Abstract
The ideal antiserum for immunohistochemical (IHC) applications contains monospecific high-affinity antibodies with little nonspecific adherence to sections. Many commercially available antibodies are “affinity” purified, but it is unknown if they meet “hard” specificity criteria, such as absence of staining in tissues genetically deficient for the antigen or a staining pattern that is identical to that of an antibody raised against a different epitope on the same protein. Reviewers, therefore, often require additional characterization. Although the affinity-purified antibodies used in our study on the distribution of muscarinic receptors produced selective staining patterns on sections, few passed the preabsorption test, and none produced bands of the anticipated size on Western blots. More importantly, none showed a difference in staining pattern on sections or Western blots between wild-type and knockout mice. Because these antibodies were used in most studies published thus far, our findings cast doubts on the validity of the extant body of morphological knowledge of the whole family of muscarinic receptors. We formulate requirements that antibody-specification data sheets should meet and propose that journals for which IHC is a core technique facilitate consumer rating of antibodies. “Certified” antibodies could avoid fruitless and costly validation assays and should become the standard of commercial suppliers. (J Histochem Cytochem 56:1099–1111, 2008)
Keywords: muscarinic receptors, antibody specificity, immunohistochemistry, Western blotting, knockout mice
The ideal antiserum for immunohistochemical (IHC) purposes should contain monospecific antibodies with a high affinity for its target epitope(s) and little nonspecific adherence to the section. The widespread availability of antibodies has made the IHC visualization of most known proteins feasible. Often, such monoclonal and polyclonal antibodies are additionally purified using protein A/G and antigen-affinity chromatography. Although these tools should yield specific, high-affinity antibodies, the quality of antisera varies. The problems of cross-reactivity of antisera that are associated with degeneracy and mimicry in immune recognition (Cohn 2005) are well established. Furthermore, the inherent drawbacks of the use of animals to produce antisera, viz., the potential presence of antibodies against contaminants of the immunogen and/or against antigens to which the animal has been exposed earlier, are well known.
For all these reasons, commercial catalogs usually extol the quality of antisera by showing their staining pattern in sections to demonstrate the signal-to-noise ratio of the antiserum-dependent staining and on Western blots to validate that only a single protein of the expected molecular mass is recognized. Often, IHC studies with antisera, including antisera against muscarinic receptors (MRs) (Danielson et al. 2006; Mukerji et al. 2006; Sakamoto et al. 2006; Tyagi et al. 2006; Coccini et al. 2007; Danielson et al. 2007; Harrington et al. 2007), are considered to be specific on the basis of the data in specification sheets only. However, such information may pertain to special cells or tissues, whereas Western blots may show reactivity with a single protein in a specific (partially purified) extract only. In fact, the catalogs often do not even provide additional information when the size of a band in a Western blot does not correspond with the expected size of the protein (see Western Blots).
Because adequate quality control data are rarely available for commercial antisera and because tissue-intrinsic controls are only applicable if previous experience with a particular antigen–tissue combination is available, additional validation of specificity of antibodies is still a crucial part of morphological studies. Many criteria have been used to evaluate specificity (Swaab et al. 1977; Van der Sluis and Boer 1986; van Leeuwen 1986; Saper and Sawchenko 2003; Holmseth et al. 2006; Zarghooni et al. 2007) (Table 1). Of these, a selective staining pattern, a single band of the expected size on Western blots, and the disappearance of staining after preabsorption of the antiserum with purified epitope are most often available for commercially available antisera, whereas the remaining, more solid criteria, such as absence of staining in tissues genetically deficient for the protein (Swaab et al. 1977; van Leeuwen 1986; Holmseth et al. 2006), identical staining patterns of antibodies raised against different epitopes on the same protein (Fischer et al. 2003), and/or correspondence between the staining pattern after ISH and IHC (Sträter et al. 2001) are rarely available. The establishment of confirmatory criteria for each and every single study is financially costly, time consuming, and requires biochemical expertise. These repetitive quality controls and the confusion that is generated if they are not properly carried out can be largely avoided if the specificity data sheets of commercially available antisera meet adequate, that is, higher-quality criteria.
Table 1.
Criteria to evaluate antiserum selectivity and specificity
| Selectivity |
| a. Highly selective staining pattern |
| b. Staining of a protein band of the expected size on Western blots |
| c. Disappearance of staining after preabsorption of the antiserum with purified epitope |
| Specificity |
| d. Absence of staining in (mouse) tissues genetically deficient for the protein |
| e. Identical staining pattern of antibodies raised against different epitopes on the same protein in consecutive sections |
| f. Correspondence between the staining pattern after ISH and IHC in consecutive sections |
In this study, we describe our experience with commercially available antisera against MRs. These antisera met more than one of the criteria for specificity described above, but none met all, and hence, none showed MR localization. Because these antibodies have been used in most studies published thus far, our findings cast doubt on the validity of the published body of morphological knowledge of the whole family of MRs. Based on our experience, we propose that one of the robust specificity criteria (Table 1, items d and e) and consumer rating be added to the qualifications that are provided by commercial suppliers as validation of the reliability of antisera in their catalogs. The availability of “certified” antisera will save end users time and the expense of additional work over and above their main line of research.
Materials and Methods
Antibodies
Commercially available antisera or antibodies directed against the five subtypes of MRs were obtained from three suppliers [Alomone Labs, Jerusalem, Israel; Research and Diagnostic Antibodies (R&D), Las Vegas, NV; Chemicon/Millipore, Billerica, MA; Table 2]. They were used to localize MRs in various organs of the lower urinary tract and gastrointestinal tract. The details of the epitopes used to generate antibodies against each MR subtype as provided by the suppliers are listed in Table 3. All antibodies were available as affinity-purified preparations. In addition, images of Western blots were provided in the product certificates to show the specificity of the antisera.
Table 2.
Muscarinic receptor antibodies used in this study
| Antigen | Host species | Dilution (WB) | Dilution (IHC) | Code | Supplier | Blocking serum |
|---|---|---|---|---|---|---|
| MR1 | Rabbit | 1:900 | 1:300 | AMR-001 | Alomone Labs | Goat or fetal calf |
| MR2 | Rabbit | 1:600 | 1:250 | AMR-002 | Alomone Labs | Goat or fetal calf |
| MR3 | Rabbit | 1:750 | 1:500 | AS-3741S | R&D | Goat or fetal calf |
| MR3 | Rabbit | 1:500 | 1:100 | AMR-006 | Alomone Labs | |
| MR4 | Mouse | 1:300 | 1:50 | MAB1576 | Chemicon | Fetal calf |
| MR5 | Rabbit | 1:1,000 | 1:1,000 | AS-3781S | R&D | Goat or fetal calf |
WB, Western blotting; MR, muscarinic receptor.
Table 3.
Epitope of muscarinic receptor antisera used
| Receptor | Epitope sequence | Corresponding residues | Fusion protein |
|---|---|---|---|
| MR1 | GSETPGKGGGSSSSSERSQPGAEGSPETPPGRCCRCCRAPRLLQAYSWKEEEEEDEGSMESLTSSEGEEPGSEVVIKMPMVDPEAQAPTKQPPRSSPNTVKRPTKKGRDRAGKGQKPRGKEQLAKRK | AA 227-353 of human MR1 | GST |
| MR2 | VANQDPVSPSLVQGRIVKPNNNNMPSSDDGLEHNKIQNGKAPRDPVTENCVQGEEKESSNDSTSVSAVASNMRDDEITQDENTVSTSLGHSKDENSKQTCIRIGTKTPKSDSCTPTNTTVEVVGSSGQNGDE | AA 225-356 of human MR2 | GST |
| MR3 (R&D) | FHKRVPEQAL | AA 580-589 of rat MR3 | KLH |
| MR3 (Alomone) | TLAKRFALKTRSQITKRKR | AA 461-479 rat MR3 | mcKLH |
| MR4 | Third intracellular loop of human MR4 | Sequence details not disclosed by company | GST |
| MR5 | EEKLYWQGNSKLP | AA 519-531 of rat MR5 | KLH |
MR, muscarinic receptor; GST, gluthathione S-transferase; KLH, keyhole limpet hemocyanin; mcKLH, maleimide-activated keyhole limpet hemocyanin.
Animals
FVB mice and Wistar rats, 4–5 weeks old, were obtained from our institute's animal center. The animals were euthanized by instant decapitation under an O2/CO2 daze in agreement with Dutch guidelines for experimental animals. In addition, we used C57/Bl6 mice that were genetically deficient for the MR1, MR2, MR3, MR4, or MR5 receptors (Wess et al. 2003,2007). These mice were made available by Dr. Jurgen Wess, Laboratory of Bioorganic Chemistry, National Institutes of Health–National Institute of Diabetes, Digestive and Kidney Diseases (NIH-NIDDK), Bethesda, MD.
Western Blotting
Protein extracts from the brain, liver, stomach, jejunum, colon, and bladder of mice and rats were prepared. Briefly, the organs were homogenized in PBS containing 1% Triton-X114, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.1 mg/ml PMSF, 5 mg/ml aprotinin, and 1 mM sodium orthovanadate. Protein concentration was measured using the bicinchoninic acid (BCA) reagent (Pierce; Rockford, IL). Ten percent SDS-PAGE gels were used to separate 50 μg protein of the respective organs. The gels were blotted for 2 hr in 25 mM ethanolamine/glycine, pH 9.5, to polyvinylidene fluoride (PVDF) membranes (Millipore; Billerica, MA). After blocking in TENG-T [10 mM Tris HCl, 5 mM EDTA, 150 mM NaCl, 0.25% (w/v) gelatin, 0.05% (v/v) Tween-20, pH 8.0], containing 10% FCS, the membranes were exposed overnight to the antisera for MR1 (1:900), MR2 (1:600), MR3 (Alomone Labs: 1:500; R&D: 1:750), MR4 (1:300), and MR5 (1:1000) at 4C, all dissolved in TENG-T/FCS. After washing, the membranes were exposed for 2.5 hr at room temperature to horseradish peroxidase–conjugated goat anti-rabbit (1:5000) or goat anti-mouse (1:2500) secondary antibody (no. 170-6516 and 170-6516; BioRad, Hercules, CA) that was dissolved in TENG-T/FCS. Chemiluminescence was recorded with a Lumi-imager (Roche; Almere, The Netherlands) after addition of LumiLight Plus (Roche).
IHC Staining
The organs of the lower urinary tract and gastrointestinal tract were removed immediately after sacrifice and dividing the pubic symphysis. The specimens were fixed overnight by immersion in 4% freshly prepared PBS-buffered formaldehyde or in an ice-cold mixture of methanol:acetone:water (2:2:1; v/v) at 4C. Thereafter, each sample was dehydrated in graded ethanols and embedded in Paraplast (Oxford; St. Louis, MO). Serial sections of 7 μm thickness were prepared, mounted on poly-l-lysine–coated slides, deparaffinized in xylene, rehydrated in graded ethanols, and washed in PBS. The sections were heated at 120C in 10 mM sodium citrate (pH 6.0) for 10 min to retrieve antigens (Yamashita 2007) and to inactivate endogenous alkaline phosphatase, cooled at room temperature for at least 15 min, washed in PBS, blocked in TENG-T, containing either 10% normal goat, fetal calf, or rabbit serum, for 30 min in a moist incubation chamber, and incubated overnight (without prior washing) at room temperature with primary antisera dissolved in the blocking solution (see Table 2 for details of antisera and dilutions; we always opted for the lowest concentration of antiserum that still produced a measurable staining intensity within 30 min). Subsequently, the sections were washed three times in 0.5 M sodium acetate and incubated for 2 hr at room temperature with the alkaline phosphatase–conjugated goat anti-mouse (Sigma; St. Louis, MO) or goat anti-rabbit immunoglobulin-G (Dako; Glostrup, Denmark). After incubation, the sections were washed once more as described. To show antibody binding, the sections were incubated with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (toluidine salt; Dako) diluted in 100 mM Tris pH 9.5, 100 mM NaCl, and 50 mM MgCl2 at room temperature. Antibody concentrations and staining times were chosen to assure a linear relation between antibody binding and staining intensity (Grube 2004; van Straaten et al. 2006). Care was taken that the maximum absorbance of the stain did not exceed an optical density of 0.8. After the reaction was stopped in bidistilled water, the sections were quickly dehydrated through an ascending series of graded ethanols, cleared in xylene, and mounted in Entellan (Merck; Whitehouse Station, NJ). Control sections, in which the primary antibody was omitted, were always included in the assays.
Results
To test the validity of the IHC staining patterns that we obtained with “affinity-purified” antisera against MRs (Figures 1 and 2) with respect to the identity and specificity of the epitopes visualized, we performed several quality tests (Table 1). The equivocal outcome of some of these tests was reason for a progressively more detailed characterization of the antisera (Figures 3–7).
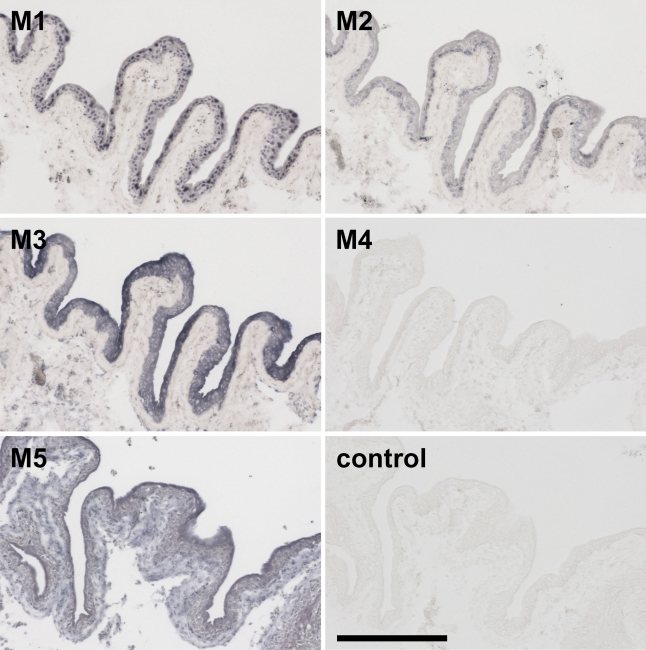
Figure 1.
Staining for the presence of muscarinic receptor (MR) subtypes in bladder urothelium of the rat. MR1 and MR2, Alomone's antisera; MR3 and MR5, R&D's antibodies; MR4, Chemicon's monoclonal antibody; Control, section incubated without primary antiserum. Unless otherwise indicated, all panels in a figure have the same magnification. Bar = 0.2 mm.
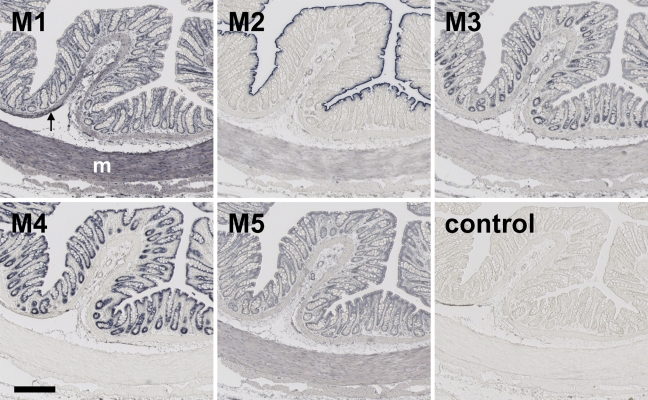
Figure 2.
Staining for the presence of MR subtypes in colon of the rat. The antisera mentioned in Figure 1 legend were used. Arrow, muscularis mucosae; M, muscularis externa. Bar = 0.2 mm.
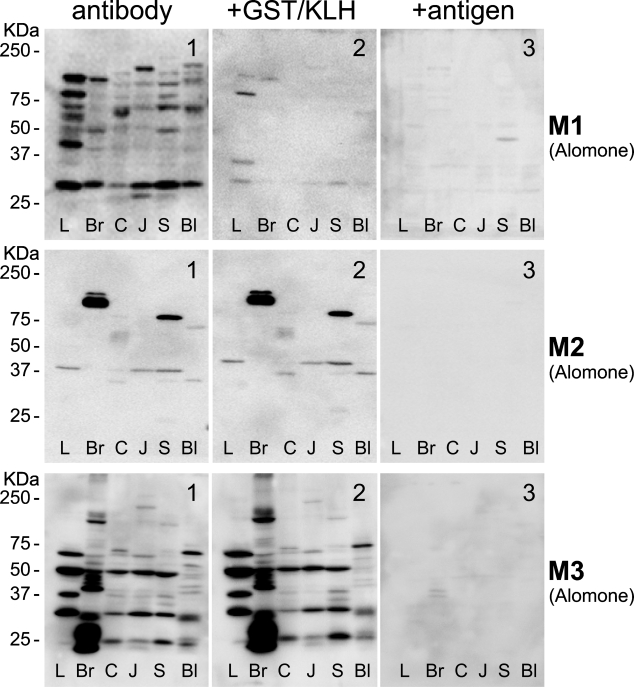
Figure 3.
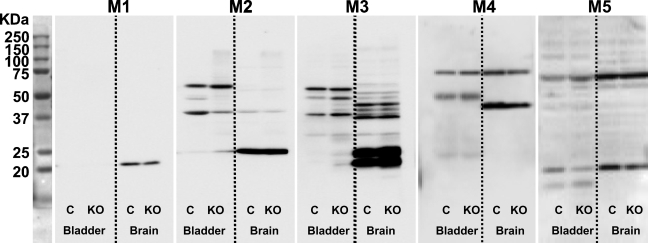
Staining pattern of MR subtypes on Western blots of rat organ extracts. Extracts of rat liver (L), brain (Br), colon (C), jejunum (J), stomach (S), and bladder (Bl) were electrophoresed, blotted, and stained for the presence of MR subtypes with antisera from Alomone (MR1-3). The calculated molecular mass for MR1, 2, and 3 is 51, 52, and 66 kDa, respectively. Fifty μg protein was applied per lane. Note that the banding pattern was different in each of the organs analyzed and that no bands of the anticipated size were visualized (see left subpanels). Preabsorption of the antibodies with glutathione S-transferase (GST), the carrier protein for the oligopeptides, did not show an effect on the staining of Western blots for MR2 or MR3, but bands disappeared for MR1 (see middle subpanels). A preabsorption test with each of the corresponding oligopeptide antigens at the concentration of 2 μg per 1 μg antibody for 2 hr at room temperature completely eliminated staining with the MR2 and MR3 antisera, whereas the MR1 antiserum produced a still acceptable decrease in staining (see right subpanels).
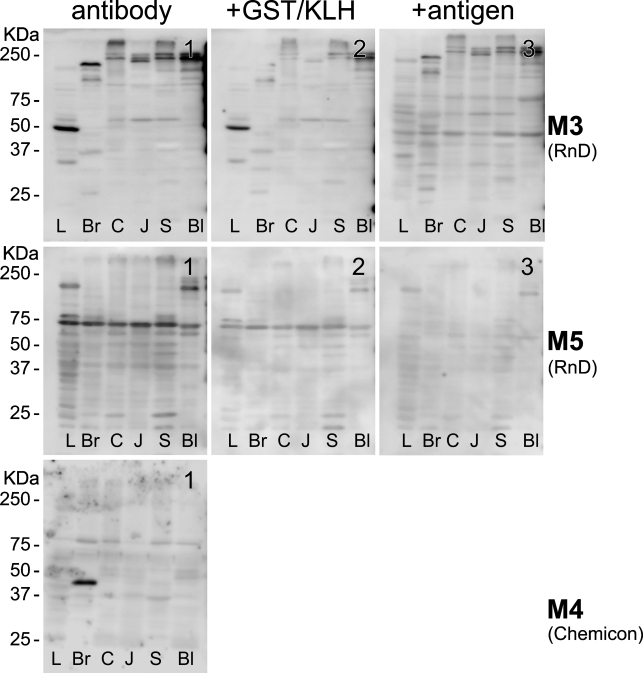
Figure 4.
Staining pattern of MR subtypes on Western blots of rat organ extracts. The legend is identical to that of Figure 3, except that the blots were stained for the presence of MR subtypes with antisera from R&D (MR3 and 5) and Chemicon (MR4). The calculated molecular mass for MR3, 4, and 5 is 66, 53, and 60 kDa, respectively. Preabsorption of the antibodies with GST caused the bands to become weaker (MR3 and MR5; see middle subpanels). A preabsorption test with 2 μg of each of the corresponding oligopeptide antigens per 1 μg antibody for 2 hr at room temperature caused a still acceptable decrease in staining with the MR5 antiserum, but the MR3 antiserum from R&D did not pass this test (right subpanels). Also note that the MR4 antiserum only produced a strong band in brain tissue.
Figure 5.
Staining pattern of MR subtypes on Western blots of knockout mice. Antibodies against MR1, MR2, and MR3 (Alomone), MR4 (Chemicon), and MR5 (R&D) were tested on Western blots containing 50 μg protein per lane from bladder and brain of wild-type (WT) or MR knockout mice (KO; each antibody was tested on extracts of the corresponding KO mice). Note that the antisera produced many common bands in WT and MR KO organ extracts and that none of the antisera produced a specific MR band, that is, a band that is present in WT and absent in MR KO organ extract.
Figure 6.
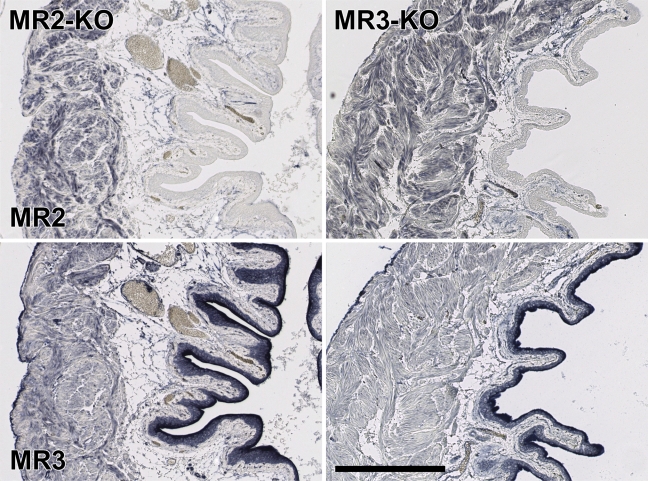
Staining pattern of MR subtypes in bladder of KO mice. The bladder wall of MR2 or MR3 KO mice was stained with Alomone's MR2 and MR3 antisera. Both antisera produce a highly selective staining pattern, but the presence or absence of the MR2 or MR3 alleles does not affect the pattern of staining. Bar = 0.2 mm.
Figure 7.
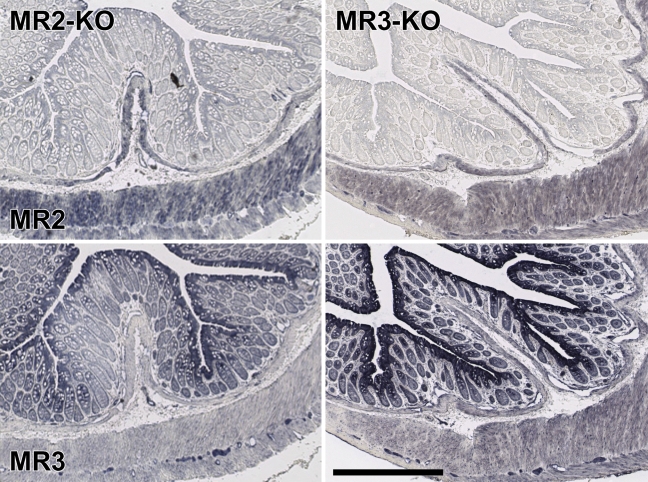
Staining pattern of MR subtypes in colon of KO mice. The colonic wall of MR2 or MR3 KO mice was stained with Alomone's MR2 or MR3 antiserum. Both antisera produce a highly selective staining pattern, but the presence or absence of the MR2 or MR3 alleles does not affect the pattern of staining. Bar = 0.2 mm.
Selective Staining Patterns, Including Low Background Staining (Criterion a)
Expecting that the claims in the product certificates were correct, we assumed we could rely on the specificity of the antisera to MR1–5. Staining patterns of the MRs in the organs of the lower urinary tract (e.g., bladder urothelium; Figure 1) and in the organs of the gastrointestinal tract (e.g., colon; Figure 2) were distinct and selective for each of the MRs, showing that the antisera used identified defined subpopulations of cells in the organs with very low or no background noise. In the bladder (Figure 1), for example, the MR1–3 antisera all stained the epithelium, but each produced a different pattern: the MR1 antiserum stained nuclei throughout the epithelium, the MR2 antiserum only the nuclei of the basal epithelial layer, and the MR3 antiserum the cytosol of all epithelial cells, in particular the umbrella cells at the surface. The MR4 antiserum did not stain any structure, whereas staining with the MR5 antiserum was strongest in the umbrella cells but this also stained the deeper layer of the epithelium and the underlying submucosa. In the colon (Figure 2), on the other hand, staining with the MR1, MR4, and MR5 antisera was similar in all epithelial cells, whereas staining with the MR2 antiserum was only detectable in the brush border of the surface epithelium, and staining with the MR3 antiserum showed a gradient toward the bottom of the crypt. MR1 showed strong staining of the smooth muscle layers, whereas MR4 failed to stain these layers.
Based on the findings shown in Figures 1 and 2, and judging by their staining patterns, we presumed that the antisera had performed satisfactorily (Table 1, criterion a). Especially, the MR2 and MR3 antisera from Alomone Labs and the MR4 monoclonal antibody from Chemicon showed an excellent immunoreactivity with a low signal-to-noise ratio. It should be noted that we obtained a substantially lower background staining when we used the denaturing fixative methanol/acetone/water than when we used the cross-linking fixative formaldehyde. Furthermore, the monoclonal antibody against MR4 stained the epithelium of the colon (Figure 2) if the tissue was fixed with methanol/acetone/water but not if the tissue was fixed with formaldehyde. We also tested all antisera on brain sections and obtained selective staining patterns in that tissue as well (data not shown).
Western Blots (Criterion b)
To assure that the staining patterns that we observed were not only “selective” but also “specific” for the respective MRs, we repeated the Western blots shown in the product certificates, as far as available, with extracts of organs of the lower urinary tract, gastrointestinal tract, and brain. The results for liver (L), brain (Br), colon (C), jejunum (J), stomach (S), and bladder (Bl) are shown in Figures 3–5. We found that, with the exception of MR5, no tissue showed a band that was present in all tissues, and no bands were shown with the anticipated molecular mass based on the amino acid sequence of the respective MRs. Initially, we ascribed these findings to post-translational processing, such as glycosylation (which increases the size of the protein; Chmelar and Nathanson 2006) and/or protein degradation (for bands smaller than the full-length protein), even though protease inhibitors were used during extraction. When we checked our findings with the data provided by the suppliers, we found that Alomone's antisera against MR1, MR2, and MR3 (Western blot data were not available for the other MR antisera and the monoclonal antibody from Chemicon) produced bands that all exceeded the expected molecular mass substantially, without any explanatory notes. We therefore concluded that none of these antibodies met criterion b (Table 1) unconditionally and that further testing was mandatory.
Preabsorption With Corresponding Antigen Followed by Western Blot (Criterion c)
Next, we investigated to what extent the staining pattern of the antisera was eliminated by preabsorption with the glutathione S-transferase (GST) carrier protein or with the antigen used to raise the antigenic response in rabbits. Carrier protein or antigen was used at a concentration of 2 μg per 1 μg of antibody for 2 hr at room temperature before use. The preabsorption procedure with antigen completely eliminated staining on bladder and colon sections (data not shown). On Western blots, the staining pattern with Alomone's MR2 and MR3 antisera did not change when the antiserum was preincubated with carrier protein and disappeared completely when the antiserum was incubated with the corresponding antigen (Figure 3). Alomone's MR1 and R&D's MR5 antisera were more (MR1; Figure 3) or less (MR5; Figure 4) sensitive to preincubation with the carrier protein but produced a detectable decrease in staining after preincubation with the corresponding antigens. R&D's MR3 antiserum, however, did not pass this test (Figure 4) and was not further tested. The MR4 monoclonal antibody was not tested.
Validation Using MR-deficient (Knockout) Mice (Criterion d)
Because neither distinct and selective staining patterns nor Western blot analysis or preabsorption of the antisera yielded unequivocal data about the specificity of the antisera, we incubated the antisera with Western blots and tissue sections of mice that are deficient for either the MR1, MR2, MR3, MR4, or MR5 muscarinic receptor (Wess et al. 2003,2007). Using PCR analysis (for primers and conditions, see Table 4), we showed for each of these mice that the gene encoding the pertinent MR was homozygously knocked out. On Western blots (for bladder and brain, in which all MRs are abundantly expressed) (Lamping et al. 2004; Aihara et al. 2005), we observed many bands (Figure 5), but none was convincingly absent in the knockout specimen, which conclusively shows that the antisera tested are mostly if not entirely reacting with non-MR proteins and did not meet criterion d.
Table 4.
Primer sequences, annealing temperature, and PCR product lengths for MR knockout mice
| Allele | Primers | Sequence 5′→3′ | Tm (°C) | PCR product (bp) |
|---|---|---|---|---|
| MR1 | M1 forward 190 | TCA GTG CCC CCT GCT GTC A | 65 | 350 |
| M1 reverse 541 | GCT GAT GAG CAG AAG ATT CAT | |||
| MR1 KO | M1 reverse 541 | GCT GAT GAG CAG AAG ATT CAT | 60 | ±490 |
| Neo forward 1789 | CTC ATT CCT CCA CTC ATG AT | |||
| MR2 | M2 forward 790 | AAG GCT CTG CGG GAC GGT | 65 | 433 |
| M2 reverse 1223 | TAG AGC ACC ATG ACA TTG TAT | |||
| MR2 KO | M2 reverse 1223 | TAG AGC ACC ATG ACA TTG TAT | 57 | ±490 |
| Neo reverse 427 | TCC TGC ACG ACG CGA GCT T | |||
| MR3 | M3 forward 1701 | GTA TGG TGG CTG TCA CTT CT | 65 | 419 |
| M3 reverse 2120 | ACC GAG GAG TTG GTG TCA GA | |||
| MR3 KO | M3 reverse 2120 | ACC GAG GAG TTG GTG TCA GA | 63 | ±410 |
| Neo reverse 427 | TCC TGC ACG ACG CGA GCT T | |||
| MR4 | M4 forward 594 | AGC CAT TGC TGC CTT CTA | 65 | 467 |
| M4 reverse 1061 | ACA TTC ACT GCC TGT CTG CT | |||
| MR4 KO | M4 reverse 1061 | ACA TTC ACT GCC TGT CTG CT | 60 | ±510a |
| Neo forward 1789 | CTC ATT CCT CCA CTC ATG AT | |||
| MR5 | M5 forward 617 | GTC TCC GTC ATG ACC ATA CTC TA | 60 | 230 |
| M5 reverse 845 | CCC GTT GTT GAG GTG CTT CTA C | |||
| MR5 KO | M5 reverse 845 | CCC GTT GTT GAG GTG CTT CTA C | 63 | ±290 |
| Neo reverse 427 | TCC TGC ACG ACG CGA GCT T | |||
| Neo fragment | Neo forward 885 | CTG TCC GGT GCC CTG AAT | 60 | 432 |
| Neo reverse 1317 | GAT ATT CGG CAA GCA GGC AT |
For MR4-KO PCR product, 32 cycles are needed.
MR, muscarinic receptor; KO, knockout.
Because Alomone's MR2 and MR3 antisera performed “best” in the preabsorption tests, we used these antisera to stain sections of wild-type and MR2 or MR3 knockout mice. Unfortunately, but as anticipated (Figure 5), we did not observe any structure in the lower urinary tract or colon that did stain in sections of wild-type mice but not in sections of either MR2 or MR3 knockout mice (Figures 6 and 7). In Alomone's MR2 antiserum, a minor band of ∼50 kDa was present in the extract of wild-type bladder but not in that of MR2 knockout bladder (Figure 3). Zarghooni et al. (2007) have reported that preabsorption of MR antisera with sections of MR knockout mice was an effective tool to produce specific staining patterns in the urothelium. Using their protocol, we preabsorbed Alomone's MR2 antiserum with MR2 knockout tissue sections, containing bladder, prostate, and colon, for 2 hr at room temperature. Thereafter, this antiserum was applied to either wild-type or MR2 knockout sections. Although this procedure weakened overall staining, it did not result in structures that were stained in sections of wild-type mice and not in sections of MR2 knockout mice (data not shown).
Discussion
The outcome of our quality tests with antisera against MRs showed that neither antisera against polypeptides (MR1 and MR2) or oligopeptides (MR3 and MR5) nor monoclonal antibodies (MR4) were specific. We first discuss the relative merits of the three most often used quality criteria. These criteria (Table 1, a–c) deal, in our view, with the selectivity rather than the specificity of the antisera. We therefore conclude by proposing “hard” criteria for specificity that should be included in the product data sheets of “certified” antisera.
Results obtained after IHC staining with all MR antisera tested seemed convincing in the sense that all showed tissue- and antiserum-dependent staining patterns with a very satisfactory signal-to-background ratio (examples of the bladder and colon are shown in Figures 1 and 2). Based on their behavior on sections only, we therefore judged that the antisera had performed satisfactorily (Table 1, criterion a). As we showed, however, such selective staining patterns do not necessarily reflect the tissue distribution of the antigen that was used to raise and purify the antiserum. Meeting criterion a is therefore only a precondition for a good antiserum.
A minimum requirement of “affinity-purified” antisera is that they should visualize a single band on Western blots of extracts of the tissues that were stained by IHC (Table 1, criterion b). The unfamiliarity of many morphologists with Western blotting transpires when this technique generates a band with a size that does not correspond with that deduced from the amino acid sequence of the corresponding antigen (see Disney et al. 2006; Hamamura et al. 2006; Qu et al. 2006; Tobin et al. 2006; Arrighi et al. 2008 for expression studies of MRs) or produces several bands (see Giglio et al. 2005; Tobin et al. 2006; Li et al. 2007; Liu et al. 2007; Arrighi et al. 2008; Ryberg et al. 2008 for expression studies of MRs). Without explanation, such findings invalidate the Western blot as a quality control test. Nevertheless, the antisera in the cited studies were used in IHC studies without further testing. Similarly, MR3 bands in partially purified membrane fractions, a procedure that enriches for the antigen and potentially dilutes contaminants, were 5 and 12 kDa bigger than expected but were nevertheless accepted without comment as specific by the investigators (Siu et al. 2006). Furthermore, bands on blots are sometimes shown without providing a molecular mass (Tong et al. 2006), making validation virtually impossible.
Preincubation with the carrier protein used to generate antibodies against oligopeptides should not decrease the staining intensity on sections or Western blots, whereas preincubation with the oligopeptide antigen should completely eliminate this staining (Table 1, criterion c). Two of the MR antisera tested met this criterion without reservations, two passed marginally, and one failed totally. Even though this preabsorption with the corresponding antigen is an often used specificity test, it is based on a circular argument: if an antibody recognizes, in addition to its specific antigen, identical epitopes (mimicry) or similar epitopes (with a lower affinity) in other proteins, the blocking test, in which excess antigen is always used will still work (cf. Petrusz et al. 1976 vs Swaab et al. 1977). The same reasoning holds if impurities are present in both the immunizing and blocking preparations. In other words, if no band is produced after absorption of the antibody by the antigen, it only proves that all antibodies were bound to the added antigen preparation. Although this conclusion about the use of blocking peptides for the establishment of antiserum specificity for IHC studies agrees with earlier reviews of this approach (Swaab et al. 1977; Burry 2000), preabsorption continues to be widely used as a quality control.
Criteria for Specificity to Be Included in Product Certificates
Our disappointing experience showed that there is room for substantial improvement of the product data sheets of commercially available antibodies and antisera. Presently, the product data sheets usually, but not always (Table 3, MR4 antigen), mention the identity of the immunogen (epitope), the purification of the antibody if applicable (removal of antibodies against the carrier protein, affinity purification with the epitope), species cross-reactivity, and the type of applications. In our view, product data sheets should contain information showing the following:
(1) the amino acid sequence of the immunogen, if this is a chemically synthesized or bacterially expressed product, and the absence of sequence similarity with other proteins as determined with a NCBI “Blast” search (http://blast.ncbi.nlm.nih.gov/blast.cgi) (Saper and Sawchenko 2003).
-
(2) the selectivity of the staining pattern of the antiserum:
- (a) a selective and unique staining pattern on sections (criterion a). To convince, the section should be shown at a low magnification with a detail at high magnification.
- (b) a Western blot of a non-purified organ extract (criterion b). The Western blot should show a band of the expected size or, if band(s) with different molecular mass are present, a reference to a reviewed study explaining the difference. A weakness of this criterion is that more than one protein can “hide” in a single band. If the specificity criteria d or e cannot be met, it may be necessary to extend criterion b to a two-dimensional Western blot.
- (3) the specificity of the antiserum for the protein of interest. This information should include:
- (a) the absence of staining in sections or on Western blots of tissue extracts of knockout animals (criterion d). The rhetorical question of Swaab et al. (1977) of whether specificity of antibodies could ever be proven was based on their experience with the Brattleboro rat, a “natural” knockout of vasopressin. Because of the ever-increasing number of genes that have been knocked out or shown to be deficiently expressed in mutants, the use of this criterion is no longer a theoretical option. Furthermore, if criterion e is not met, the use of a mutant or knockout animal model will unambiguously identify which of the antisera, if any, is specific. For this very reason, we decided to obtain the MR knockout mice rather than ordering a series of antisera to show (the absence of) specificity.
- (b) the presence of identical staining patterns of antibodies/antisera directed against two or more different epitopes on the same protein in consecutive sections or on Western blots (criterion e; Fischer et al. 2003). This criterion is both a reasonable and a feasible requirement, because many suppliers carry several antisera against different epitopes of the same protein (these antisera detect the amino- and carboxy-terminal portion or distinct functional regions of a protein). An inherent weakness of this criterion is that a lack of correspondence in the staining patterns of antibodies directed against different epitopes of the same protein does not show which antibody is to blame.
- (c) If criterion d or e cannot be met, a correspondence between the staining pattern after ISH and IHC in consecutive sections (criterion f) can also serve the purpose. ISH as a technique depends on the sequence of mRNA molecules rather than on the functional properties of antibodies and, as such, is therefore a more straightforward visualization technique. A restriction on this criterion is that not all mRNAs are (well) translated and that conformational antibodies are not directly represented in the mRNA sequence. Identical staining patterns, therefore, support the claim of specificity, but the lack of an identical staining pattern does not prove the absence of specificity.
There may be no all-inclusive rules for establishing the specificity of antisera or antibodies. However, some of our criteria, in particular criteria d and e, represent rather compelling evidence for specificity. In contrast, qualifications that suggest specificity, such as “affinity purified,” are no longer warranted. We do, therefore, argue for practical information in the specification data sheets that informs users which specificity tests have actually been carried out. Because such information may not become spontaneously available from the suppliers any time soon, we suggest that journals for which IHC is a core technique in the mean time open up their websites to enable consumer rating of antisera, an approach that is already available for many household commodities. Ranking will enable readers to quickly assess the value of the most positive or negative scores and make their choice accordingly. The establishment of the website “Biorating.com” is an important first step in this direction. Of course, it would be even more desirable that “certified” antisera meeting our criteria of specificity d or e become commercially available. A wide sharing of experience with antisera can avoid much of the confusion that is generated by antisera that lack sufficient specificity, whereas the introduction of certified antisera could avoid many fruitless and costly validation assays. Such “premium” antisera should, therefore, become the standard in the catalogs of commercial suppliers.
A final question that has to be raised is why none of the MR antisera we tested qualified. Unfortunately, we cannot answer this question at the moment. We do not think the problem is caused by cross-reactivity with other members of MR family, because the staining patterns seen on the Western blots differ per antiserum without common bands throughout the series. Furthermore, the oligopeptides used to raise the antisera (with the exception of M4, which could not be tested) do not share sequence similarity with the other MRs in a NCBI “Blast” search, even at low stringency. Unfortunately, cross-reactivity cannot be ruled out completely in silico, because a one amino acid difference in an epitope may affect the affinity of the antiserum for these oligopeptides to different extents and hence make the degree of cross-reactivity also sensitive to abundance. We have encountered similar specificity problems as described for the MRs with antisera against the adrenergic receptors but have not gone so far as to include knockout animals in the analysis. Because both the muscarinic and adrenergic receptors belong to the family of G protein–coupled receptors, this group of proteins may exhibit peculiar immunogenic properties.
Addendum
Recently, Lorincz and Nusser (2008) have put forward two caveats on the use of knockout animals in specificity tests, viz. the remaining expression of truncated parts of the inactivated genes and a downregulation of the expression of a cross-reacting related gene, such as another subunit of the same protein.
Acknowledgments
This study was financed through a grant from the John L. Emmett Foundation for Urology, The Netherlands, and by research grants from the Thai government (008/2550 and 125/2550).
We thank Dr. Jurgen Wess (Laboratory of Bioorganic Chemistry, NIH-NIDDK, Bethesda, MD) for making MR-deficient mice available for our study and Dr. Martin Michel (Department of Pharmacology, Academic Medical Center) for advice during the study.
References
- Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S (2005) Cholinergically stimulated gastric acid secretion is mediated by M3 and M5 but not M1 muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol 288:G1199–1207 [DOI] [PubMed] [Google Scholar]
- Arrighi N, Bodei S, Peroni A, Mirabella G, Zani D, Simeone C, Cunico SC, et al. (2008) Detection of muscarinic receptor subtypes in human urinary bladder mucosa: age and gender-dependent modifications. Neurourol Urodyn 27:421–428 [DOI] [PubMed] [Google Scholar]
- Burry RW (2000) Specificity controls for immunocytochemical methods. J Histochem Cytochem 48:163–166 [DOI] [PubMed] [Google Scholar]
- Chmelar RS, Nathanson NM (2006) Identification of a novel apical sorting motif and mechanism of targeting of the M2 muscarinic acetylcholine receptor. J Biol Chem 281:35381–35396 [DOI] [PubMed] [Google Scholar]
- Coccini T, Roda E, Castoldi AF, Goldoni M, Poli D, Bernocchi G, Manzo L (2007) Perinatal co-exposure to methylmercury and PCB153 or PCB126 in rats alters the cerebral cholinergic muscarinic receptors at weaning and puberty. Toxicology 238:34–48 [DOI] [PubMed] [Google Scholar]
- Cohn M (2005) Degeneracy, mimicry and cross-reactivity in immune recognition. Mol Immunol 42:651–655 [DOI] [PubMed] [Google Scholar]
- Danielson P, Alfredson H, Forsgren S (2006) Immunohistochemical and histochemical findings favoring the occurrence of autocrine/paracrine as well as nerve-related cholinergic effects in chronic painful patellar tendinosis. Microsc Res Tech 69:808–819 [DOI] [PubMed] [Google Scholar]
- Danielson P, Andersson G, Alfredson H, Forsgren S (2007) Extensive expression of markers for acetylcholine synthesis and of M2 receptors in tenocytes in therapy-resistant chronic painful patellar tendon tendinosis: a pilot study. Life Sci 80:2235–2238 [DOI] [PubMed] [Google Scholar]
- Disney AA, Domakonda KV, Aoki C (2006) Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas V1 and V2 of the macaque monkey. J Comp Neurol 499:49–63 [DOI] [PubMed] [Google Scholar]
- Fischer DF, De Vos RA, Van Dijk R, De Vrij FM, Proper EA, Sonnemans MA, Verhage MC, et al. (2003) Disease-specific accumulation of mutant ubiquitin as a marker for proteasomal dysfunction in the brain. FASEB J 17:2014–2024 [DOI] [PubMed] [Google Scholar]
- Giglio D, Ryberg AT, To K, Delbro DS, Tobin G (2005) Altered muscarinic receptor subtype expression and functional responses in cyclophosphamide induced cystitis in rats. Auton Neurosci 122:9–20 [DOI] [PubMed] [Google Scholar]
- Grube D (2004) Constants and variables in immunohistochemistry. Arch Histol Cytol 67:115–134 [DOI] [PubMed] [Google Scholar]
- Hamamura M, Marostica E, de Avellar MCW, Porto CS (2006) Muscarinic acetylcholine receptor subtypes in the rat seminal vesicle. Mol Cell Endocrinol 247:192–198 [DOI] [PubMed] [Google Scholar]
- Harrington AM, Hutson JM, Southwell BR (2007) Immunohistochemical localisation of cholinergic muscarinic receptor subtype 1 (M1r) in the guinea pig and human enteric nervous system. J Chem Neuroanat 33:193–201 [DOI] [PubMed] [Google Scholar]
- Holmseth S, Lehre KP, Danbolt NC (2006) Specificity controls for immunocytochemistry. Anat Embryol (Berl) 211:257–266 [DOI] [PubMed] [Google Scholar]
- Lamping KG, Wess J, Cui Y, Nuno DW, Faraci FM (2004) Muscarinic (M) receptors in coronary circulation: gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler Thromb Vasc Biol 24:1253–1258 [DOI] [PubMed] [Google Scholar]
- Li GQ, Kevetter GA, Leonard RB, Prusak DJ, Wood TG, Correia MJ (2007) Muscarinic acetylcholine receptor subtype expression in avian vestibular hair cells, nerve terminals and ganglion cells. Neuroscience 146:384–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li J, Tan DT, Beuerman RW (2007) Expression and function of muscarinic receptor subtypes on human cornea and conjunctiva. Invest Ophthalmol Vis Sci 48:2987–2996 [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z (2008) Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci 28:9083–9086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, Anand P (2006) Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol 176:367–373 [DOI] [PubMed] [Google Scholar]
- Petrusz P, Sar M, Ordronneau P, DiMeo P (1976) Specificity in immunocytochemical staining. J Histochem Cytochem 24:1110–1112 [DOI] [PubMed] [Google Scholar]
- Qu J, Zhou X, Xie R, Zhang L, Hu D, Li H, Lu F (2006) The presence of M1 to M5 receptors in human sclera: evidence of the sclera as a potential site of action for muscarinic receptor antagonists. Curr Eye Res 31:587–597 [DOI] [PubMed] [Google Scholar]
- Ryberg AT, Warfvinge G, Axelsson L, Soukup O, Gotrick B, Tobin G (2008) Expression of muscarinic receptor subtypes in salivary glands of rats, sheep and man. Arch Oral Biol 53:66–74 [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Suri D, Rajasekaran M (2006) Characterization of muscarinic receptor subtypes in human ureter. J Endourol 20:939–942 [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE (2003) Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol 465:161–163 [DOI] [PubMed] [Google Scholar]
- Siu ER, Yasuhara F, Marostica E, Avellar MC, Porto CS (2006) Expression and localization of muscarinic acetylcholine receptor subtypes in rat efferent ductules and epididymis. Cell Tissue Res 323:157–166 [DOI] [PubMed] [Google Scholar]
- Sträter J, Walczak H, Hasel C, Melzner I, Leithäuser F, Möller P (2001) CD95 ligand (CD95L) immunohistochemistry: a critical study on 12 antibodies. Cell Death Differ 8:273–278 [DOI] [PubMed] [Google Scholar]
- Swaab DF, Pool CW, Van Leeuwen FW (1977) Can specificity ever be proved in immunocytochemical staining? J Histochem Cytochem 25:388–391 [DOI] [PubMed] [Google Scholar]
- Tobin G, Ryberg AT, Gentle S, Edwards AV (2006) Distribution and function of muscarinic receptor subtypes in the ovine submandibular gland. J Appl Physiol 100:1215–1223 [DOI] [PubMed] [Google Scholar]
- Tong YC, Cheng JT, Hsu CT (2006) Alterations of M(2)-muscarinic receptor protein and mRNA expression in the urothelium and muscle layer of the streptozotocin-induced diabetic rat urinary bladder. Neurosci Lett 406:216–221 [DOI] [PubMed] [Google Scholar]
- Tyagi S, Tyagi P, Van-le S, Yoshimura N, Chancellor MB, de Miguel F (2006) Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol 176:1673–1678 [DOI] [PubMed] [Google Scholar]
- Van der Sluis PJ, Boer GJ (1986) The relevance of various tests for the study of specificity in immunocytochemical staining: a review. Cell Biochem Funct 4:1–17 [DOI] [PubMed] [Google Scholar]
- van Leeuwen F (1986) Pitfalls in immunocytochemistry with special reference to the specificity problems in the localization of neuropeptides. Am J Anat 175:363–377 [DOI] [PubMed] [Google Scholar]
- van Straaten HW, He Y, van Duist MM, Labruyere WT, Vermeulen JL, van Dijk PJ, Ruijter JM, et al. (2006) Cellular concentrations of glutamine synthetase in murine organs. Biochem Cell Biol 84:215–231 [DOI] [PubMed] [Google Scholar]
- Wess J, Duttaroy A, Zhang W, Gomeza J, Cui Y, Miyakawa T, Bymaster FP, et al. (2003) M1–M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Receptors Channels 9:279–290 [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6:721–733 [DOI] [PubMed] [Google Scholar]
- Yamashita S (2007) Heat-induced antigen retrieval: mechanisms and application to histochemistry. Prog Histochem Cytochem 41:141–200 [DOI] [PubMed] [Google Scholar]
- Zarghooni S, Wunsch J, Bodenbenner M, Bruggmann D, Grando SA, Schwantes U, Wess J, et al. (2007) Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci 80:2308–2313 [DOI] [PubMed] [Google Scholar]