Abstract
The proinflammatory enzyme 5-lipoxygenase (5-LOX) is upregulated in Alzheimer's disease (AD), but its localization and association with the hallmark lesions of the disease, β-amyloid (Aβ) plaques and neurofibrillary tangles (NFTs), is unknown. This study examined the distribution and cellular localization of 5-LOX in the medial temporal lobe from AD and control subjects. The spatial relationship between 5-LOX immunoreactive structures and AD lesions was also examined. We report that, in AD subjects, 5-LOX immunoreactivity is elevated relative to controls, and its localization is dependent on the antibody-targeted portion of the 5-LOX amino acid sequence. Carboxy terminus–directed antibodies detected 5-LOX in glial cells and neurons, but less frequently in neurons with dystrophic (NFT) morphology. In contrast, immunoreactivity observed using 5-LOX amino terminus–directed antibodies was virtually absent in neurons and abundant in NFTs, neuritic plaques, and glia. Double-labeling studies showed a close association of 5-LOX–immunoreactive processes and glial cells with Aβ immunoreactive plaques and vasculature and also detected 5-LOX in tau immunoreactive and amyloid containing NFTs. Different immunolabeling patterns with antibodies against carboxy vs amino terminus of 5-LOX may be caused by post-translational modifications of 5-LOX protein in Aβ plaques and NFTs. The relationship between elevated intracellular 5-LOX and hallmark AD pathological lesions provides further evidence that neuroinflammatory pathways contribute to the pathogenesis of AD. (J Histochem Cytochem 56:1065–1073, 2008)
Keywords: leukotrienes, inflammation, amyloid, neurodegeneration, hippocampus, dementia
Alzheimer's disease (AD), the most common form of dementia in the elderly, is characterized neuropathologically by the presence of β-amyloid (Aβ)-containing senile plaques and tau protein–containing neurofibrillary tangles (NFTs). These lesions are associated with brain inflammation and oxidative imbalance resulting in lipid peroxidation, DNA and RNA damage, and neuronal degeneration (Hensley et al. 1995; Akiyama et al. 2000; Pratico and Delanty 2000; Nunomura et al. 2007; Moreira et al. 2008). Understanding the status of proinflammatory molecules, especially in relation to the pathological hallmarks of AD, is necessary for the development of novel therapeutic strategies. 5-lipoxygenase (5-LOX), which catalyzes the conversion of arachidonic acid to proinflammatory mediators leukotrienes, is involved in chronic inflammatory diseases in the periphery (Rådmark et al. 2007). This enzyme is also expressed in the brain, with particularly high levels in the hippocampus (Lammers et al. 1996), and its expression increases during aging and in neurodegenerative disorders (Manev et al. 2000; Tomimoto et al. 2002; Zhang et al. 2006; Zhou et al. 2006; Chinnici et al. 2007). Several lipoxygenases, including 5-LOX and 12/15-LOX, are elevated in brain tissues from AD subjects and Aβ-overproducing transgenic mice (Praticò et al. 2004; Yao et al. 2005; Firuzi et al. 2008). Furthermore, inhibition of 5-LOX reduces Aβ production in vitro (Firuzi et al. 2008) and is protective against Aβ-mediated neurotoxicity (Goodman et al. 1994). These observations suggest that 5-LOX plays a role in AD and is a potential target for novel therapeutic agents (Sugaya et al. 2000; Klegeris and McGeer 2002).
In this IHC study, we characterized the distribution and cellular localization of 5-LOX–immunoreactive (ir) structures in the hippocampal formation of AD patients and aged non-demented (control) subjects, with emphasis on the association of these structures with Aβ plaques and NFTs.
Materials and Methods
Subjects
Eleven AD patients [age range, 60–93 years; four men and seven women; post-mortem interval (PMI) = 4–14 hr] were followed in the University of Pittsburgh Alzheimer's Disease Research Center (ADRC). All had clinical diagnoses of AD and autopsy-confirmed AD with advanced stages of neuropathology (Braak V/VI) (Braak and Braak 1991). Five of the 11 AD subjects had cortical Lewy bodies. Five control subjects (age range, 49–75 years; three men and two women; PMI = 10–20 hr), drawn from autopsy cases at the University of Pittsburgh Medical Center, had no history of dementia and no disease-specific pathology on neuropathological examination (Braak stage 0), except for ischemic events observed in two cases.
Tissue Preparation and Single-label IHC
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise indicated. Tissue blocks of the medial temporal lobe including the hippocampus, parahippocampal gyrus, and inferior temporal cortex were fixed in 4% paraformaldehyde, cryoprotected in graded sucrose concentrations, and sectioned at 40 μm. A portion of unfixed hippocampal tissue from three AD and two control cases was flash frozen for Western blotting (see below). Free-floating single-label IHC was performed as described previously (Ikonomovic et al. 2004). Briefly, three sections from each case were incubated with antibodies generated against different regions of human 5-LOX protein (see Table 1 for complete list of 5-LOX antibodies) for 24 hr at 4C. Sections were incubated for 1 hr at 21C in affinity-purified biotinylated secondary antisera (Jackson ImmunoResearch; West Grove, PA) generated against the primary antisera host. Immunoreactivity was visualized using the VectaStain Elite kit (Vector Laboratories; Burlingame, CA) with nickel-enhanced DAB (NiDAB) as the chromogen. The reaction was absent in sections that were exposed to the incubation solution lacking the primary antibody. Sections from all AD and control cases were processed together.
Table 1.
Specifications of 5-LOX antisera used for IHC experiments and semiquantitative assessment of frequencies of 5-LOX–immunoreactive structures in the hippocampus from subjects with AD
| Antibody (host species) | Working dilution | Epitope/antigen | Pyramidal neurons | Dystrophic cells/NFT | Glial cells | Plaque-like structures |
|---|---|---|---|---|---|---|
| Santa Cruz | 1:500 | N-terminal | ||||
| (goat) | aa 1–80 | 0 | ++++ | + | ++ | |
| RDI-II | 1:500 | N-terminal | ||||
| (goat) | aa 1–80 | 0 | ++++ | + | ++ | |
| Cayman | 1:600 | N-terminal | ||||
| (rabbit) | aa 130–149 | + | +++ | ++ | +++ | |
| Transduction | 1:40 | C-terminal | ||||
| (mouse) | aa 442–590 | ++ | ++ | ++ | 0 | |
| Santa Cruz | 1:500 | C-terminal | ||||
| (goat) | aa 574–673 | +++ | + | ++++ | 0 | |
| RDI-I | 1:100 | C-terminal | ||||
| (goat) | aa 574–673 | ++ | + | ++++ | 0 |
Frequency of 5-LOX immunoreactive structures was graded in a semiquantitative manner as follows: 0, no immunoreactive structure; +, very sparse (<5–10 per section); ++, sparse; +++, moderate; ++++, frequent. 5-LOX, 5-lipoxygenase; AD, Alzheimer's disease; NFT, neurofibrillary tangle; RDI, Research Diagnostics (Flanders, NJ); Transduction, Transduction Laboratories (Lexington, KY).
Dual-labeling IHC/histochemistry
Chromogen-based dual labeling was performed as described previously (Desai et al. 2005) using the 10D5 monoclonal antibody generated against the NH2 terminus of human Aβ (Athena Neurosciences; South San Francisco, CA) (Hyman et al. 1992) and the Cayman 5-LOX antisera (Cayman; Ann Arbor, MI) (Table 1). 5-LOX immunoreactivity was visualized using NiDAB as described above; 10D5 immunoreactivity was visualized using Vector Red (Vector), an alkaline phosphatase substrate. NFTs were detected using X-34 histofluorescence as previously described (Ikonomovic et al. 2006). X-34 is an amyloid binding compound and a particularly sensitive marker of neurofibrillary pathology (Styren et al. 2000). Sections were histostained with X-34 (generously provided by Dr. William E. Klunk, University of Pittsburgh, Pittsburgh, PA), imaged, and processed for 5-LOX IHC as described above. Matching images were captured to assess the degree of codistribution of 5-LOX– and X-34–labeled NFTs. Dual immunofluorescence labeling was performed as described previously (Desai et al. 2005), using antibodies directed against the NH2 terminus of 5-LOX (goat polyclonal; Santa Cruz–N, Santa Cruz, CA), tau (clone K9JA; DAKO, Carpinteria, CA; rabbit polyclonal), and Alexa 594–conjugated donkey anti-goat and Alexa 488-conjugated donkey anti-rabbit secondary antibodies (Molecular Probes; Eugene OR), respectively.
Antibody Characterization by Peptide Preabsorption and Western Blot Analysis
Antibody preabsorption testing was performed by preincubating 5-LOX antisera (1:600; Cayman) with excess 5-LOX peptide (1 μg/ml; Cayman) for 1 hr at 4C, followed by the single-labeling IHC protocol outlined above. For Western blot analysis of frozen hippocampal homogenates, proteins were separated electrophoretically on a 10% Bis-Tris-HCl gel for 1 hr at 21C, blotted onto nitrocellulose membranes, and incubated in 5-LOX antisera (1:1000; Cayman) overnight at 4C. 5-LOX–immunoreactive bands were visualized with a horseradish peroxidase–conjugated goat anti-mouse secondary antisera (1:2500; Vector; 1 hr at 21C) and a chemiluminescence detection system (Pierce Biotechnology; Rockford, IL).
Statistical Analysis
The mean values of demographics in AD and control groups were compared using t-test for independent samples, with statistical difference set at p<0.05 (one-tailed).
Results
In AD hippocampus, 5-LOX antibodies immunolabeled a variety of elements including neuronal cell bodies and processes, glial cells, blood vessels, and NFT- and plaque-like structures. Semiquantitative evaluation of the frequency with which each antibody labeled different immunoreactive structures is summarized in Table 1, and their overall localization/distribution patterns are shown schematically in Figure 1 and described below. 5-LOX immunoreactivity was largely absent in control subjects (see below; Figure 2). Although AD subjects were older, had shorter PMI (both p<0.001), and included more women than the control group, similar labeling patterns were observed when evaluating 5-LOX-ir structures in AD subjects of different age, sex, or postmortem delay.
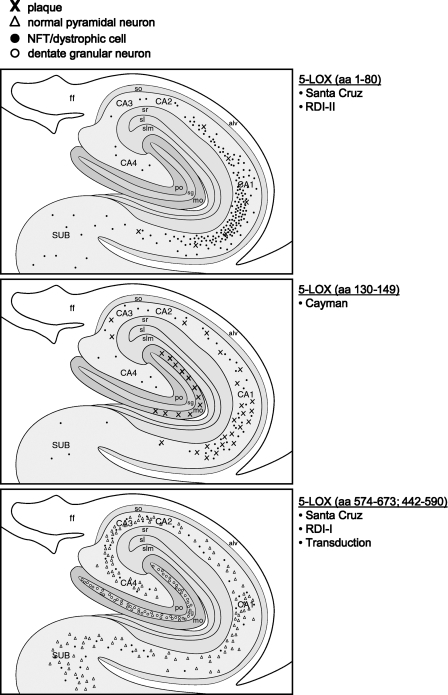
Figure 1.
Schematic illustration of representative distributions of 5-lipoxygenase (5-LOX)-labeled elements, using the 5-LOX antibodies shown in Table 1, in the hippocampus of an AD subject.
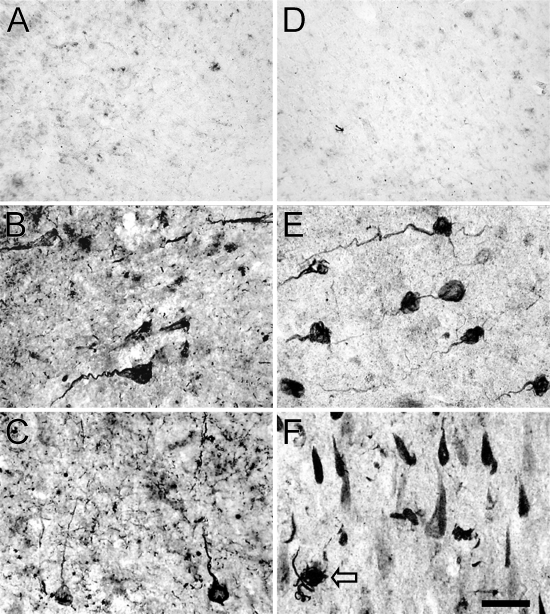
Figure 2.
IHC detection of 5-LOX protein in the hippocampus of control individuals with no cognitive impairment (A,D) and patients with Alzheimer's disease (AD) (B,C,E,F) using antisera recognizing the NH2 terminus (A–C; Santa Cruz-N) and aa 130–149 (D–F; Cayman) of 5-LOX. With either antibody, there is a very low level of immunoreactivity in the subiculum/CA1 from a control case (A,D). In the CA1 (B,E) and subiculum (C,F) of a representative AD case, neurons with twisted apical dendrites and “tangled” material in cell bodies are strongly immunoreactive with either the aa 130–149 (E,F)– or NH2 terminus (B,C)–specific antibodies. 5-LOX immunoreactive tangled neuritic structures are also seen in the neuropil (F, arrow). Most 5-LOX–immunostained neurons in subiculum resemble neurofibrillary tangles (NFTs) (F). Bar = 25 μm.
IHC Using NH2 Terminus–directed 5-LOX Antibodies
Using antisera against the N terminus (aa 1–80) and a region proximal to the N terminus (aa 130–149), 5-LOX immunoreactivity was not detectable, or was very sparse, in lightly labeled pyramidal cells in CA1/subiculum of control subjects (Figures 2A and 2D). In AD hippocampus, however, these antibodies intensely labeled dystrophic-looking pyramidal neurons, plaque-like clumps of cell processes, and, less frequently, glial cells (Table 1; Figures 2 and 3). In the CA1 region (Figures 2B, 2E, and 4A) and the subiculum (Figures 2C and 2F) of AD subjects, numerous dystrophic neurons with twisted apical dendrites and tangled intracellular material, resembling paired helical filaments, were strongly 5-LOX-ir. A subset of these cells, particularly those in the subiculum, had the morphology of classic NFTs (Figure 2F). 5-LOX-ir plaque-like clumps of small cell bodies and processes (Figure 2F) were observed in all hippocampal fields but were particularly numerous in the molecular layer of the dentate gyrus (Figure 3). Similar structures were seen in the inferior temporal cortex (Figures 3C and 3D), where 5-LOX-ir cell structures were distributed both focally and in more dispersed clusters (Figure 3).
Figure 3.
5-LOX immunoreactivity in the dentate gyrus (A), CA3 hippocampus (B), and temporal cortex (C,D) from an AD patient, using the antiserum recognizing 5-LOX aa 130–149 (Cayman). Accumulations of 5-LOX–immunoreactive (ir) plaque-like clumps of cells and cell processes are evident in all three regions. Bar = 50 μm.
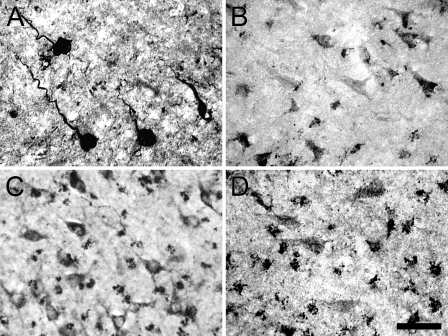
Figure 4.
A comparison of IHC detection of 5-LOX protein in the CA1 hippocampus of patients with AD using antisera recognizing the NH2 terminus (A, RDI-2), aa 442–590 (B, Transduction), and COOH terminus (C, RDI-1; D, Santa Cruz-C). The NH2 terminus antibody shows robustly stained cells with twisted apical dendrites (A), whereas the aa 442–590 antibody detects sparse tangle-looking cells (B). Both of the C terminus antibodies recognize neuronal and glial cells (C,D). Bar = 25 μm.
IHC Using COOH Terminus–directed 5-LOX Antibodies
In control subjects, immunolabeling with carboxy terminus 5-LOX antibodies was scarce and very light (not shown), similar to that seen with amino terminus 5-LOX antibodies. In AD cases, the pattern of immunolabeling with carboxy terminus 5-LOX antibodies differed compared with that observed with amino terminus 5-LOX antibodies. No plaque-like clumps of cell processes were observed, and dystrophic neurons and tangle-like cells were observed infrequently (Table 1; Figure 4B). In contrast, these antibodies detected moderate numbers of normal looking pyramidal neurons and numerous glial cells (Table 1; Figure 4).
5-LOX Antibody Characterization
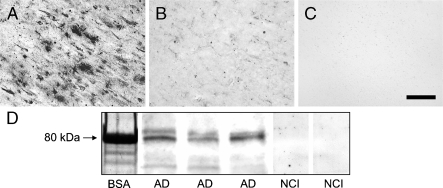
We next examined the binding specificity of the antibody directed to 5-LOX aa 130–149 (Cayman), because it detected the full array of 5-LOX–immunoreactive elements observed using the other antibodies (Table 1). Preabsorption of this antiserum with excess 5-LOX peptide dramatically reduced 5-LOX immunostaining (Figure 5). With the same antisera, Western blot analysis of AD hippocampal homogenates showed a prominent band at ∼80 kDa; under these experimental conditions, the same signal was not observed in hippocampal homogenates from control subjects (Figure 5).
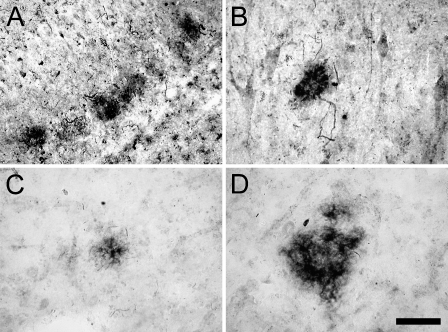
Figure 5.
IHC study of 5-LOX protein expression in the AD hippocampus using antisera recognizing aa 130–149 (Cayman) of 5-LOX. Compared with the regular IHC protocol (A), preabsorbing the antisera with purified 5-LOX peptide (B) or omission of the primary antisera (C) reduced immunostaining signal to background levels. Western blot analysis (D) of 5-LOX protein expression in the hippocampus from AD patients (AD, three different individuals) and control subjects with no cognitive impairment (NCI, two different individuals), using the same (Cayman) antibody, shows a prominent 80-kDa band (matching the known molecular mass of BSA) in AD tissues, whereas this band is hardly visible in control hippocampi. Bar = 50 μm.
5-LOX Colocalization With Aβ Plaques and NFTs
We next assessed the degree to which 5-LOX-ir structures codistributed with Aβ plaques and NFT. The Cayman and Santa Cruz N terminus 5-LOX antibodies most robustly labeled plaque structures and dystrophic neurons, respectively; consequently they were used for dual labeling with markers of Aβ plaques and NFTs.
In sections dual-immunolabeled for 5-LOX and Aβ, we observed a close association between 5-LOX-ir glial cells and Aβ-ir plaques, as well as cerebrovascular Aβ deposits (Figure 6). Many of these cells had the morphological characteristics of astrocytes and were often highly ramified. Within Aβ-ir plaques, 5-LOX immunoreactivity was restricted to clusters of cell bodies and processes; these were absent in more diffuse Aβ deposits. In the entorhinal cortex lamina II, we observed Aβ immunoreactivity as a diffuse haze around the soma of a subset of NFTs that were also 5-LOX-ir (Figure 6D).
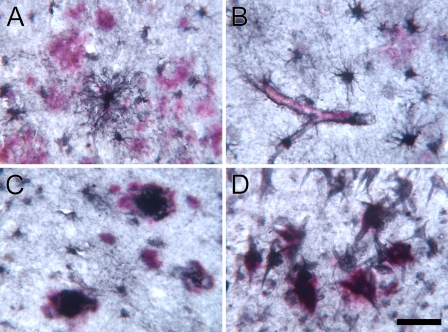
Figure 6.
Dual chromogen IHC for 5-LOX (Cayman; black, DAB/nickel) and amyloid-beta (Aβ; red; Vector red) in the hippocampus (A,B), inferior temporal (C), and entorhinal cortex (D) from an AD patient. Numerous large astrocytes surround Aβ plaques (A,B) and Aβ-containing blood vessels (B) in the hippocampus. A subset of plaques colocalized both peptides in the temporal cortex (C). In the entorhinal cortex lamina II, double IHC shows intensely 5-LOX–immunoreactive NFTs codistributed with small Aβ aggregates (D). Bar: A,C = 50 μm; B,D = 25 μm.
Dual labeling of tissue sections with 5-LOX and an antibody generated against human tau showed 5-LOX immunoreactivity in a subset of tau-ir NFTs in the CA regions of the hippocampus (Figure 7). In the entorhinal cortex, almost all of the robustly 5-LOX–immunoreactive dystrophic neurons within lamina II cell islands contained fibrillar amyloid, shown using the amyloid-binding dye X-34 (Figure 7).
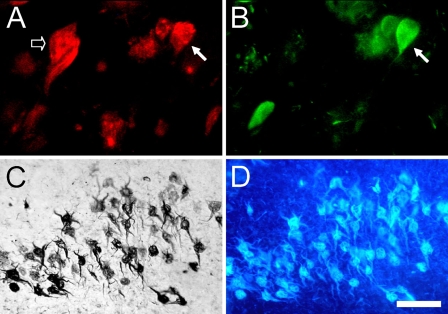
Figure 7.
Dual immunofluorescence for 5-LOX (Santa Cruz-N; red) and tau (green) in the hippocampus (A,B) from an AD patient. Solid arrows point to the same cell that is positive for 5-LOX and tau. Some of the 5-LOX-ir cells (A, empty arrow) do not contain tau accumulation. Combined 5-LOX IHC (C, DAB/nickel) and X-34 histochemistry (D, blue fluorescence) in the same entorhinal cortex tissue section show that, in advanced AD, all of the 5-LOX-ir lamina II neurons also contain pathological β-pleated sheet conformed protein aggregates. Bar: A,B = 25 μm; C,D = 50 μm.
Discussion
This IHC analysis showed that, in the AD medial temporal lobe, there are robust intracellular increases in 5-LOX protein. These changes might be caused by disease-related increases in cytokine production and increased Aβ production, both of which can activate p38–mitogen-activated protein kinase (p38-MAPK) (Raingeaud et al. 1995), which in turn may activate 5-LOX protein (Hanaka et al. 2005) and facilitate translocation of 5-LOX from the cytosol to the nuclear membrane, where it achieves its active state through interactions with the 5-LOX–activating protein (Luo et al. 2003). In further support of its role in AD pathogenesis, 5-LOX upregulation localized primarily to glial and neuronal cells that were associated with Aβ-ir plaques and NFTs, the pathological hallmarks of AD. 5-LOX-ir glial cell bodies were frequently observed within and surrounding Aβ plaques, reflecting the typical distribution of reactive astrocytes and macrophages in AD brains. Upregulation of 5-LOX could mediate the proinflammatory effect of Aβ-containing senile plaques or, conversely, influence their development in AD (Firuzi et al. 2008). The pattern of 5-LOX immunoreactivity in this study is nearly identical to what was observed previously in AD brain using antibodies against p38-MAPK (Hensley et al. 1999), which is also detected in tau-containing NFTs (Ferrer et al. 2001). The idea that this inflammatory cascade is a potential therapeutic target is underscored by the observation that lipoxygenase inihibition is beneficial in relation to elevated oxidative stress caused by normal aging (Bishnoi et al. 2005), after experimental stroke (Jatana et al. 2006), and in models of amyotrophic lateral sclerosis (West et al. 2004), likely through suppression of toxic action of microglia (Klegeris and McGeer 2002). The potential benefit of 5-LOX inhibition in AD is further supported by the fact that elevated 5-LOX expression, together with increased levels of other LOX enzymes (e.g., 12/15-LOX), likely contributes to lipid peroxidation and oxidative stress (Pratico 2002; Praticò et al. 2004), which are believed to play an important role in the formation of AD lesions (Smith et al. 1996). Proinflammatory cascades involving neutrophil infiltration, expression of cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, and production of reactive oxygen species are substantially reduced in 5-LOX–deficient mice (Cuzzocrea et al. 2005; Genovese et al. 2005), further supporting the idea that 5-LOX has an important regulatory role in these potentially pathologic processes.
Robust 5-LOX immunoreactivity was also associated with clumps of cell processes in plaques and with intracellular fibrillar aggregates inside NFTs. The exact mechanism of how 5-LOX contributes to the development of neurofibrillary pathology is unclear, however, it may involve its affinity for the coactosin/actin protein complex, where it can regulate actin dynamics (Provost et al. 2001). Polymerized actin aggregates are abundant in AD brain, where they are associated with virtually all NFTs and amyloid plaques (Minamide et al. 2000). The close association of 5-LOX with dystrophic neurons and neuritic plaque-like structures suggests that increased 5-LOX production and/or activity could be involved in the actin-related alterations in neuronal cytoskeleton and might contribute to neurodegenerative changes in AD. In the entorhinal cortex, the initial site of NFT formation, we found that almost all of the 5-LOX–immunoreactive lamina II cells contained fibrillar amyloid, indicative of advanced NFTs. In other areas of the medial temporal cortex, we observed partial overlapping of 5-LOX–immunoreactive neurons with tau-immunoreactive NFTs. Colocalization of 5-LOX with antibodies specific for different stages of NFT development is currently being assessed. It is not clear why a subset of neurons with normal pyramidal cell morphology in the AD brain also upregulated 5-LOX; these cells could be undergoing prodromal (pre-tangle) degenerative changes.
This study showed that antibodies generated against different portions of 5-LOX amino acid sequence, particularly the catalytic carboxyl terminus and the ligand binding N terminus (Rådmark et al. 2007), revealed different, although partially overlapping, patterns of immunolabeling. The carboxy terminus 5-LOX antisera labeled primarily neurons with normal appearing morphology, as well as numerous glial cells. The observed punctuate pattern of immunolabeling, most abundant in the peri-nuclear area (Figure 4C), is consistent with cytosolic and nuclear location of this enzyme. In contrast, amino terminus–directed 5-LOX antibodies labeled primarily dystrophic cells and neuritic elements, as well as reactive glia associated with Aβ plaques. The immunolabeling was particularly robust using the latter antibodies, and, with the exception of glia, they appeared to label almost exclusively fibrillar structures in plaques. The different labeling patterns of carboxy and amino terminus 5-LOX antibodies could reflect post-translational protein modifications. Conformational changes and/or proteolytic processing of 5-LOX could have shown some, and masked other, epitopes specific to the 5-LOX antibodies used in this study. The effect of such changes on 5-LOX enzyme activity, and their role in the pathogenesis of AD, remains to be determined.
In conclusion, 5-LOX protein is upregulated in AD hippocampus, where it is primarily associated with neurofibrillary structures and Aβ-containing plaques. 5-LOX upregulation may result from increased oxidative stress (Pratico and Delanty 2000), which occurs early in AD in association with the development of amyloid lesions (Nunomura et al. 2001; Moreira et al. 2008). In addition, Aβ peptide fibrilization and tau overphosphorylation themselves may stimulate lipoxygenase activity, creating a vicious cycle of pathological cascades that could perpetuate neuronal degeneration and loss of synapses in AD. Our data provide further support for the exploration of therapeutic interventions that target lipoxygenase activity, especially in the early stages of AD.
Acknowledgments
This work was supported by National Institute on Aging Grants P50-AG-05133 and AG-15347.
We thank Barbara A. Isanski, William R. Paljug, and Laurie Nicholson.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21:383–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishnoi M, Patil CS, Kumar A, Kulkarni SK (2005) Protective effects of nimesulide (COX inhibitor), AKBA (5-LOX inhibitor), and their combination in aging-associated abnormalities in mice. Methods Find Exp Clin Pharmacol 27:465–470 [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259 [DOI] [PubMed] [Google Scholar]
- Chinnici CM, Yao Y, Praticò D (2007) The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging 28:1457–1462 [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Rossi A, Mazzon E, Di Paola R, Genovese T, Muià C, Caputi AP, et al. (2005) 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab Invest 85:808–822 [DOI] [PubMed] [Google Scholar]
- Desai PP, Ikonomovic MD, Abrahamson EE, Hamilton RL, Isanski BA, Hope CE, Klunk WE, et al. (2005) Apolipoprotein D is a component of compact but not diffuse amyloid-beta plaques in Alzheimer's disease temporal cortex. Neurobiol Dis 20:574–582 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm 108:1397–1415 [DOI] [PubMed] [Google Scholar]
- Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D (2008) 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB J 22:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Rossi A, Di Paola R, Cannavò G, Muià C, Crisafulli C, et al. (2005) Involvement of 5-lipoxygenase in spinal cord injury. J Neuroimmunol 166:55–64 [DOI] [PubMed] [Google Scholar]
- Goodman Y, Steiner MR, Steiner SM, Mattson MP (1994) Nordihydroguaiaretic acid protects hippocampal neurons against amyloid beta-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res 654:171–176 [DOI] [PubMed] [Google Scholar]
- Hanaka H, Shimizu T, Izumi T (2005) Stress-induced nuclear export of 5-lipoxygenase. Biochem Biophys Res Commun 338:111–116 [DOI] [PubMed] [Google Scholar]
- Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, et al. (1999) p38 kinase is activated in the Alzheimer's disease brain. J Neurochem 72:2053–2058 [DOI] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, et al. (1995) Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem 65:2146–2156 [DOI] [PubMed] [Google Scholar]
- Hyman BT, Tanzi RE, Marzloff K, Barbour R, Schenk D (1992) Kunitz protease inhibitor-containing amyloid-beta protein precursor immunoreactivity in Alzheimer's disease. J Neuropathol Exp Neurol 51:76–83 [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Abrahamson EE, Isanski BA, Debnath ML, Mathis CA, DeKosky ST, Klunk WE (2006) X-34 labeling of abnormal protein aggregates during the progression of Alzheimer's disease. Methods Enzymol 412:123–144 [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM-Y, Clark RSB, et al. (2004) Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 190:192–203 [DOI] [PubMed] [Google Scholar]
- Jatana M, Giri S, Ansari MA, Elango C, Singh AK, Singh I, Khan M (2006) Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia. J Neuroinflammation 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL (2002) Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging 23:787–794 [DOI] [PubMed] [Google Scholar]
- Lammers CH, Schweitzer P, Facchinetti P, Arrang JM, Madamba SG, Siggins GR, Piomelli D (1996) Arachidonate 5-lipoxygenase and its activating protein: prominent hippocampal expression and role in somatostatin signaling. J Neurochem 66:147–152 [DOI] [PubMed] [Google Scholar]
- Luo M, Jones SM, Peters-Golden M, Brock TG (2003) Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc Natl Acad Sci USA 100:12165–12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T, Sugaya K, Qu T (2000) Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J 14:1464–1469 [DOI] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR (2000) Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol 2:628–636 [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, et al. (2008) Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets 7:3–10 [DOI] [PubMed] [Google Scholar]
- Nunomura A, Moreira PI, Takeda A, Smith MA, Perry G (2007) Oxidative RNA damage and neurodegeneration. Curr Med Chem 14:2968–2975 [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, et al. (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60:759–767 [DOI] [PubMed] [Google Scholar]
- Pratico D (2002) Alzheimer's disease and oxygen radicals: new insights. Biochem Pharmacol 63:563–567 [DOI] [PubMed] [Google Scholar]
- Pratico D, Delanty N (2000) Oxidative injury in diseases of the central nervous system: focus on Alzheimer's disease. Am J Med 109:577–585 [DOI] [PubMed] [Google Scholar]
- Praticò D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, et al. (2004) 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am J Pathol 164:1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost P, Doucet J, Hammarberg T, Gerisch G, Samuelsson B, Radmark O (2001) 5-Lipoxygenase interacts with coactosin-like protein. J Biol Chem 276:16520–16527 [DOI] [PubMed] [Google Scholar]
- Rådmark O, Werz O, Steinhilber D, Samuelsson B (2007) 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci 32:332–341 [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270:7420–7426 [DOI] [PubMed] [Google Scholar]
- Smith MA, Sayre LM, Monnier VM, Perry G (1996) Oxidative posttranslational modifications in Alzheimer disease. A possible pathogenic role in the formation of senile plaques and neurofibrillary tangles. Mol Chem Neuropathol 28:41–48 [DOI] [PubMed] [Google Scholar]
- Styren SD, Hamilton RL, Styren GC, Klunk WE (2000) X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer's disease pathology. J Histochem Cytochem 48:1223–1232 [DOI] [PubMed] [Google Scholar]
- Sugaya K, Uz T, Kumar V, Manev H (2000) New anti-inflammatory treatment strategy in Alzheimer's disease. Jpn J Pharmacol 82:85–94 [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Shibata M, Ihara M, Akiguchi I, Ohtani R, Budka H (2002) A comparative study on the expression of cyclooxygenase and 5-lipoxygenase during cerebral ischemia in humans. Acta Neuropathol (Berl) 104:601–607 [DOI] [PubMed] [Google Scholar]
- West M, Mhatre M, Ceballos A, Floyd RA, Grammas P, Gabbita SP, Hamdheydari L, et al. (2004) The arachidonic acid 5-lipoxygenase inhibitor nordihydroguaiaretic acid inhibits tumor necrosis factor alpha activation of microglia and extends survival of G93A–SOD1 transgenic mice. J Neurochem 91:133–143 [DOI] [PubMed] [Google Scholar]
- Yao Y, Clark CM, Trojanowski JQ, Lee VM, Praticò D (2005) Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol 58:623–626 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang WP, Hu H, Wang ML, Sheng WW, Yao HT, Ding W, et al. (2006) Expression patterns of 5-lipoxygenase in human brain with traumatic injury and astrocytoma. Neuropathology 26:99–106 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wei EQ, Fang SH, Chu LS, Wang ML, Zhang WP, Yu GL, et al. (2006) Spatio-temporal properties of 5-lipoxygenase expression and activation in the brain after focal cerebral ischemia in rats. Life Sci 79:1645–1656 [DOI] [PubMed] [Google Scholar]