Abstract
The findings of this study show that Class III β-tubulin is a component of the mitotic spindle in multiple cell types. Class III β-tubulin has been widely used as a neuron-specific marker, but it has been detected also in association with breast and pancreatic cancers. In this study, we describe a novel finding of Class III β-tubulin in a subpopulation of cells in malignant peripheral nerve sheath tumor. The findings of this study also show that Class III β-tubulin is expressed by normal mesenchymal and epithelial cells (fibroblasts and keratinocytes), two transitional cell carcinoma cell lines, and neurofibroma Schwann cells, as shown by immunolabeling and Western transfer analysis using two different Tuj-1 antibodies that are specific for Class III β-tubulin. The corresponding mRNA was detected using RT-PCR and whole human genome microarrays. Both antibodies localized Class III β-tubulin to the mitotic spindle and showed a colocalization with α-tubulin. The immunoreaction became visible in early prophase, and the most intense immunoreaction was detected during metaphase and anaphase when microtubules were connected to the kinetochores on chromosomes. Class III β-tubulin–specific immunoreaction lasted to the point when the midbody of cytokinesis became detectable. (J Histochem Cytochem 56:1113–1119, 2008)
Keywords: Class III β-tubulin, malignancy, mitosis, malignant peripheral nerve sheath tumor, neurofibromas, TUBB3, Tuj1
Microtubules are hollow tubes that take part in various modes of cellular movement and in the maintenance and changing of cell morphology in an interphase cell. The cellular movements include molecular trafficking along microtubules through dynein and kinesin-type ATPases, movement of cilia and flagella, and translocation of chromosomes during the formation and disassembly of the mitotic spindle (Honore et al. 2005; Walczak and Heald 2008). Microtubules are composed of two structural tubulin subunit proteins, α- and β-tubulins. During the formation of microtubules, the 50-kDa α- and β-tubulins form heterodimers, which in turn constitute the building blocks of tubules.
In humans, seven β-tubulin isotypes exist with different tissue distributions (Ludueña 1998). These isotypes differ primarily in their C-terminal variable domain composed of 15 amino acids (Sullivan 1988; McKean et al. 2001). Class III β-tubulin has been considered a neuron-specific marker molecule encoded by a gene located at the long arm of chromosome 16 in humans (Katsetos et al. 2003a). In addition to neurons, Class III β-tubulin has been detected in selected malignancies, such as in breast cancers and other malignant epithelial tumors (Hasegawa et al. 2003; Jirásek et al. 2007). Some studies have associated expression of Class III β-tubulin with histologically high grade of malignancy in non-neuronal tumors (Katsetos et al. 2003a), and recent studies have described Class III β-tubulin as a marker of cancer cell resistance to taxanes (Hasegawa et al. 2003; Ferlini et al. 2005; Lee et al. 2007). Expression of Class III β-tubulin has also been shown to represent a useful tool to study early phases of neuronal differentiation in human embryonic development (Easter et al. 1993; Katsetos et al. 1993,2003b; Kukharskyy et al. 2004). The expression of Class III β-tubulin in mitotic spindles of neuronal precursors has been documented (Memberg and Hall 1995). However, the association of Class III β-tubulin in the mitotic spindle of epithelial cells, mesenchymal cells, and neural crest–derived Schwann cells has not been described previously.
In this study, we first analyzed malignant peripheral nerve sheath tumors (MPNSTs) of patients with neurofibromatosis 1 (NF1) with respect to Class III β-tubulin expression to seek informative biomarkers for these malignant tumors. High magnification showed a positive immunoreaction for Class III β-tubulin in dividing tumor cells. Confocal microscopy suggested localization of Class III β-tubulin in the mitotic spindle. To study the expression of Class III β-tubulin in mitotic cells in more detail, we analyzed normal human skin fibroblasts, keratinocytes, neurofibroma Schwann cells, and two transitional cell carcinoma cell lines in vitro and showed that Class III β-tubulin is a component of the mitotic spindle also in non-neuronal cells.
Materials and Methods
Tissue Samples and Cell Lines
All human tissue material was obtained from Turku University Central Hospital, Turku, Finland, with the permission of the Ethical Committee of the Hospital District of Southwest Finland and with appropriate written consent from the patients. Normal human skin samples were obtained from plastic surgeries of healthy persons, the MPNSTs and neurofibromas were obtained from the Department of Pathology, fresh neurofibroma tissue for culturing of Schwann cells was from the Department of Dermatology, and the great auricular nerve was provided by the Department of Otorhinolaryngology-Head and Neck Surgery. 5637 and T24 human urinary bladder cancer cell lines were purchased from American Type Culture Collection (ATCC; Rockville, MD). Cell line 5637 represents Grade 2 and T24 represents Grade 3 carcinomas. Red blood cells were obtained from a voluntary healthy female.
Cell Cultures
Keratinocytes were cultured and maintained essentially as described earlier (Ylä-Outinen et al. 2002). In brief, the cells were seeded on 25-cm2 cell culture flasks and grown until ∼40–60% confluence in serum-free low calcium keratinocyte growth medium (KGM; Gibco BRL, Paisley, UK). The medium was subsequently changed to defined KGM (Gibco BRL) containing either low (<0.1 mM) or high (1.8 mM) calcium concentration.
Fibroblast cultures were initiated from skin samples obtained from healthy volunteers (Ylä-Outinen et al. 1998). The cultures were maintained in DMEM supplemented with antibiotics (penicillin G, 100 U/ml; streptomycin sulfate, 50 μg/ml) and 10% FBS (Gibco BRL). For Western transfer analysis, fibroblasts were cultured in 1% FBS to expose the cells to the condition that provides low level of mitotic stimulus. FBS (5%) was chosen to represent a clearly higher concentration but within the same order of magnitude. Fibroblasts were harvested at time points of 0, 2, 4, and 24 hr.
Neurofibroma-derived Schwann cell cultures were initiated as described (Rosenbaum et al. 2000). The cancer cell lines 5637 and T24 were grown in DMEM supplemented with antibiotics (penicillin G, 100 U/ml; streptomycin sulfate, 50 μg/ml) and 10% FBS.
Antibodies
The following antibodies were used: mouse monoclonal antibody, Tuj-1, to rat Class III β-tubulin (MMS-435P; Covance, Princeton, NJ; referred to as an Antibody 1), mouse monoclonal antibody, Tuj-1, to human Class III β-tubulin (ab53234; Abcam, Cambridge, UK; referred to as an Antibody 2), rabbit monoclonal antibody to α-tubulin (ab52866; Abcam), and rat monoclonal anti-tubulin YL 1/2 (ab6160; Abcam). Secondary antibodies for indirect immunofluorescence were Alexa Fluor 488–conjugated goat anti-mouse IgG (A11029; Molecular Probes, Eugene, OR), Alexa Fluor 568–conjugated goat anti-rabbit IgG (A11011; Molecular Probes), and Cy3 conjugated goat anti-rat IgG (112-165-167; Jackson ImmunoResearch Europe, Suffolk, UK). Hoechst (H3570; Invitrogen, Eugene, OR) was used to visualize nuclei.
Immunolabeling of Paraffin-embedded Tissues
Formalin-fixed and paraffin-embedded malignant tumor specimens were immunolabeled with the avidin-biotin method and indirect immunofluorescence. The sections were cut on SuperFrost Plus microscope slides (Menzel-Gläser; Braunschweig, Germany), deparaffinized, and hydrated in descending ethanol series. To retrieve Tuj-1 antigens, the samples were boiled for 10 min in a microwave oven in 10 mM Tris, 1 mM EDTA, pH 9, and subsequently cooled in the same solution at room temperature for 30 min. For indirect immunofluorescence, the samples were blocked in PBS supplemented with 1% BSA. The samples were incubated overnight at 4C with antibody to Class III β-tubulin (Antibody 1, dilution 1:1000) and with secondary antibody Alexa 488 (dilution 1:200) for 1 hr at room temperature and DAPI (100 ng/ml) for DNA staining. For avidin-biotin immunolabeling, endogenous peroxidase activity was quenched by treating the sections in 0.3% H2O2 for 30 min. To prevent nonspecific binding, the sections were incubated in horse serum diluted in PBS. Antibodies to Class III β-tubulin were diluted in PBS supplemented with 1% BSA in dilutions 1:10,000 (Antibody 1) and 1:1000 (Antibody 2), and incubated on the samples overnight at 4C. The bound antibodies were visualized using the appropriate avidin-biotin peroxidase kit (Vectastain; Vector Laboratories, Burlingame, CA) with 3.3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen (DAB peroxidase substrate kit; Vector Laboratories). Sections were counterstained with Mayer's hematoxylin. In negative control reactions, primary antibody was replaced with 1% BSA-PBS.
Indirect Immunofluorescence of Cultured Cells
Samples were fixed in 4% paraformaldehyde at room temperature for 10 min and permeabilized with 0.1% Triton-X-100 in PBS. To prevent nonspecific binding, the samples were preincubated in 1% BSA-PBS for 30 min. The primary antibodies, Tuj-1 (dilutions Antibody 1, 1:250; Antibody 2, 1:200), anti-α-tubulin (dilution 1:250), and anti-tubulin YL 1/2 (dilution 1:100) were diluted in 1% BSA-PBS and incubated on the samples overnight at 4C. After five 5-min washes in PBS, the slides were incubated with secondary antibodies, Alexa Fluor 488 (dilution 1:200), Alexa Fluor 568 (dilution 1:300), and Cy3 (dilution 1:500), and Hoechst for nuclear staining (dilution 1:10,000), at room temperature for 2 hr. After incubation, the samples were washed five times in PBS for 5 min and mounted with IMMU-Mount (Thermo Shandon; Pittsburgh, PA). In control immunoreactions, primary antibodies were replaced with 1% BSA-PBS.
Microscopy
Confocal laser scanning microscopy was carried out using a Zeiss LSM 510 META confocal microscope equipped with argon-ion and helium-neon lasers (Zeiss; Jena, Germany) and LSM 3.2 software. The objectives were ×63 (oil immersion; numeric aperture, 1.4) and ×100 (oil immersion; numeric aperture, 1.4). For excitation, the 405-nm line was used for Hoechst, the 488-nm line for Alexa Fluor 488, and the 543-nm line for Alexa Fluor 568 and Cy3.
Western Transfer Analysis
The cells were rinsed with PBS, lysed in lysis buffer containing 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton-X-100, protease inhibitors (Complete, Mini, EDTA-free Protease Inhibitors cocktail tablets, 1 tablet per 10 ml; Boehringer, Mannheim, Germany), 1 mM dithiothreitol, and 1 mM Na3VO4. Protein concentration was measured using a protein assay (Bio-Rad; Hercules, CA), and equal amounts of protein (4 μg) were run on 10% SDS-PAGE gels. After electrophoresis, the proteins were transferred to Immobilon–P transfer membrane (Millipore; Bedford, MA). Membranes were first blocked with 5% skimmed milk in 50 mM Tris, pH 8.0, 2 mM CaCl2, and 80 mM NaCl and incubated with primary antibodies. Both antibodies to Class III β-tubulin were used in the 1:1000 dilution and β-actin antibody in the 1:5000 dilution. Horse anti-mouse horseradish peroxidase conjugated antibody (7076; Cell Signaling Technology, Beverly, MA) was used as secondary antibody and detected with enhanced chemiluminescence (Amersham Life Sciences; Little Chalfont, UK).
RT-PCR
Total RNA was isolated from human peripheral nerve, cultured keratinocytes, and skin fibroblasts using the RNeasy Mini Kit (cat no 74104; Qiagen, Venlo, The Netherlands) according to the protocol provided by the manufacturer. Total RNA was transcribed into single-stranded DNA in a 20-μl reaction volume containing 200 U of SuperScript II reverse transcriptase (18064-022; Invitrogen, Carlsbad, CA), 0.5 mM of each of the four deoxynucleotides, random primers (C1181; Promega, San Luis Obispo, CA), 20 U of RNase inhibitor (rRNasin; Promega), and 0.01 M dithiothreitol in the first-strand buffer. The concentrations of cDNA were measured using a NanoDrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies; Wilmington, DE). One hundred ng of the cDNA was used as a template for PCR in a 20-μl reaction containing 20 pmol of Class III β-tubulin–specific primers (5′-CTCAGGGGCCTTTGGACATC-3′ and 5′-CAGGCAGTCGCAGTTTTCAC-3′) or β-actin–specific primers (5′-CTGTCTGGCGGCACCACCAT-3′ and 5′-GCAACTAAGTCATAGTCCGC-3′), 0.2 mM of each of the four deoxynucleotides, and 0.4 U of Phusion Hot Start DNA Polymerase (Finnzymes Oy; Espoo, Finland). Amplification was performed in 25 cycles of denaturation (30 sec at 95C), annealing (30 sec at 60C), and extension (30 sec at 72C). In the negative control samples, the template was omitted. The primer sequences for Class III β-tubulin and β-actin and the RT-PCR reactions were previously published (Hasegawa et al. 2003; Ramani et al. 2005). The primers for Class III β-tubulin were determined so that they are located in different exons to prevent amplification from contaminating genomic DNA. They were also designed to maximize the sequence difference from other β-tubulin isotypes (Hasegawa et al. 2003). The PCR products were analyzed electrophoretically on 2% agarose gels and stained with ethidium bromide.
Detection of Hybridization Signal in Whole Human Genome Microarray
For microarray analysis, RNA was isolated using the cesium chloride gradient centrifugation method. Quantitation and quality control of RNA was performed before sample labeling using BioRad Experion system (Cat no. 700-7002; Bio-Rad Life Science Research Group, Hercules, CA). Agilent whole human genome microarray (G4112A; Agilent Technologies, Palo Alto, CA) was used as a microarray platform. Twenty μg of total RNA was used for cDNA synthesis and labeling with Cy3 and Cy5 using a fluorescent direct label kit (G2557A; Agilent Technologies), and the hybridizations and washes were performed according to the protocol provided by the manufacturer. A total of 21 arrays were hybridized. Arrays were scanned with an Agilent Technologies Scanner, model G2505B. Numerical results were extracted with Agilent Feature Extraction software.
Results
Class III β-Tubulin Is Expressed in MPNST
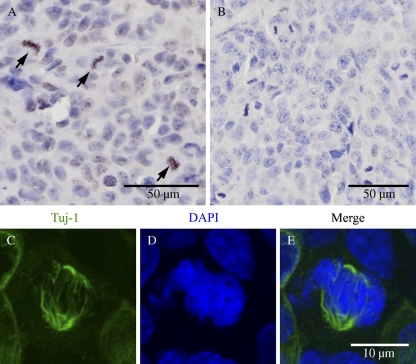
Class III β-tubulin has been detected in selected carcinomas, and expression of Class III β-tubulin has been associated with high grade of these malignancies. A novel finding of this study was detection of Class III β-tubulin in an MPNST (Figure 1). Examination of this MPNST with higher resolution showed Class III β-tubulin in a subpopulation of cells. Furthermore, confocal laser scanning microscopy showed a fibrillar Class III β-tubulin–specific immunoreaction in association with mitotic spindles. In benign neurofibromas with no mitoses, antibodies specific for Class III β-tubulin showed a positive immunoreaction exclusively in axons (data not shown).
Figure 1.
Formalin-fixed, paraffin-embedded malignant peripheral nerve sheath tumor (MPNST). Avidin-biotin immunolabeling with antibody to Class III β-tubulin (Tuj-1, Antibody 1) (A). Arrows point to positive immunoreaction in a subpopulation of cells. Negative control; primary antibody omitted (B). Confocal laser scanning microscopy of the same tumor sample shows a fibrillar Class III β-tubulin immunoreaction in association with mitotic spindles (C–E). Indirect immunofluorescence labeling for Class III β-tubulin: Antibody 1 (C), DAPI staining (D), and a merged image of C and D (E).
Class III β-Tubulin Is Expressed Throughout Mitosis in Various Cell Types
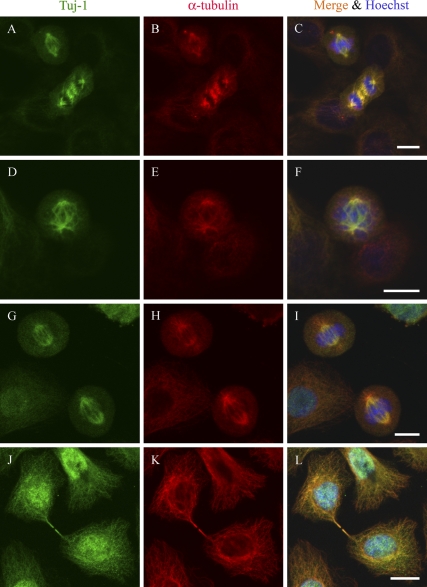
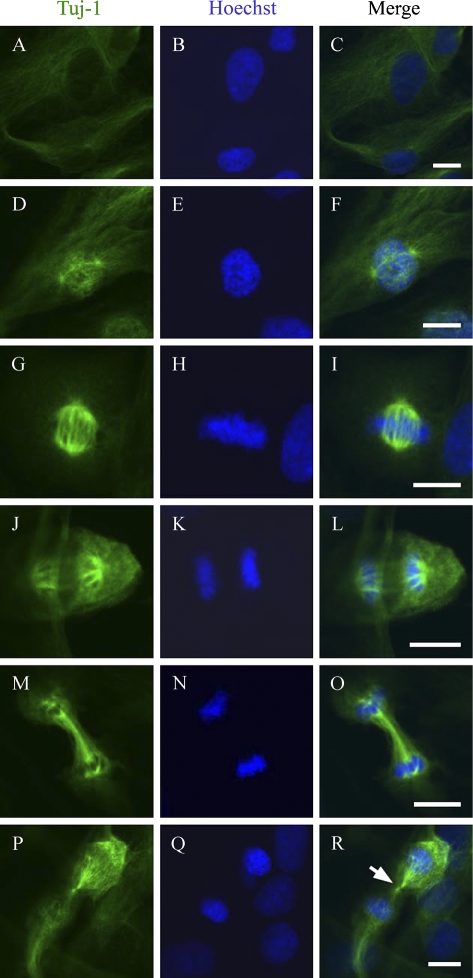
Double immunolabeling of normal human keratinocytes and two transitional cell carcinoma cell lines showed colocalization of α-tubulin and Class III β-tubulin in mitotic spindle (Figure 2). Further analyses on the different phases of mitosis showed that Class III β-tubulin became detectable in microtubules connecting centrosomes in early prophase (Figure 3). The most intense immunoreaction with antibodies specific for Class III β-tubulin was detected during metaphase and anaphase when microtubules were attached to centrosomes and kinetochores. Specific immunoreaction lasted to the point when the midbody of cytokinesis became detectable (Figure 3).
Figure 2.
Colocalization of Class III β-tubulin and α-tubulin in two transitional cell carcinoma cell lines and in normal human keratinocytes. Two transitional cell carcinoma cell lines, T24 (A–C) and 5637 (D–F), were immunolabeled for Class III β-tubulin (Tuj-1, Antibody 2) (A,D) and α-tubulin (B,E). Normal human keratinocytes were immunolebeled with Tuj-1 Antibody 1 for Class III β-tubulin (G,J) and α-tubulin (H,K). (C,F,I,L) Merged image of Class III β-tubulin, α-tubulin, and Hoechst stain. Bar = 10 μm.
Figure 3.
Visualization of Class III β-tubulin at different stages of mitosis. Confocal laser scanning analysis of normal human skin fibroblasts immunolabeled for Class III β-tubulin (Tuj-1, Antibody 1) (left) and stained with Hoechst (middle). Merged images are shown on the right. Interphase (A–C), prophase (D–F), metaphase (G–I), early anaphase (J–L), late anaphase (M–O), and early cytokinesis (P–R). Arrow points to the site of the midbody. Bar = 10 μm.
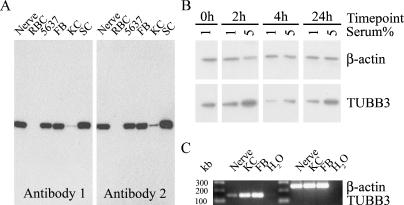
The presence of Class III β-tubulin in normal human skin fibroblasts, keratinocytes, neurofibroma Schwann cells, and two transitional cell carcinoma cell lines was shown also by Western transfer analysis using two different antibodies specific for Class III β-tubulin. Both antibodies showed an expected ∼50-kDa band that corresponds to that described for Class III β-tubulin (Figure 4A). Normal peripheral nerve and red blood cells were used as positive and negative controls, respectively. In further analyses, normal human fibroblasts were cultured in medium with 1% or 5% FBS for varying time periods. Medium supplemented with 1% FBS was chosen to expose the cells to the condition that provides low level of mitotic stimulus, whereas 5% FBS represented a clearly higher concentration but within the same order of magnitude. At all time points, the levels of Class III β-tubulin were higher in cultures maintained in medium with 5% FBS compared with those maintained in medium with 1% FBS (Figure 4B). At time point 4 hr, the Class III β-tubulin levels were lower than at the 2- or 24-hr time points. These data suggest that the expression of Class III β-tubulin can be regulated, and it thus cannot be considered as a housekeeping gene.
Figure 4.
Expression of Class III β-tubulin (TUBB3) as shown by Western transfer analysis and RT-PCR. (A) Western analysis for Class III β-tubulin with two different antibodies to Class III β-tubulin (Antibodies 1 and 2); the samples included human peripheral nerve, human red blood cells (RBC), transitional cell carcinoma cell line 5637, human skin fibroblasts (FB), normal human keratinocytes (KC), and neurofibroma Schwann cells (SC). (B) Western transfer analysis of normal human fibroblasts cultured in medium with 1% or 5% FBS for varying time periods and immunolabeled for β-actin and Class III β-tubulin (Antibody 1). (C) RT-PCR with Class III β-tubulin and β-actin sequence-specific primers. RNA was isolated from human peripheral nerve, skin fibroblasts (FB), and keratinocytes (KC); the results show predicted 254- and 160-bp bands for β-actin and Class III β-tubulin mRNA, respectively.
To verify the presence of Class III β-tubulin mRNA in cultured fibroblasts and keratinocytes, total RNA was isolated and subjected to RT-PCR analysis. RNA extract from the human peripheral nerve was used as a positive control. The results showed a single band of predicted size apparently representative of Class III β-tubulin mRNA (Figure 4C). Furthermore, Class III β-tubulin (TUBB3, NM_006086) expression by normal human epidermal keratinocytes was detected in the Agilent whole human genome microarray platform. This study was originally designed to analyze differentiating keratinocytes, and the bulk of this material will be reported independently.
Discussion
Class III β-tubulin has been considered one of the first cytoskeletal proteins with neuron specificity, and it has been detected in a wide variety of neural tissues. In agreement with previous findings, we found Class III β-tubulin exclusively in axons of benign tissues, normal skin, and cutaneous neurofibromas. Previous studies have detected Class III β-tubulin in human malignancies with neuroepithelial and non-neuronal origins. This study is the first to report the expression of Class III β-tubulin in MPNSTs. Surprisingly, cultured normal human mesenchymal and epithelial cells also expressed Class III β-tubulin.
When seeking informative biomarkers for MPNSTs, we found that Class III β-tubulin–specific antibody labels a subpopulation of tumor cells. A more detailed analysis showed a positive immunoreaction for Class III β-tubulin in the mitotic spindle of dividing MPNST cells. To further study the unexpected association of Class III β-tubulin and the mitotic spindle of non-neuronal cells, we cultured normal human skin fibroblasts representing mesenchymal cells, and keratinocytes and two carcinoma cell lines representing epithelial cells of different non-neural origins. The presence of Class III β-tubulin in all cell types was shown by Western transfer analysis using two different antibodies specific for Class III β-tubulin, both recognizing the C-terminal domain of human Class III β-tubulin. Furthermore, we verified the presence of Class III β-tubulin mRNA in cultured cells by RT-PCR using Class III β-tubulin–specific primers. Class III β-tubulin expression by keratinocytes was also detected in whole human genome microarrays. Immunolabeling showed that Class III β-tubulin is present in mitotic cells and that it is detectable in all phases of mitosis. Double immunolabeling showed colocalization of α-tubulin and Class III β-tubulin in the mitotic spindle.
In summary, we suggest that the positivity for Class III β-tubulin in malignant tumors described earlier may be explained, at least in part, by the high rate of cell divisions in malignant tissues. We can only speculate that the presence of Class III β-tubulin may relate to the intense cellular movement taking place during mitosis. An analogous situation may be operative in the fast axonal trafficking that transports molecules and organelles for very long distances on a cellular scale.
Acknowledgments
This study was financially supported by grants from the Academy of Finland, University of Turku, Finland, the Turku University Foundation, the Finnish Medical Foundation, the Hospital District of Southwest Finland, and Northern Ostrobothnia Hospital District of Finland.
References
- Easter SJ, Ross L, Frankfurter A (1993) Initial tract formation in the mouse brain. J Neurosci 13:285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlini C, Raspaglio G, Mozzetti S, Cicchillitti L, Filippetti F, Gallo D, Fattorusso C, et al. (2005) The seco-taxane IDN5390 is able to target class III beta-tubulin and to overcome paclitaxel resistance. Cancer Res 65:2397–2405 [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Miyoshi Y, Egawa C, Ishitobi M, Taguchi T, Tamaki Y, Monden M, et al. (2003) Prediction of response to docetaxel by quantitative analysis of class I and III beta-tubulin isotype mRNA expression in human breast cancers. Clin Cancer Res 9:2992–2997 [PubMed] [Google Scholar]
- Honore S, Pasquier E, Braguer D (2005) Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci 62:3039–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirásek T, Písaríková E, Viklický V, Mandys V (2007) Expression of class III beta-tubulin in malignant epithelial tumours: an immunohistochemical study using TU-20 and TuJ-1 antibodies. Folia Histochem Cytobiol 45:41–45 [PubMed] [Google Scholar]
- Katsetos C, Frankfurter A, Christakos S, Mancall E, Vlachos I, Urich H (1993) Differential localization of class III, beta-tubulin isotype and calbindin-D28k defines distinct neuronal types in the developing human cerebellar cortex. J Neuropathol Exp Neurol 52:655–666 [DOI] [PubMed] [Google Scholar]
- Katsetos C, Herman M, Mörk S (2003a) Class III beta-tubulin in human development and cancer. Cell Motil Cytoskeleton 55:77–96 [DOI] [PubMed] [Google Scholar]
- Katsetos C, Legido A, Perentes E, Mörk S (2003b) Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol 18:851–866 [DOI] [PubMed] [Google Scholar]
- Kukharskyy V, Sulimenko V, Macůrek L, Sulimenko T, Dráberová E, Dráber P (2004) Complexes of gamma-tubulin with nonreceptor protein tyrosine kinases Src and Fyn in differentiating P19 embryonal carcinoma cells. Exp Cell Res 298:218–228 [DOI] [PubMed] [Google Scholar]
- Lee K, Cao D, Itami A, Pour P, Hruban R, Maitra A, Ouellette M (2007) Class III beta-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology 51:539–546 [DOI] [PubMed] [Google Scholar]
- Ludueña R (1998) Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol 178:207–275 [DOI] [PubMed] [Google Scholar]
- McKean P, Vaughan S, Gull K (2001) The extended tubulin superfamily. J Cell Sci 114:2723–2733 [DOI] [PubMed] [Google Scholar]
- Memberg SP, Hall AK (1995) Dividing neuron precursors express neuron-specific tubulin. J Neurobiol 27:26–43 [DOI] [PubMed] [Google Scholar]
- Ramani P, Thomas G, Ahmed S (2005) Use of RT-PCR in detecting disseminated cancer cells after incisional biopsy among oral squamous cell carcinoma patients. J Cancer Res Ther 1:92–97 [DOI] [PubMed] [Google Scholar]
- Rosenbaum T, Rosenbaum C, Winner U, Müller HW, Lenard HG, Hanemann CO (2000) Long-term culture and characterization of human neurofibroma-derived Schwann cells. J Neurosci Res 61:524–532 [DOI] [PubMed] [Google Scholar]
- Sullivan K (1988) Structure and utilization of tubulin isotypes. Annu Rev Cell Biol 4:687–716 [DOI] [PubMed] [Google Scholar]
- Walczak C, Heald R (2008) Mechanisms of mitotic spindle assembly and function. Int Rev Cytol 265:111–158 [DOI] [PubMed] [Google Scholar]
- Ylä-Outinen H, Aaltonen V, Björkstrand A, Hirvonen O, Lakkakorpi J, Vähä-Kreula M, Laato M, et al. (1998) Upregulation of tumor suppressor protein neurofibromin in normal human wound healing and in vitro evidence for platelet derived growth factor (PDGF) and transforming growth factor-beta1 (TGF-beta1) elicited increase in neurofibromin mRNA steady-state levels in dermal fibroblasts. J Invest Dermatol 110:232–237 [DOI] [PubMed] [Google Scholar]
- Ylä-Outinen H, Koivunen J, Nissinen M, Björkstrand A, Paloniemi M, Korkiamäki T, Peltonen S, et al. (2002) NF1 tumor suppressor mRNA is targeted to the cell-cell contact zone in Ca(2+)-induced keratinocyte differentiation. Lab Invest 82:353–361 [DOI] [PubMed] [Google Scholar]