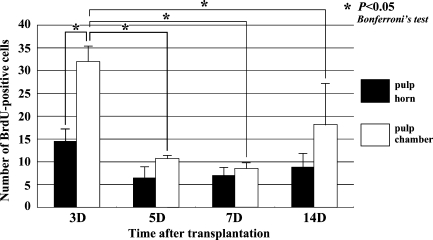
Although few BrdU-positive cells were recognizable in the pulp chamber on Day 1 (data not shown), a large number of BrdU-positive cells were observed in both the pulp horn and chamber on Day 3 (Figures 4A and 5). On Days 5–14, the BrdU-positive cells were significantly decreased in number in the pulp chamber (Figures 4B–4D and 5). The number of BrdU-labeled cells was statistically analyzed separately in two areas such as the pulp horn and chamber (Figure 5).
Figure 4.
5-Bromo-2′-deoxyuridine (BrdU)-labeled sections of the transplanted teeth at 3 (A), 5 (B), 7 (C), and 14 (D) days after surgery (B, bone-like tissue; D, dentin; PC, pulp chamber; PH, pulp horn). (A) A large number of BrdU-positive cells are observed in both the pulp horn and chamber. (B–D) The BrdU-positive cells are decreased in number in the pulp chamber. Bar = 100 μm.
Figure 5.
Quantitative analysis of cell proliferation in the dental pulp of replanted teeth at 3, 5, 7, and 14 days after tooth transplantation. BrdU-positive cells in the pulp chamber are significantly increased in number at Day 3 compared with the other stages.
Allogenic Transplantation Between lacZ Transgenic and Wild-type ROSA26 Mice
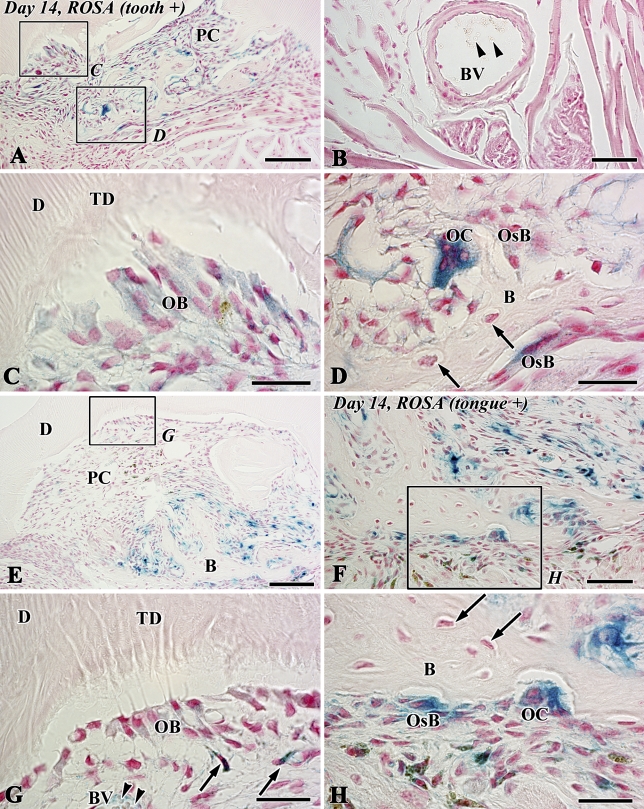
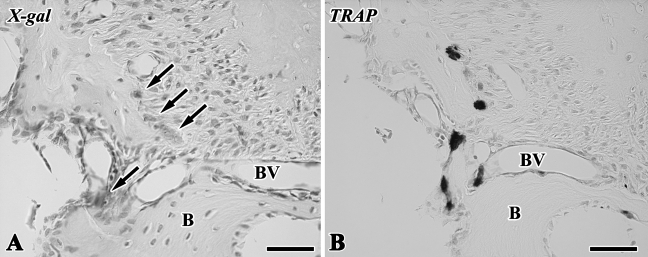
The blue-stained cells after X-gal staining corresponded to the lacZ transgenic cells from the lacZ transgenic mice (Kim et al. 2006), although stainability differed depending on the types of cells and their cellular activity. In tooth transplantation using lacZ transgenic ROSA 26 mice as the donor and wild type as the host, newly differentiated odontoblast-like cells beneath the dentin matrix contained granular depositions with the blue color. Osteoblast-like cells also showed the blue color in their cytoplasm in addition to intense stainability in polynuclear giant cells and osteoclast-like cells, although osteocytes and red blood cells in blood vessels showed negative reactions (Figures 6A–6D). In the experiment using lacZ transgenic ROSA 26 mice as the host and wild type as the donor, blue-stained cells were recognized in the osteoblast- and osteoclast-like cells, mesenchymal cells, and red blood cells in blood vessels in contrast with odontoblast-like cells and osteocytes with negative reactions (Figures 6E–6H). The results of X-gal stainability in the above two types of experiments are summarized in Table 1. To confirm the specificity of X-gal staining in the osteoclast-like cells, we performed both X-gal staining and TRAP histochemistry in the periodontal tissue of the wild-type ROSA26 mice. TRAP-positive osteoclast-like cells showed blue color in their cytoplasm, suggesting that X-gal staining was unable to determine the lacZ transgenic osteoclast-like cells in our experiment (Figure 7).
Figure 6.
X-gal staining in sections of the transplants at 14 days after allograft of the tooth of LacZ transgenic ROSA26 mice into the sublingual region of wild-type mice (A–D) and vice versa (E–H) (B, bone-like tissue; D, dentin; PC, pulp chamber; TD, tubular dentin). (A,C,D) Newly differentiated odontoblast-like cells (OB) beneath the dentin matrix contain granular depositions with the blue color. Osteoblast-like cells (OsB) also show blue color in their cytoplasm in addition to the intense stainability in the polynuclear giant cells, osteoclast-like cells (OC). Note osteocytes with negative reactions (arrows) in the bone matrix. (B) A blood vessel in the lingual tissue contains red blood cells with negative reactions (arrowheads). (C,D) Higher magnification of boxed areas labeled by C and D in A, respectively. (E–H) Blue-stained cells are recognized in osteoclast-like cells (OC), osteoblast-like cells (OsB), mesenchymal cells (arrows in G), and the red blood cells (arrowheads) in blood vessels (BV) in contrast with odontoblast-like cells (OB) and osteocytes (arrows in H) with negative reactions. (G,H) Higher magnification of boxed areas labeled by G and H in E and F, respectively. Bars: A,E = 100 μm; B,F = 50 μm; C,D,G,H = 25 μm.
Table 1.
X-gal stainability in each cell in the dental pulp after tooth transplantation using lacZ transgenic ROSA26 and wild-type mice
| LacZ transgenic tooth (donor) and wild-type tongue (host) | Wild-type tooth (donor) and lacZ transgenic tongue (host) | |
|---|---|---|
| Odontoblast-like cell | + | − |
| Osteoblast-like cell | + | ++ |
| Osteoclast-like cell | ++ | ++ |
| Osteocyte | − | − |
| Mesenchymal cell in the transplanted pulp chamber | + | ++ |
| Red blood cell | − | + |
++, intensive; +, weak; −, negative.
Figure 7.
X-gal staining (A) and TRAP reaction (B) in periodontal tissue of wild-type mice (B, alveolar bone; BV, blood vessel). (A,B) TRAP-positive osteoclast-like cells showed positive reactions in their cytoplasm (arrows). Bar = 50 μm.
Discussion
An experimental mouse model for allogenic transplantation of tooth crown into the sublingual region was established in this study. This model is useful for clarifying the capacity of dental pulp differentiation in the regenerative process after tooth transplantation, because the tooth root and periodontal tissues including root dentin, periodontal ligament, and cementum have already been removed from the donor transplant before allograft into the sublingual region. In contrast, the experimental model for conventional tooth replantation or transplantation includes the commitment of the periodontal tissue for pulp regeneration. Actually, the vascular supply from the periodontal tissue reaches the coronal portion of dental pulp until 5 days after tooth replantation (Shimizu et al. 2000). Another advantage of this model is that immunological rejection does not occur in the case of the use of littermates, despite allogenic tooth transplantation. The obtained results on the histological changes occurring in this study were almost the same as those in autogenic tooth transplantation (Ogawa et al. 2006). The summarized pulpal changes in the regenerative process are as follows: odontoblasts showed the degenerative features and inflammatory reactions including neutrophil infiltration and hemorrhage occurred in the pulp chamber on Day 1, cell proliferation in the pulp chamber became most active on Day 3, nestin-positive and OPN-negative newly differentiated odontoblast-like cells were arranged along the pulp–dentin border to deposit the dentin matrix in the pulp horn on Days 5–7, and bone-like tissue occurred in the pulp chamber apart from the continuous deposition of the dentin matrix on Day 14. Thus, this observation in allogenic tooth transplantation confirms that the dental pulp contains two types of competent progenitor cells capable of differentiating into either odontoblast- or osteoblast-like cells, as shown in autogenic tooth transplantation.
With respect to the capacity of dental pulp differentiation, it is well established that undifferentiated mesenchymal cells exist in the dental pulp and have the ability to differentiate into odontoblast-like cells, which are responsible for reparative dentin formation after tooth injury (Yamamura 1985; Ohshima 1990; Tziafas 1995; About et al. 2000a; Nakakura-Ohshima et al. 2003; Ohshima et al. 2003). Other studies have shown that isolated pulp tissue contributes to bone tissue formation in transplants (Zussman 1966; Luostarinen and Ronning 1977; Yamamura 1985; Takei et al. 1988; Inoue and Shimono 1992; Laino et al. 2005). In contrast, the pulpal mesenchymal cells from postnatal mouse incisors can give rise to odontoblasts and osteoblasts and functionally produce matrix when transplanted into kidney capsules (Braut et al. 2002,2003). Thus, it is reasonable to suppose that dental pulp is composed of various progenitor populations including odontoblast and osteoblast lineage cells and/or one multipotent stem cell, which gives rise to differentiation of both the odontoblasts and osteoblasts, and that certain regulatory mechanisms for determining the fate of these different lineage cells may exist in the microenvironment or niche. One important regulatory factor may be the existence of scaffolds such as preexisting dentin, because the differentiation of odontoblast-like cells always occurred beneath the preexisting dentin, and new dentin formation never occurred apart from the preexisting dentin matrix in this study. This notion is supported by evidence that signals similar to those involved in physiological dentinogenesis (Ruch et al. 1995) might be sequestered within the dentin matrix and released under pathological conditions (Tziafas et al. 2000; Smith and Lesot 2001; Goldberg and Smith 2004). Another important regulatory factor may be the type of cells appearing along the pulp–dentin border before the differentiation of hard tissue-formative cells. The close relationship between the appearance of intense TRAP-positive cells and the induction of bone-like tissue formation was shown in this study. The bone tissue formation occasionally occurred even beneath the preexisting dentin, where TRAP-positive osteoclast lineage cells appeared and extended their cellular processes into the dentinal tubules (Figures 2D and 2F). Thus, the appearance of osteoclast lineage cells may be involved in the induction of bone-like tissue formation in the allogenic transplanted tooth and the autografted tooth.
A recent study has shown that odontoblast and osteoblast lineage cells reside in the dental pulp in autogenic tooth transplantation (Ogawa et al. 2006). Regarding the origin of osteoblast lineage cells, we still cannot exclude the possibility that progenitor cells from the surrounding lingual tissue migrate into the pulp chamber and proliferate to give rise to hard tissue-forming cells. These allograft transplantation experiments using ROSA26 reporter mice clearly showed that both donor and host mesenchymal cells differentiated into osteoblasts with the assistance of osteoclast lineage cells, whereas newly differentiated odontoblasts were exclusively derived from donor pulpal cells. The findings are supported by a recent study describing allogenic tooth transplantation experiments using GFP reporter rats, although the previous study failed to clarify the capacity of dental pulp differentiation because the donor-transplanted tooth contained the periodontal tissue (Zhao et al. 2007). However, we have not been able to determine the origin of osteoclast lineage cells, because X-gal staining was unable to determine lacZ transgenic osteoclast-like cells in our experiment, in which TRAP-positive osteoclast-like cells in the periodontal tissue of wild-type ROSA mice showed LacZ-positive reactions in their cytoplasm. This osteoclast pitfall was mentioned in a recent study (Kopp et al. 2007). It is reasonable to suppose that osteoclast lineage cells are derived from the circulatory system in the host tissue, because TRAP-positive osteoclast-lineage cells first appear 5 days after transplantation, and they are associated with blood vessels. In contrast, the osteocytes in both donor and host tissues showed LacZ-negative reactions, probably because of their decreased cellular activity. Thus, the results suggest that the odontoblast and osteoblast lineage cells reside in the dental pulp and that both donor and host cells contribute to the bone-like tissue formation in the regenerated pulp tissue. With advances in stem cell biology and emerging concepts of tissue engineering (Langer and Vacanti 1993), biological teeth (Sharpe and Young 2005) may become an alternative for replacing missing teeth. The idea is to cultivate stem cells with odontogenic induction signals through epithelial–mesenchymal interactions, thereby programming the stem cells to adopt dental lineage and, with the help of scaffold/extracellular matrix, to become part of the tooth (Yen and Sharpe 2008). Thus, exact knowledge of the capacity of dental pulp differentiation would provide useful information for future regenerative treatment of the dental pulp and the application of this dental pulp capacity to biological teeth. Further studies are needed to clarify the relationship between the biological properties of dental pulp and the localization and differentiation capacity of DPSCs.
Acknowledgments
This work was supported in part by grants from KAKENHI (B) (16390523 and 19390462 to HO) and KAKENHI (C) (18592232 to KN-O) from MEXT and the Japan-Korea Joint Research Project from JSPS and KOSEF (F01-2005-000-10210-0).
The authors thank Shin-ichi Kenmotsu for technical assistance.
References
- About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA (2000a) Human dentin production in vitro. Exp Cell Res 258:33–41 [DOI] [PubMed] [Google Scholar]
- About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA (2000b) Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol 157:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, Robey PG, et al. (2003) Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 82:976–981 [DOI] [PubMed] [Google Scholar]
- Bonucci E, Nanci A (2001) Alkaline phosphatase and tartrate-resistant acid phosphatase in osteoblasts of normal and pathologic bone. Ital J Anat Embryol 106:129–133 [PubMed] [Google Scholar]
- Braut A, Kalajzic I, Kalajzic Z, Rowe DW, Kollar EJ, Mina M (2002) Col1a1-GFP transgene expression in developing incisors. Connect Tissue Res 43:216–219 [DOI] [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M (2003) Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1–2.3-GFP transgene. Int J Dev Biol 47:281–292 [PubMed] [Google Scholar]
- Byers MR, Kvinnsland I, Bothwell M (1992) Analysis of low affinity nerve growth factor receptor during pulpal healing and regeneration of myelinated and unmyelinated axons in replanted teeth. J Comp Neurol 326:470–484 [DOI] [PubMed] [Google Scholar]
- Goldberg M, Smith AJ (2004) Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med 15:13–27 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, et al. (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Suzuki H, Yoshie H, Ohshima H (2007) Influence of extended operation time and of occlusal force on determination of pulpal healing pattern in replanted mouse molars. Cell Tissue Res 329:259–272 [DOI] [PubMed] [Google Scholar]
- Haylock DN, Nilsson SK (2006) Osteopontin: a bridge between bone and blood. Br J Haematol 134:467–474 [DOI] [PubMed] [Google Scholar]
- Inoue T, Shimono M (1992) Repair dentinogenesis following transplantation into normal and germ- free animals. Proc Finn Dent Soc 88:183–194 [PubMed] [Google Scholar]
- Kim E, Cho SW, Yang JY, Cai J, Lee SL, Ohshima H, Jung HS (2006) Tooth survival and periodontal tissues healing of allogenic-transplanted teeth in the mice. Oral Dis 12:395–401 [DOI] [PubMed] [Google Scholar]
- Kopp HG, Hooper AT, Shmelkov SV, Rafii S (2007) Beta-galactosidase staining on bone marrow. The osteoclast pitfall. Histol Histopathol 22:971–976 [DOI] [PubMed] [Google Scholar]
- Kvinnsland I, Heyeraas KJ, Byers MR (1991) Regeneration of calcitonin gene-related peptide immunoreactive nerves in replanted rat molars and their supporting tissues. Arch Oral Biol 36:815–826 [DOI] [PubMed] [Google Scholar]
- Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, et al. (2005) A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res 20:1394–1402 [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926 [DOI] [PubMed] [Google Scholar]
- Liu H, Gronthos S, Shi S (2006) Dental pulp stem cells. Methods Enzymol 419:99–113 [DOI] [PubMed] [Google Scholar]
- Luostarinen V, Ronning O (1977) Differences in the osteoinductive potential of transplanted isogeneic dental structures of the rat. Acta Anat (Basel) 99:76–83 [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakakura-Ohshima K, Watanabe J, Kenmotsu S, Ohshima H (2003) Possible role of immunocompetent cells and the expression of heat shock protein-25 in the process of pulpal regeneration after tooth injury in rat molars. J Electron Microsc (Tokyo) 52:581–591 [DOI] [PubMed] [Google Scholar]
- Nanci A (2008) Ten Cate's Oral Histology: Development, Structure, and Formation. 7th ed. St. Louis, MO, Mosby, 379–395
- Ogawa R, Saito C, Jung HS, Ohshima H (2006) Capacity of dental pulp differentiation after tooth transplantation. Cell Tissue Res 326:715–724 [DOI] [PubMed] [Google Scholar]
- Ohshima H (1990) Ultrastructural changes in odontoblasts and pulp capillaries following cavity preparation in rat molars. Arch Histol Cytol 53:423–438 [DOI] [PubMed] [Google Scholar]
- Ohshima H, Nakakura-Ohshima K, Takeuchi K, Hoshino M, Takano Y, Maeda T (2003) Pulpal regeneration after cavity preparation, with special reference to close spatio-relationships between odontoblasts and immunocompetent cells. Microsc Res Tech 60:483–490 [DOI] [PubMed] [Google Scholar]
- Ohshima H, Nakakura-Ohshima K, Yamamoto H, Maeda T (2001) Alteration in the expression of heat shock protein (HSP)-25-immunoreactivity in the dental pulp of rat molars following tooth replantation. Arch Histol Cytol 64:425–437 [DOI] [PubMed] [Google Scholar]
- Ruch JV, Lesot H, Begue-Kirn C (1995) Odontoblast differentiation. Int J Dev Biol 39:51–68 [PubMed] [Google Scholar]
- Rungvechvuttivittaya S, Okiji T, Suda H (1998) Responses of macrophage-associated antigen-expressing cells in the dental pulp of rat molars to experimental tooth replantation. Arch Oral Biol 43:701–710 [DOI] [PubMed] [Google Scholar]
- Sharpe PT, Young CS (2005) Test-tube teeth. Sci Am 293:34–41 [DOI] [PubMed] [Google Scholar]
- Shimizu A, Nakakura-Ohshima K, Noda T, Maeda T, Ohshima H (2000) Responses of immunocompetent cells in the dental pulp to replantation during the regeneration process in rat molars. Cell Tissue Res 302:221–233 [DOI] [PubMed] [Google Scholar]
- Smith AJ (2002) Dentin formation and repair. In Hargreaves KM, Goodis HE, eds. Seltzer and Bender's Dental Pulp. Chicago, IL, Quintessence Publishing, 41–62
- Smith AJ, Lesot H (2001) Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med 12:425–437 [DOI] [PubMed] [Google Scholar]
- Takei K, Inoue T, Shimono M, Yamamura T (1988) An experimental study of dentinogenesis in autografted dental pulp in rats. Bull Tokyo Dent Coll 29:9–19 [PubMed] [Google Scholar]
- Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J (1995) Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol 39:947–956 [PubMed] [Google Scholar]
- Tsukamoto-Tanaka H, Ikegame M, Takagi R, Harada H, Ohshima H (2006) Histochemical and immunocytochemical study of hard tissue formation in dental pulp during the healing process in rat molars after tooth replantation. Cell Tissue Res 325:219–229 [DOI] [PubMed] [Google Scholar]
- Tziafas D (1995) Basic mechanisms of cytodifferentiation and dentinogenesis during dental pulp repair. Int J Dev Biol 39:281–290 [PubMed] [Google Scholar]
- Tziafas D, Smith AJ, Lesot H (2000) Designing new treatment strategies in vital pulp therapy. J Dent 28:77–92 [DOI] [PubMed] [Google Scholar]
- Yamamura T (1985) Differentiation of pulpal cells and inductive influences of various matrices with reference to pulpal wound healing. J Dent Res 64:530–540 [DOI] [PubMed] [Google Scholar]
- Yen AH, Sharpe PT (2008) Stem cells and tooth tissue engineering. Cell Tissue Res 331:359–372 [DOI] [PubMed] [Google Scholar]
- Zhao C, Hosoya A, Kurita H, Hu T, Hiraga T, Ninomiya T, Yoshiba K, et al. (2007) Immunohistochemical study of hard tissue formation in the rat pulp cavity after tooth replantation. Arch Oral Biol 52:945–953 [DOI] [PubMed] [Google Scholar]
- Zussman WV (1966) Osteogenic activity of odontoblasts in transplanted tooth pulps. J Dent Res 45:144–151 [DOI] [PubMed] [Google Scholar]