Abstract
Water content within the epididymis of the male reproductive system is stringently regulated to promote sperm maturation. Several members of the aquaporin (AQP) family of water channel–forming integral membrane proteins have been identified in epididymal cells, but expression profiling for this epithelium is presently incomplete, and no AQP isoform has yet been identified on basolateral plasma membranes of these cells. In this study, we explored AQP expression by RT-PCR and light microscopy immunolocalizations using peroxidase and wide-field fluorescence techniques. The results indicate that several AQPs are coexpressed in the epididymis including AQP 5, 7, 9, and 11. Immunolocalizations suggested complex patterns in the spatial distribution of these AQPs. In principal cells, AQP 9 and 11 were present mainly on microvilli, whereas AQP 7 was localized primarily to lateral and then to basal plasma membranes in a region-specific manner. AQP 5 was also expressed regionally but was associated with membranes of endosomes. Additionally, AQPs were expressed by some but not all basal (AQP 7 and 11), clear (AQP 7 and 9), and halo (AQP 7 and 11) cells. These findings indicate unique associations of AQPs with specific membrane domains in a cell type– and region-specific manner within the epididymis of adult animals. (J Histochem Cytochem 56:1121–1135, 2008)
Keywords: aquaporins, efferent ducts, epididymis, principal and clear cells, RT-PCR, immunohistochemistry
Water moves across lipid bilayers of plasma membranes in mammalian cells by simple diffusion or bulk flow as driven by osmotic gradients established through hydrophilic pores created by proteins called aquaporins (AQPs) (Preston and Agre 1991; King and Agre 1996; Wintour 1997; Verkman and Mitra 2000; King et al. 2004). These channels provide a mechanism for rapid and sustained movement of water across tightly sealed epithelium with low activation costs (Borgnia et al. 1999; King et al. 2000; van Os et al. 2000; Sansom and Law 2001; Nielsen 2002; Verkman 2002). AQPs have been shown to be essential for regulation of cell volume, transepithelial water transport, and whole body homeostasis (Verkman 2002).
AQPs are a family of 13 small (25–34 kDa) hydrophobic integral membrane proteins that assemble as homotetramers (Preston and Agre 1991; King and Agre 1996). Each monomer is composed of six α-helical domains that enclose a central pore that allows the bidirectional movement of water (Verkman and Mitra 2000; King et al. 2004). AQPs are expressed in many epithelia involved in fluid transport (Li et al. 1994; Ishibashi et al. 1997,2000; Page et al. 1998; Beitz and Schultz 1999; Shanahan et al. 1999). The orthodox AQPs, consisting of AQP 0, 1, 2, 4, and 5, are highly water selective, whereas the aquaglyceroporins, composed of AQP 3, 7, 9, and 10, are non-selective water channels permeable to glycerol, urea, and other small uncharged molecules. AQP 6, 8, 11, and 12 are classified as unorthodox (Tsukaguchi et al. 1998; Borgnia et al. 1999; van Os et al. 2000; Sansom and Law 2001; Agre et al. 2002; Morishita et al. 2004; Yasui 2004; Verkman 2005; Zardoya 2005; Hara-Chikuma and Verkman 2006; Wang et al. 2006; Rojek et al. 2007). AQP 6 is permeable to glycerol and anions (Morishita et al. 2004), whereas AQP 8 may represent another phylogenetic branch permeable to urea, hydrogen peroxide, ammonia, formamide, and methylammonium (Liu et al. 2006; Bienert et al. 2007; Saparov et al. 2007).
The more recently defined AQP 11 and 12 in mammals contain poorly conserved asparagine-proline-alanine (NPA) boxes that are signature sequences for other AQPs in the formation of water-permeating pores. AQP 11 and AQP 12 are subgrouped as the AQP superfamily or superaquaporin subfamily (Morishita et al. 2004; Ishibashi 2006a,b; Nozaki et al. 2008). AQP 11 has 20–29% identity with other AQPs (highest with AQP 8), whereas AQP 12 has low homology with other AQPs (highest with AQP 11). AQP 11 has efficient water channel activity comparable to AQP 1 (Yakata et al. 2007). AQP 11 is expressed highest in the testis and moderately in the kidney, whereas AQP 12 is selectively expressed in the pancreas. In the testis, AQP 11 is found in spermatogonia (Morishita et al. 2004,2005).
The epididymis, a highly coiled tube connecting the efferent ducts (EDs) to the vas deferens, plays a crucial role in sperm concentration, maturation, transport, protection, and storage (Robaire and Hermo 1988; Hermo and Robaire 2002; Robaire et al. 2006). Divided into several distinct regions [i.e., the initial segment, caput (head), corpus (body), and cauda (tail)], the epididymal epithelium consists of different cell types that may be present in one (narrow/apical), several (clear), or all regions (principal, basal), with each often showing region-specific functions and regulatory factors (Hamilton 1975; Robaire and Hermo 1988; Hermo et al. 1994; Cornwall et al. 2002; Robaire et al. 2006). The EDs link the anastomotic rete testis to the initial segment of the epididymis and serve to remove up to 90% of the water coming from the testis. Water is reabsorbed by the epithelial cells of the EDs, and this process concentrates sperm (25-fold increase) as they enter the epididymis (Crabo 1965; Johnson and Howards 1977; Turner and Cesarini 1983; Clulow et al. 1994). Impairment of water reabsorption in the EDs leads to abnormally diluted sperm, resulting in reduced, or even complete loss of, fertility (Hess et al. 2002). Thus far, AQP 1, 9, and 10 have been shown to be expressed within non-ciliated cells of the adult EDs, with AQP 1 being localized both apically and basolaterally and AQP 9 and 10 being localized on microvilli (Brown et al. 1993; Fisher et al. 1998; Pastor-Soler et al. 2001; Badran and Hermo 2002; Hermo et al. 2004).
Considerable fluid reabsorption also occurs in the epididymis as reflected by significant increases in sperm concentration observed in distal epididymal regions and by creation of luminal fluid that is hypertonic (Levine and Marsh 1971; Yeung et al. 1993; Turner 1995). Several AQPs have already been identified in the adult rat epididymis, albeit with conflicting results. AQP 1 has been localized to myoid cells surrounding epididymal tubules of the initial segment and in endothelial cells of the vascular channels throughout the epididymis (Brown et al. 1993; Badran and Hermo 2002). AQP 9 is expressed on microvilli of principal cells in all regions of the tubule (Elkjaer et al. 2000; Pastor-Soler et al. 2001; Badran and Hermo 2002). AQP 3 has been localized to basal cells of the epididymal epithelium (Hermo et al. 2004) as has AQP 8 (Elkjaer et al. 2001). Recently, RT-PCR analyses have shown mRNA specific for AQP 5, 7, 9, and 11 in epididymal epithelial cells (Da Silva et al. 2006a). Thus, although many AQPs are clearly associated with the epididymis as a whole tissue (epithelium + connective tissue + vascular), there remains inconsistencies about the exact cellular distribution of some of these AQPs (e.g., AQP 3 and 8) and no information about the localization patterns of others (e.g., AQP 7 and 11).
Considering embryological similarities of the EDs and epididymis to different segments of the kidney (Hinton and Turner 1988), where a multitude of AQPs have been identified in a cell type– and segment-specific manner (Verkman 2002), it seems reasonable to expect that the male reproductive tract might also show similar diversity. Notable in existing descriptions of AQP distribution within the epididymal epithelium is the absence of any reported association of an AQP with the basolateral plasma membranes of principal cells. The purpose of this study therefore was to discover new AQPs in the EDs and epididymis of both immature and adult rats. This was accomplished by RT-PCR, which defined AQP mRNAs in the adult EDs and epididymis. In addition, light microscope (LM) IHC using both peroxidase and fluorescence approaches on tissues mildly fixed with zinc ions (Beckstead 1994) was used to determine the specific cell types, regions, and membrane domains associated with AQP 5, 7, and 11. AQP 9, which has been reported in the EDs and epididymis in previous publications (Brown et al. 1993; Fisher et al. 1998; Pastor-Soler et al. 2001; Badran and Hermo 2002), was used as a positive control.
Materials and Methods
Animals
The Sprague-Dawley rats used in this study were obtained from Charles River Laboratories (St. Constant, QC, Canada) and were maintained on 12-hr light:dark cycles with food and water provided ad libitum. They were purchased as adults (3–4 months old; 350–450 g) or as young rats (30 days old; 100 g). Experimentation on all animals was performed according to guidelines maintained by the Animal Care Committees of McGill University and the Université de Montréal.
RT-PCR Experiments
Efferent ducts and epididymal tissues of four adult rats were pooled and homogenized, and total RNA was extracted in 2.5 ml per tube of Trizol (Invitrogen Canada; Burlington, ON, Canada) using a Polytron (Brinkmann Instruments; Mississauga, ON, Canada). RNA pellets were resuspended in diethyl polycarbonate (DEPC)-treated deionized water and stored at −80C. RT-PCR amplifications were carried out with aliquots of total RNA using the Thermoscript RT kit (Invitrogen Canada) as follows: 2 mg of total RNA from each sample was incubated at 65C for 10 min in the presence of dNTPs and oligodT primer. After this, 4 ml cDNA synthesis buffer and 1 ml of each 0.1 M DTT, RNase out, and Thermoscript RT enzyme were added to the mixture and further incubated at 55C for 1 hr and then at 85C for an additional 5 min. One μl RNase H was added to each reaction tube, which were incubated again for 20 min at 37C. PCR was carried out using the Hot Start PCR kit (Qiagen; Mississauga, ON, Canada) by adding 2 μl of the RT product to a tube containing 4 μl dNTPs, 0.25 μl Taq polymerase, 5 μl 10× PCR buffer, and 1 μl of each appropriate forward and reverse primers specific for AQPs 1–9 (Table 1). Twenty-five cycles of PCR were performed with a primer annealing temperature of 55C and an elongation temperature of 72C. Aliquots of reaction mixture (8 μl) were loaded onto 1.8% agarose gels, electrophoresed, and stained with ethidium bromide. Controls for these reactions included (a) using standard oligonucleotide probes for the general housekeeping protein, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), (b) using RNA extracted from rat kidney, and (c) running reactions without an RT step.
Table 1.
RT-PCR primers for EDs and Epi in adult rats
| Detected in
|
|||||
|---|---|---|---|---|---|
| Gene | Primer sequence (forward/reverse) | Predicted fragment size (bp) | Tested (this study) | EDs | Epi |
| AQP 0 | CCCCACCAGCTGTCCGAGGAAACC | 248 | X | ||
| GAGCAAAGGAGCGGGCGGGATTC | |||||
| AQP 1 | TGGCCAGCGAAATCAAGAAGA | 249 | √ | √ | √ |
| GAGGATGGCGGAGGCAACGAT | |||||
| AQP 2 | GCCATCCTCCATGAGATTACTCCA | 323 | √ | X | X |
| CAGGGGTCCGATCCAGAAGA | |||||
| AQP 3 | CTTCGCTGTCACCTTGCCATCTT | 228 | √ | X | √ |
| GGGCCGGAGACAACAAGCTCATT | |||||
| AQP 4 | CTGGCCATGCTCATCTTTGTTC | 821 | √ | X | X |
| AGACGAGTCCTTCCCCTTCTTCTC | |||||
| AQP 5 | TCTTCTTTGGCCTGGGCTCAGCAC | 388 | √ | X | √ |
| CGGCGAGAGTCGGTGGAGGAGA | |||||
| AQP 6 | CTACAGTTGGGGCTGCTCTTCTTT | 397 | √ | X | X |
| GGCTACGGTCTTGGTGTCAGG | |||||
| AQP 7 | GGCAGGCGGCATCTCTGGAG | 397 | √ | √ | √ |
| GGACACCCCAAGAACGCAAACAAG | |||||
| AQP 8 | CTTGGGGCTCATCATTTGCTACTT | 330 | √ | X | X |
| AGGGGACCCATTGTCTTCTCATTG | |||||
| AQP 9 | ATCGCAGTCGTGATGGCTCTCTA | 296 | √ | √ | √ |
| CTACAAAGGCACCTGGCGTGGAT | |||||
| AQP 10 | X | ||||
| AQP 11 | X | √a | |||
| AQP 12 | X | ||||
As reported by Da Silva et al. 2006b.
EDs, efferent ducts; Epi, epididymis; AQP, aquaporin.
Tissue Preparation for LM
Initial experiments were carried out using EDs and epididymal samples obtained from 16 adult rats. The tissues from eight rats were fixed in situ by perfusion with Bouin's (n=4) or Ste. Marie's (n=4) fixative through the abdominal aorta, whereas tissues from the other eight rats were rapidly dissected from anesthetized rats and immersed in either Bouin's (n=4) or Ste. Marie's (n=4) solution (95% ethanol, 1% acetic acid) as described previously (Cyr et al. 1999). Preliminary experiments with this material indicated that one or both fixatives were suitable for immunolocalizations of AQP 5, 9, and 11 but not AQP 7. Consequently, a third group of eight adult and two immature (100 g) male rats were fixed with the IHC Zinc Fixative solution from BD Biosciences (Cat 550523; BD Biosciences, Mississauga, ON, Canada). Of the eight adult rats, four were fixed by perfusion for 10 min with the zinc fixative, after which samples were removed and immersed in fresh fixative for an additional 2 hr at room temperature. The tissues from the other four adult rats and the two 100-g rats were removed quickly and immersed for 2 hr in zinc fixative at room temperature. Immersion fixation in some cases was enhanced by microwaving for periods of 4 min in six cycles as described by Laboux et al. (2004). All tissues were subsequently placed in alcohol for several days before being dehydrated and embedded in paraffin. Paraffin sections were cut at a 5-μm thickness and mounted on “Posi-Plus” slides (Fisher Scientific Company; Ottawa, ON, Canada).
IHC
Rabbit polyclonal primary antibodies against AQP 5 (cytoplasmic region) were purchased from Calbiochem (Cat 178615; EMD Biosciences, La Jolla, CA), for AQP 7 (N-terminal), AQP9 (C-terminal), and AQP11 (C-terminal) from Alpha Diagnostics (Cat AQP71-A, AQP91-A, AQP115-A; Alpha Diagnostic International, San Antonio, TX), and for AQP 7 from Santa Cruz (residues 169-269 as R-101, Cat sc-28625; Santa Cruz Biotechnology, Santa Cruz, CA) and FabGennix (C-terminal, Cat AQP7-701AP; FabGennix, Frisco, TX). Negative controls for all experiments (data not shown) consisted of substituting PBS for primary antibody or incubating sections in preimmune serum or an affinity-purified normal IgG fraction diluted at the same concentration as that used for a given primary antibody. For some experiments, aliquots of primary antibody were preincubated for 1 hr with a 1000-fold molar excess of the specific peptide used to elicit the antibody (e.g., AQP71-A antibody from Alpha Diagnostic incubated with its AQP71-P blocking peptide).
Peroxidase Method
Sections of Bouin's- and Ste. Marie–fixed tissues were deparaffinized with Histoclear (Fisher brand 22-143975; Fisher Scientific, Ottawa, ON, Canada) and rehydrated in a series of 100%, 100%, 95%, 80%, 70%, and 50% ethanol solutions, 0.3 M glycine, and PBS. During hydration, residual picric acid was neutralized by using a 70% ethanol solution containing 1% lithium carbonate for tissues that had been fixed with Bouin's. Antigen retrieval was accomplished by boiling sections in a microwave at full power for 2–3 min in 0.1 M citrate buffer, pH 6.0, followed by heating for 7 min at 60% power of the microwave. Immunostaining was performed with the Envision+ System-HRP (DAB) anti-rabbit Kit (Cat K4010; Dako Canada, Mississauga, ON, Canada) as per manufacturer's instructions and using a washing buffer solution containing 0.05 M Tris, 0.3 M NaCl, and 0.1% Tween 20, pH 7.4. Dilutions for each primary antibody were as follows—AQP 5, 1:20; AQP 7 (Santa Cruz), 1:30; AQP 9 and 11, 1:100—all of which were incubated at 37C for 1.5 hr. The sections were counterstained for 1 min in a 1:5 diluted solution of 0.1% methylene blue and 0.1% thionin, washed, and quickly dehydrated through graded ethanols to Histoclear. Coverslips were mounted on slides with Permount.
Fluorescence Method
Sections were deparaffinized in hexane (Fisher Scientific), rehydrated in a graded ethanol series, and washed in distilled water followed by 50 mM Tris-buffered saline (TBS), pH 7.4. In some cases (mostly for AQP 11), antigen retrieval was carried out by heating sections for a total of 15 min at 85–95C in 10 mM sodium citrate buffer (pH 6.0) containing 0.1% Tween 20 (Sigma-Aldrich Canada; Oakville, ON, Canada). Sections were washed in TBS containing 0.1% Tween 20 (TBST) and incubated for 30 min with a casein blocking solution (Dako Canada). Sections were incubated for 3 hr at room temperature with a primary antibody diluted at 1:20 (anti-AQP 5), 1:30 (anti-AQP 11), or 1:50 (anti-AQP 7 from all three suppliers and anti-AQP 9) in TBS. In some cases, incubations were done using tissues other than EDs or epididymis as positive (e.g., salivary gland for AQP 5) and negative (e.g., enamel organ of tooth for AQP 7 and 11) controls. In other cases, incubations were done with blocking peptides as additional positive/negative controls. Sections were washed with TBST, blocked for 20 min in a casein solution, and incubated for 30 min at room temperature with Alexafluor 594–labeled goat anti-rabbit IgG antibody (Invitrogen Canada) diluted 1:500 in TBS. Samples were washed with TBST, rinsed in TBS, and counterstained for 1–3 min at room temperature with 300 nM 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen Canada) in TBS. Samples were rinsed in TBS, and coverslips were mounted using Prolong Gold antifade reagent (Invitrogen Canada). Sections were examined and photographed on a Zeiss Axioskop 2 motorized light microscope equipped with variable intensity FluorArc epifluoresence mercury lighting and AxioCam HR color digital camera (Carl Zeiss Canada; Montreal, QC, Canada).
Results
RT-PCR of AQPs
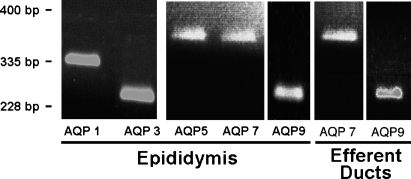
Using specific oligonucleotide primers to amplify mRNAs for AQP 1–9 (Table 1), we determined which of these were present in the adult rat epididymis. RT-PCR showed mRNA for AQP 1, 3, 5, 7, and 9, corresponding to expected band lengths of 335, 228, 388, 397, and 296 bp, respectively (Table 1; Figure 1). ED cDNA amplified by RT-PCR showed three transcripts at 249 and 296 bp indicative of AQP 1 and AQP 9, respectively (controls), and a band at 397 bp indicative of an AQP 7 transcript (Table 1; Figure 1).
Figure 1.
Ethidium bromide–stained agarose gels illustrating amplification fragments detected by RT-PCR (Table 1) for aquaporin (AQP) 1, 3, 5, 7, and 9 (positive control) in adult rat epididymis and for AQP 7 and 9 in efferent ducts.
LM Immunolocalizations of AQPs
Overview
In broad terms, the most consistently intense and uniform immunostaining in the EDs and epididymis irrespective of fixative type was obtained with anti-AQP 9. This contrasted with anti-AQP 5, which gave consistent results only in tissues fixed with Ste. Marie's solution, and anti-AQP 7 (Santa Cruz), which gave strong principal cell membrane reactions only in zinc-fixed tissues. Anti-AQP 11, like anti-AQP 9, did not appear sensitive to fixation, but adequate immunostaining was obtained only after antigen retrieval.
Immunolocalization of AQP 5
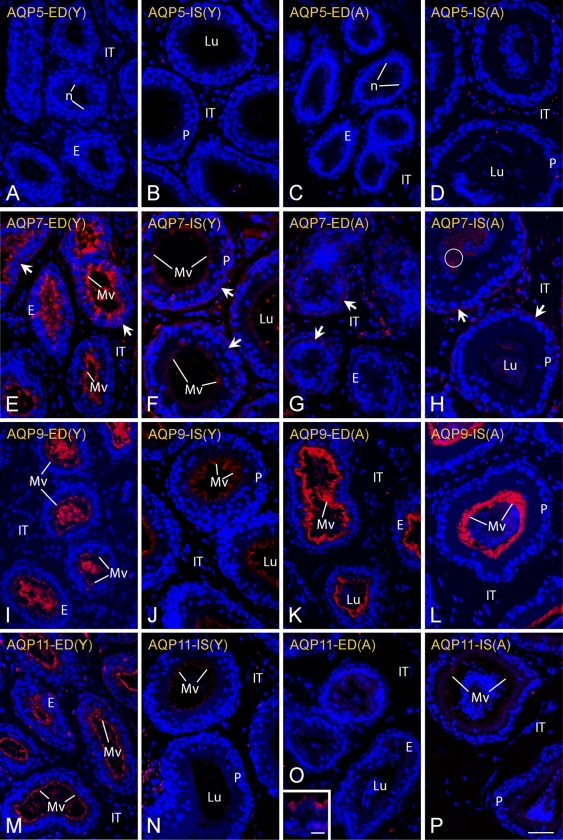
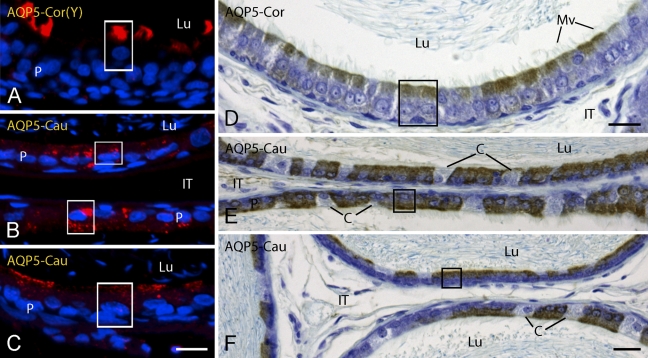
Consistent with findings from RT-PCR (Table 1; Figure 1), the epithelium of the EDs were negative for AQP 5 (Figures 2A and 2C) as were epithelial cells of the initial segment (Figures 2B and 2D) and caput regions of the epididymis (data not shown) in young and adult rats. In the corpus and cauda epididymis of young and adult rats, immunostaining was observed primarily over apical and supranuclear domains of principal cells, although not all principal cells in these regions appeared reactive (Figures 3A–3F). In the corpus epididymidis, randomly reactive principal cells showed intense staining over their apical cytoplasmic domains (Figures 3A and 3D). Many more principal cells appeared reactive within the cauda epididymidis, where they showed by fluorescence intense, punctuate cytoplasmic immunostaining that often included apical, and supranuclear domains (Figures 3B and 3C). The reaction was more uniform and diffuse with the peroxidase method (Figures 3E and 3F). Microvilli of principal cells throughout these regions appeared unreactive in both young and adult rats. No reaction was observed in any region over narrow, basal, clear, or halo cells. Spermatozoa in the epididymal lumen were unreactive, as were myoid cells and cells of the intertubular spaces (Figures 2C, 2D, and 3).
Figure 2.
Light micrographs of efferent ducts (EDs; columns 1 and 3) and initial segment of the epididymis (IS; columns 2 and 4) in young rats (Y; columns 1 and 2) and adult rats (A; columns 3 and 4) in zinc-fixed tissues immunostained for AQP 5 (A–D), AQP 7 (E–H), AQP 9 (I–L), and AQP 11 (M–P) by fluorescence method. Differences in the intensities of immunostaining between young and adult rats are evident in EDs for AQP 7 and 11 and in IS for AQP 9. E, epithelium; IT, intertubular space; Lu, lumen; Mv, microvilli; n, nuclei; P, principal cells; arrows, weak reactions along the base of the epithelium; circle, reactive sperm. Bars: A–P = 40 μm; inset = 10 μm.
Figure 3.
Light micrographs of corpus (Cor) (A,D) and cauda (Cau) (B,C,E,F) regions of epididymis from young (Y) (A) and adult (B–F) rats in zinc-fixed (A–C) and Ste. Marie–fixed (D–F) tissues immunostained for AQP 5. In adult rats, AQP 5 appears localized primarily to endodomes. C, clear cells; IT, intertubular space; Lu, lumen; Mv, microvilli; P, principal cells; boxes, apical/supranuclear reactions. Bars: A–C = 15 μm; D–F = 20 μm.
Immunolocalization of AQP 7
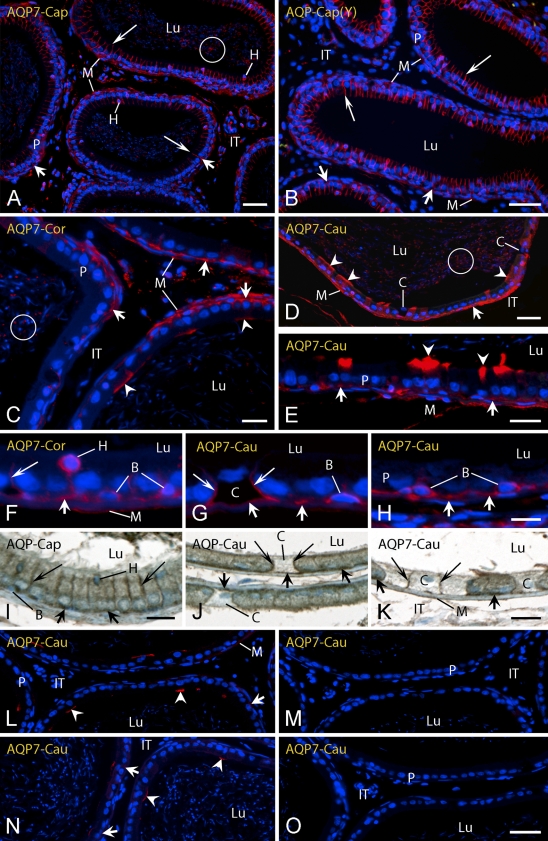
Zinc-fixed EDs and epididymal tissues were overall very reactive with the R-101 AQP 7 antibody from Santa Cruz in both young and adult rats (Figures 2E–2H and 4). A unique feature of immunolocalizations with this antibody was that the sites on plasma membranes where this protein was localized in principal cells changed in progressing from proximal to more distal regions of the epididymis (Figures 2E–2H and 4). The EDs of young rats showed intense immunostaining over microvilli of their epithelial cells and occasional staining of basolateral plasma membranes and cytoplasm (Figure 2E). This was in contrast to the EDs of adult rats where the epithelium appeared essentially unreactive for AQP 7, although there was sometimes the impression of patchy reactions in its basal area and over myoid cells (Figure 2G). The proximal initial segment of the epididymis was weakly reactive or negative for AQP 7 over microvilli and basal areas of principal cells in young and adult rats (Figures 2F and 2H). Relatively uniform immunostaining for AQP 7 began in the distal initial segment of both young and old rats and continued through the caput to the cauda epididymidis in a plasma membrane-specific manner (Figure 4). In the distal initial segment and caput epididymidis, the most intense immunostaining was observed over the lateral plasma membranes of principal cells, with virtually all cells being reactive (Figures 4A, 4B, and 4I). Immunostaining was also evident over basal plasma membranes of a few principal cells (Figures 4A and 4B). In the corpus epididymidis, immunostaining for AQP 7 was seen as isolated, intense bands over the apical plasma membrane of some principal cells, whereas most principal cells showed immunostaining only along their basal and occasionally lateral plasma membranes (Figure 4C). In the cauda region, some principal cells showed an apical cytoplasmic reaction, whereas most principal cells showed immunostaining in the area of their basal plasma membrane (Figures 4D, 4E, 4J, and 4K). The microvilli of the majority of principal cells in all regions appeared unreactive for AQP 7 (Figure 4). Many, but not all, basal cells showed immunostaining along their plasma membranes (Figures 4F–4H) as did many, but not all, halo cells (Figures 4A and 4F). Immunostaining was also evident along the lateral, and sometimes basal, plasma membranes of some clear cells (Figures 4G, 4J, and 4K).
Figure 4.
Light micrographs of caput (Cap) (A,B,I), corpus (Cor) (C,F), and cauda (Cau) (D,E,G,H,J–O) regions of epididymis from adult (A) (C–O) and young (Y) (B) rats in zinc-fixed (A–H,L–O) and Ste. Marie–fixed (I–K) tissues immunostained for AQP 7. Three different antibodies to rat AQP 7 were used including an R-101 antibody from Santa Cruz (A–K) and synthetic peptide antibodies to short sequences at the N-terminal (L–N) and C-terminal (O) ends of AQP 7. In M, the N-terminal antibody was preincubated with its specific blocking peptide, whereas in N, the same antibody was preincubated with an unrelated peptide of similar length. The site of localization for AQP 7 in principal cells varies by antibody, region, and cell type. B, basal cells; C, clear cells; H, halo cells; IT, intertubular space; Lu, lumen; M, myoid cells; P, principal cells; arrowheads, apical membrane reaction; long arrows, lateral membrane reaction; short arrows, basal membrane reaction; circle, reactive sperm. Bars: A,B,D = 40 μm; C,E,J–O = 20 μm; F–H = 10 μm; I = 15 μm.
AQP 7 expression was also detected in the mid pieces of the tails of spermatozoa filling the lumen of all epididymal regions in adult rats (Figures 4A and 4C–4E). There was also strong immunostaining of myoid cells enveloping the periphery of epididymal tubules extending from the proximal initial segment (Figure 2H) to the cauda epididymidis (Figures 4A–4E), with the latter being more strongly immunostained (Figure 4A versus 4D). The testis used as a positive control for AQP 7 expression showed immunostaining over the cytoplasm of elongating spermatids and their flagella (data not shown), in agreement with distributions reported by previous investigators (Ishibashi et al. 1997; Suzuki-Toyota et al. 1999; Calamita et al. 2001). Unexpectedly, the AQP 7 N-terminal synthetic peptide antibody from Alpha Diagnostics showed only a patchy apical cytoplasmic reaction over principal cells in the cauda (Figure 4L). This reaction was eliminated when the antibody was coincubated in the presence of its specific peptide (Figure 4M) but not when it was coincubated with an unrelated synthetic peptide of similar length (Figure 4N). The AQP 7 C-terminal antibody from FabGennix showed no immunostaining for AQP 7 in zinc-fixed epididymal tissues (Figure 4O).
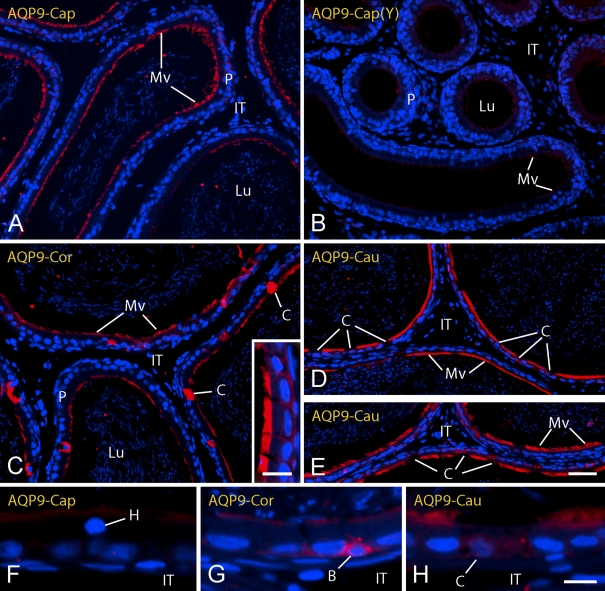
Immunolocalization of AQP 9
The EDs and epididymis of young and adult rats were intensely immunoreactive for AQP 9 (Figures 2I–2L and 5). In the EDs of young rats, intense immunostaining for AQP 9 was evident on microvilli of the non-ciliated cells (Figure 2I). In the epididymis of young rats, microvilli of principal cells in the initial segment of the epididymis were weakly reactive (Figure 2J), and reactivity was weak to moderate in the caput region (Figure 5B) and became very strong again in the corpus and cauda regions (data not shown). Adult rats, in contrast, showed intense immunostaining for AQP 9 on microvilli of non-ciliated cells in the EDs and initial segment of the epididymis (Figures 2K and 2L), as well as strong, although somewhat patchy, immunostaining of microvilli in the caput and corpus regions followed by very intense immunostaining of microvilli in the cauda region (Figures 5A and 5C–5E). Throughout the epididymis from caput to cauda regions, the occasional group of principal cells showed basal and slight lateral plasma membrane immunostaining for AQP 9 (Figure 5C, inset), and in some rare cases, basal cells appeared highly immunoreactive (Figure 5G). In the corpus and cauda regions, some sections were found where the cytoplasm of clear cells showed strong immunostaining for AQP 9 (Figure 5C), although in the majority of cases, clear cells appeared unreactive (Figures 5D, 5E, and 5H) as did halo cells (Figure 5F) in all regions of the epididymis. There was no reaction over spermatozoa in the lumen, myoid cells, or cells of the intertubular space.
Figure 5.
Light micrographs of caput (Cap) (A,B,F), corpus (Cor) (C,G), and cauda (Cau) (D,E,H) regions of epididymis from adult (A) (C–H) and young (Y) (B) rats in zinc-fixed tissues immunostained for AQP 9. AQP 9 is localized primarily to microvilli (Mv) of principal cells (P). B, basal cells; C, clear cells; H, halo cells; IT, intertubular space; Lu, lumen. Bars: A–E = 40 μm; inset to C = 20 μm; F–H = 10 μm.
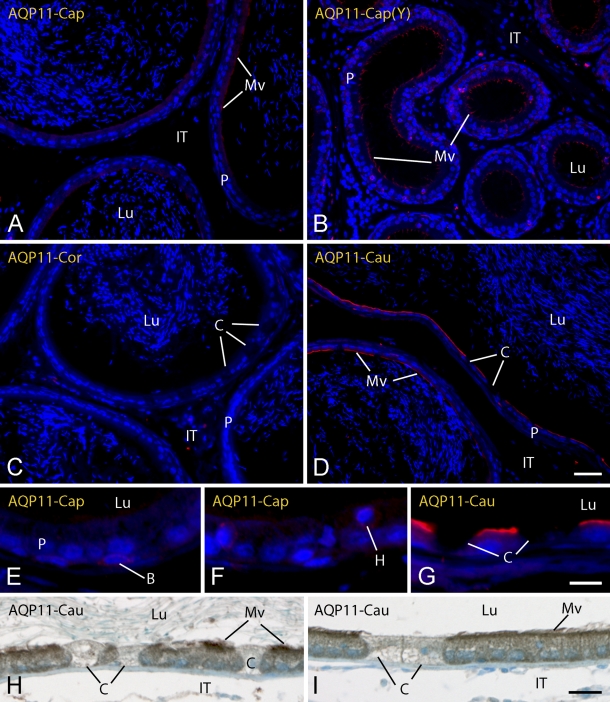
Immunolocalization of AQP 11
AQP 11, like AQP 9, appeared to be localized primarily to microvilli of principal cells, especially in the more distal caudal regions of the epididymis (Figure 6). However, unlike AQP 9, immunolocalizations of AQP 11 in young and old rats were distinctly different in the EDs and proximal regions of the epididymis (Figures 2M–2P), and this antigen proved the only instance where results by the peroxidase visualization method suggested more extensive expression of AQP 11 across the epididymis than was indicated by the fluorescence approach, which indicated mostly caudal expression for AQP 11 in young and adult rats (Figures 6A, 6C, and 6D). In addition, relatively intense immunolocalizations of AQP 11 were obtained in zinc-fixed tissues taken directly from young rats (Figures 2M and 2N), whereas it was necessary to do antigen retrieval on sections of zinc-fixed tissues to adequately immunolocalize AQP 11 in adult rats. In the EDs of young rats, strong immunostaining for AQP 11 was evident on microvilli (Figure 2M), but the reaction became absent or reduced to small focal cells in adult animals (Figure 2O and inset). In the epididymis of young and adult rats, microvilli of principal cells showed only very weak and patchy immunostaining for AQP 11 in the initial segment of the epididymis (Figures 2N and 2P) and caput region (Figures 6A and 6B), little to no immunostaining on microvilli in the corpus region (Figure 6C), and then increased strongly and more uniformly approaching more distal regions of the cauda (Figures 6D and 6G–6I). Although rare, some basal and halo cells were weakly immunoreactive for AQP 11 (Figures 6E and 6F). Clear cells were consistently unreactive (Figures 6C, 6D, and 6G–6I), and no reaction was seen over myoid cells, luminal contents, or cells of the intertubular spaces of the entire epididymis (Figure 6).
Figure 6.
Light micrographs of caput (Cap) (A,B,E,F), corpus (Cor) (C), and cauda (Cau) (D,G–I) regions of epididymis from adult (A) (C–I) and young (Y) (B) rats in zinc-fixed (A–G) and Ste. Marie–fixed (H,I) tissues immunostained for AQP 11. AQP 11, like AQP 9, is localized to microvilli (Mv) of principal cells (P) and is most intensely reactive in the distal cauda (cau) (D,E). B, basal cells; C, clear cells; H, halo cells; IT, intertubular space; Lu, lumen. Bars: A–D = 40 μm; E–G = 10 μm; H,I = 20 μm.
Discussion
Results from this study agree with conclusions reached by a number of investigators that epithelial cells of the epididymis simultaneously express many AQPs to facilitate movement of water as part of the sperm transport and maturation process (Huang et al. 2006; Da Silva et al. 2006b). This includes AQP 5 and 9 by principal cells and AQP 3 by basal cells (Brown et al. 1993; Fisher et al. 1998; Elkjaer et al. 2000,2001; Pastor-Soler et al. 2001; Zhou et al. 2001; Badran and Hermo 2002; Hermo et al. 2004; Da Silva et al. 2006a). Our findings expand this list to include AQP 7 and AQP 11 by principal cells and some basal cells and AQP 7 and AQP 9 by some clear cells (Table 2). In addition, transient halo cells passing into and through the epithelium also frequently express AQP 7 and occasionally AQP 11 (Table 2). Our RT-PCR results agree with Da Silva et al. (2006a) that AQP 6 and 8 are not expressed in adult rat epididymis, but in contrast to Da Silva et al. (2006a), we found expression of AQP 3 in the epididymis (Table 1). It should be noted that at the time this study was done, we did not have suitable primers for rat AQP 11 and for this reason do not have RT-PCR data for AQP 11 (Table 1). However, Da Silva et al. (2006a) reported that AQP 11 is expressed in the epididymis of adult rats by RT-PCR, which is consistent with our LM IHC findings of moderate and uniform immunostaining on microvilli of principal cells especially within the cauda region of the epididymis (Table 2). Western blot analyses have confirmed the presence of AQP 5, 7, and 9 proteins in adult epididymis (Pastor-Soler et al. 2001; Da Silva et al. 2006a).
Table 2.
Distribution Of AQP 5, 7, 9, and 11 in the adult rat epididymis
| Initial segment | Caput | Corpus | Cauda | |
|---|---|---|---|---|
| AQP 5 | ||||
| Principal | − | − | +/− (AS) | ++/− (PC) |
| Clear | NP | − | − | − |
| Basal | − | − | − | − |
| Halo | − | − | − | − |
| AQP 7 | ||||
| Principal | +/− (Mv) | ++ (L); +/−− (B) | ++/− (B); +/−− (A) | ++ (B); +/−− (AS) |
| Clear | NP | − | +/−− (BL) | +/− (BL) |
| Basal | − | − | +/−− | +/− |
| Halo | − | +/− | +/− | +/− |
| AQP 9 | ||||
| Principal | ++ (Mv) | ++ → + (Mv) | + → ++ (Mv) | ++ (Mv) |
| Clear | NP | − | +/−− (C) | +/− (C) |
| Basal | − | − | R | R |
| Halo | − | − | − | − |
| AQP 11 | ||||
| Principal | +/− (Mv) | +/− (Mv) | +/− (Mv) | + (Mv) |
| Clear | NP | − | − | − |
| Basal | − | R | R | R |
| Halo | R | R | R | R |
AQP, aquaporin; NP, cell type is not present; A, reaction is on apical plasma membrane; BL, reaction is on basal/lateral plasma membrane; B, reaction is on basal plasma membrane; Mv, reaction is on microvilli; L, reaction is on lateral plasma membrane; PC, punctate cytoplasmic; AS, apical/supranuclear cytoplasmic; C, cytoplasmic; R, a rare cell is reactive. −, unreactive; +/−, some cells are reactive, others are not; ++, all cells show intense reaction; +, all cells show moderate reaction; ++/−, more cells are reactive than unreactive; +/−−, more cells are unreactive than reactive; ++ → +, reaction decreases from proximal to distal caput; + → ++, reaction increases from proximal to distal corpus.
Results from this study also indicated that epithelial cells of the EDs, the narrow and highly coiled tubule that connects the rete testis to the epididymis, likewise express many AQPs including AQP 7 and 11 in young rats (Tables 1 and 3; Figure 2), in addition to the already well-defined expression of AQP 1 and 9 in young and adult rats and AQP 10 in adults (Brown et al. 1993; Fisher et al. 1998; Pastor-Soler et al. 2001; Zhou et al. 2001; Badran and Hermo 2002; Hermo et al. 2004). In adults, AQP 1, 9, and 10 are all localized to microvilli, and AQP 1 is additionally distributed along basolateral plasma membranes. Figure 2 suggests that some AQP 11 may also be present on microvilli but AQP 7 is clearly absent, or only very weakly expressed, in the epithelium of the EDs in adult rats. This suggests that the strong AQP 7 expression detected by RT-PCR in whole homogenates of EDs in adult rats (Table 1) arises in part from a carryover of spermatozoa in the lumen, which together with myoid cells enveloping the tubules, are both strongly AQP 7 positive (Figure 2).
Table 3.
Distribution of AQP 5, 7, 9, and 11 in non-ciliated cells of efferent ducts
| Microvilli
|
Basolateral
|
|||
|---|---|---|---|---|
| Location | Young | Adult | Young | Adult |
| AQP 5 | − | − | − | − |
| AQP 7 | ++ | − | +/− | − |
| AQP 9 | − | ++ | − | − |
| AQP 11 | + | +/−− | +/−− | +/−− |
AQP, aquaporin. ++, all cells show intense reaction; +, all cells show moderate reaction; +/−−, more cells are unreactive than reactive; +/−, some cells are reactive, others are not; −, unreactive.
We were initially very surprised to find strong microvillar immunostaining for AQP 7 and 11 in the epithelium of the EDs in young rats and their relatively low expression (AQP 11) or complete absence (AQP 7) in the epithelium of the EDs in adult rats (Figure 2). This is somewhat the opposite of what one might expect on a functional basis considering that spermatozoa are not fully developed in the testis or present in the lumen of the EDs in the young animals (Hermo et al. 1992). Irrespective of the issue of the absence/presence of sperm in the lumen, it is of interest that the microvilli of epithelial cells forming the EDs are associated with exactly four AQPs in both young [AQP 1, 7, 9 (moderate), and 11 (moderate)] and adult [AQP 1, 9 (intense), 10, and 11 (weak)] animals. It is further of interest that, in both cases, two of the AQP isoforms expressed are highly selective water channels (AQP 1 and 11), and the others are from the aquaglyceroporin subfamily (AQP 7, 9, and 10): permeable, as noted in the Introduction, not only to water but also to a variety of small uncharged molecules such as glycerol and urea. This emphasizes the importance and substantial redundancy that exists in the EDs in the capacity to transport water and small solutes like glycerol, as well as draws attention to the likelihood of subtle differences in the types and amounts of molecules that are being moved in the EDs before and after sperm start entering the epididymis from the testis.
Figures 3–6 document that these subtle differences apply equally to the types and spatial distributions of AQPs expressed by principal cells in the adult epididymis, which are strikingly different in the proximal, middle, and distal segments of this tubular system (Table 2). For example, the proximal initial segment of the epididymis is associated primarily only with AQP 9, which is located on microvilli. In the caput epididymidis, AQP 9 is also located on microvilli along with AQP 7 along lateral plasma membranes of all principal cells. In the corpus, AQP 9 remains on microvilli, but AQP 7 shifts to a more basal and less lateral distribution, and AQP 5 appears within the apical cytoplasm in what likely corresponds to membranes of endosomes (Table 2), similar to what has been described for AQP 5 in airway epithelium (Sidhaye et al. 2005). It is in the cauda region and especially in its more distal areas where expression of AQPs appears the greatest, suggestive of major movement of water in this region compared with more proximal regions (Table 2). This includes AQP 9 supplemented with AQP 11 on microvilli, AQP 9 cytoplasmically in some clear cells, AQP 7 on basal and occasional apical plasma membranes of principal cells, as well as along basal and/or lateral plasma membranes of some clear cells, AQP 5 in endosomes of some principal cells, and AQP 7 in plasma membranes of many basal cells and halo cells. In addition, basal cells express AQP 3 in all regions of the epididymis (Hermo et al. 2004). Collectively, this adds up to massive potential for movement of water and/or small uncharged molecules throughout the epididymis and especially within the distal cauda.
Such redundancy in expression of AQPs within the epididymis (and EDs) is presumably in part a means to ensure that dysfunction of any one AQP will not shut down the capacity of this epithelium to transport water and maintain ionic fluid balances at levels that are optimum for sperm transport, maturation, and storage. This is clearly shown by findings from several studies that knockout mice for individual AQPs such as AQP 1, AQP 7, or AQP 9 seem to have little effect on fertility in homozygotes (Zhou et al. 2001; Rojek et al. 2007; Sohara et al. 2007). AQP 11 knockout mice have been difficult to assess in this regard because they die progressively from 10 days to 2 months, making fertility estimates unreliable (Morishita et al. 2004,2005).
A key aspect of this study was the fortuitous discovery that the epididymis was tolerant of immersion/perfusion fixation with zinc ions, which is not often the case for many tissues (e.g., the testis does not fix well and small intestine deteriorates badly sitting in this solution). Although introduced in 1994 by Beckstead, zinc fixative has not received wide recognition or use in biology. It has served mostly as an alternate method for preparing small biopsies for pathological analyses, and in recent years, it has been promoted as an “improved” approach for preserving DNA and RNA for microarray PCR and real-time PCR analyses (Wester et al. 2003; Benavides et al. 2006; Lykidis et al. 2007). Little is presently know about how this fixative works, because it is merely a mixture of two zinc salts in the 23- to 37-mM range with ∼3 mM calcium acetate in 80 mM Tris buffer at pH 7.4 (Beckstead 1994; commercial product sold by BD Biosciences). Proteins do not chemically cross-link in this fixative, but the tissues remain soft and pliable. No special precautions have to be taken, however, regarding temperature for handling or storage, and the postfixed tissues seem to remain very stable in paraffin blocks or as cut sections on glass slides. The problem with the zinc fixative is that only a limited number of whole tissues preserve well enough to be acceptable for microscopic purposes, with the EDs and especially the epididymis being among these exceptions.
As noted earlier, we experienced considerable problems trying to localize AQP 7 with the Santa Cruz antibody in the EDs and epididymis of samples fixed by conventional methods including Bouin's and Ste. Marie's solutions. We initially observed in tissues processed with these fixatives that anti-AQP 7 immunoreactivity appeared localized exclusively to elongating spermatids in the testis and spermatozoa in the lumens of the epididymis as reported by others (Ishibashi et al. 1997; Suzuki-Toyota et al. 1999; Calamita et al. 2001). However, because both this study using whole epididymal extracts and the study of Da Silva et al. (2006a) using epididymal epithelial cells isolated by laser capture detected mRNA for AQP 7 by RT-PCR and translated protein by Western blotting (Da Silva et al. 2006a), it seemed reasonable to conclude that AQP 7 expression is endogenous to the epididymal epithelium but the protein itself is extremely sensitive to fixation and/or antigen masking. The spatially complex and very strong immunolocalizations obtained with the anti-AQP 7 antibody from Santa Cruz from caput to cauda regions after mild fixation with zinc (Figure 4) suggest that extensive expression of AQP 7 occurs throughout most of the epididymis. We feel this conclusion is validated by findings that anti-AQP 9 antibody localizes in zinc-fixed epididymal tissues in a manner identical to distributions reported by many investigators using many different fixatives and immunostaining methods (Elkjaer et al. 2000; Pastor-Soler et al. 2001; Badran and Hermo 2002).
However, it is also evident from the widely varying results obtained with each of the three commercial antibodies to rat AQP 7 tested in this study (Figure 4) that there are factors other than sensitivity to fixation influencing immunolocalizations of this protein in paraffin sections. Of the antibodies used, the one from Santa Cruz is the closest to representing a “whole protein,” antibody with its immunogen (R-101) comprising a very long 101 amino acid sequence from residue 169 to the C-terminal end of rat AQP 7. The other two are synthetic peptide antibodies raised against short sequences (∼18 amino acids) at the N-terminal (Alpha Diagnostics) and C-terminal (FabGennix) ends of rat AQP 7. Because of its length and extreme cost to produce, Santa Cruz does not offer a blocking peptide for the R-101 antibody, and it was for this reason that we tried the synthetic peptide antibodies that both have blocking peptides available as shown in Figures 4L and 4M (for the N-terminal antibody). It is not surprising that the R-101 antibody gave better overall results compared with the shorter synthetic peptide antibodies, because such an outcome can happen and is not unique to AQP 7 (Craig et al. 1998). What is surprising is that neither of the synthetic peptide antibodies showed lateral or basal reactions characteristic of the R-101 antibody, and the C-terminal antibody was completely negative for AQP 7 in epididymis. Such results not only provide a good example of problems that can arise in trying to show certain antigens with synthetic peptide antibodies, but they also indicate that the three-dimensional conformational folding of AQP 7 is likely very critical to its antigenicity and is different in the membrane spanning region compared with its N- and C-terminal ends (Craig et al. 1998).
One distinct advantage of working with multiple antibodies in the same study is that they can serve as their own internal antigen specificity controls when they localize to different cellular domains or in distinctly different global distribution patterns as was the case for every antibody used in this study (Table 2; Figures 2–6). Nevertheless, potential cross-reactivity is always an important issue, especially for synthetic peptide antibodies. Based on sequence information provided by the companies that manufactured the antibodies used and using alignment tools and reference sequences for rat AQPs available at NCBI (www.ncbi.nlm.nih.gov), we estimate there is a very low probability that the anti-AQP 5 antibody has much potential to cross-react with AQP 7, 9, or 11 or that the anti-AQP 9 antibody reacts against AQP 5, 7, or 11. The same low potential for cross-reactivity applies for the anti-AQP 7 antibody (Santa Cruz) against AQP 5 or 11 and for anti-AQP 11 antibody against AQP 5 or 7. However, based on sequence similarities, there is a 65% chance that the anti-AQP 11 antibody could react against AQP 9 as well as a 72% chance that anti-AQP 7 antibody (Santa Cruz) could react against AQP 9. It is on this basis we conclude that some of the weak immunoreactions seen occasionally on microvilli with the anti-AQP 7 antibody (Santa Cruz) and at some more proximal sites in the epididymis with the anti-AQP 11 antibody could be caused by cross-reactivity of these antibodies with AQP 9 antigen, which is associated with microvilli throughout the epididymis. There is no question—based on RT-PCR findings and the extremely strong immunoreactions detected with anti-AQP 7 antibody (Santa Cruz) at sites not associated with AQP 9, and the strong immunoreactivity seen on microvilli of principal cells in the distal cauda with anti-AQP 11 antibody—that these two sets of data result from anything other than recognition of a specific antigen (AQP 7 or AQP 11) by a reasonably specific antibody (anti-AQP 7 or anti-AQP 11).
In summary, this study showed a diverse and varied cell type– and region-specific expression for AQP 5, 7, 9, and 11 in the EDs and epididymis of young and adult rats. The data also showed localizations to specific membrane domains, including microvilli and apical, lateral, and basal membranes, as well as endosomes. These results suggest that the EDs and epididymis, like the kidney, have built in safeguards to ensure maximum efficiency, redundancy, and selectivity in the ability of its constituent epithelial cells to transport water and small uncharged solute molecules at critical sites serving to support and maintain spermatozoa in the lumen.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research to L.H. and C.A.M.
We thank Amanda Murphy for assistance with RT-PCR and Dr. Antonio Nanci and Micheline Fortin from the Laboratory for the Study of Calcified Tissues and Biomaterials at the Université de Montréal for contributing the paraffin sections of zinc-fixed tissue from young rats (100 g) used in this study.
References
- Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, et al. (2002) Aquaporin water channels-from atomic structure to clinical medicine. J Physiol 542:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran HH, Hermo L (2002) Expression and regulation of aquaporins 1, 8, and 9 in the testes, efferent ducts, and epididymis of adult rats and during postnatal development. J Androl 23:358–373 [PubMed] [Google Scholar]
- Beckstead JH (1994) A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 42:1127–1134 [DOI] [PubMed] [Google Scholar]
- Beitz E, Schultz JE (1999) The mammalian aquaporin water channel family: A promising new drug target. Curr Med Chem 6:457–467 [PubMed] [Google Scholar]
- Benavides J, Garcia-Pariente C, Gelmetti D, Fuertes M, Ferreras MC, Garcia-Marin JF, Perez V (2006) Effects of fixative type and fixation time on the detection of Maedi Visna virus by PCR and immunohistochemistry in paraffin-embedded ovine lung samples. J Virol Methods 137:317–324 [DOI] [PubMed] [Google Scholar]
- Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192 [DOI] [PubMed] [Google Scholar]
- Borgnia M, Nielsen S, Engel A, Agre P (1999) Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68:425–458 [DOI] [PubMed] [Google Scholar]
- Brown D, Verbavatz JM, Valenti G, Lui B, Sabolic I (1993) Localization of the CHIP28 water channel in reabsorptive segments of the rat male reproductive tract. Eur J Cell Biol 61:264–273 [PubMed] [Google Scholar]
- Calamita G, Mazzone A, Bizzoca A, Svelto M (2001) Possible involvement of aquaporin-7 and -8 in rat testis development and spermatogenesis. Biochem Biophys Res Commun 288:619–625 [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA (1994) Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol 79:915–928 [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Lareyre J-J, Matusik RJ, Hinton BT, Orgebin-Crist M-C (2002) Gene expression and epididymal function. In Robaire B, Hinton BT, eds. The Epididymis: From Molecules to Clinical Practice. New York, Kluwer Academic/Plenum Publishers, 169–199
- Crabo B (1965) Studies on the composition of epididymal content in bulls and boars. Acta Vet Scand 6:8–94 [PubMed] [Google Scholar]
- Craig L, Sanschagrin PC, Rozek A, Lackie A, Kuhn LA, Scott JK (1998) The role of structure in antibody cross-reactivity between peptides and folded proteins. J Mol Biol 281:183–201 [DOI] [PubMed] [Google Scholar]
- Cyr DG, Hermo L, Egenberger N, Mertineit C, Trasler JM, Laird DW (1999) Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology 140:3815–3825 [DOI] [PubMed] [Google Scholar]
- Da Silva N, Piétrement C, Brown D, Breton S (2006a) Segmental and cellular expression of aquaporins in the male excurrent duct. Biochim Biophys Acta 1758:1025–1033 [DOI] [PubMed] [Google Scholar]
- Da Silva N, Silberstein C, Beaulieu V, Piétrement C, Van Hoek AN, Brown D, Breton S (2006b) Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biol Reprod 74:427–438 [DOI] [PubMed] [Google Scholar]
- Elkjaer ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, Frokiaer J, Nielsen S (2001) Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am J Physiol Renal Physiol 281:F1047–F1057 [DOI] [PubMed] [Google Scholar]
- Elkjaer ML, Vajda Z, Nejsum LN, Kwon TH, Jensen UB, Amiry-Moghaddam M, Frokiaer J, et al. (2000) Immunolocalization of AQP-9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 276:1118–1128 [DOI] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Fraser HM, Saunders PT, Brown D, Sharpe RM (1998) Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology 139:3935–3945 [DOI] [PubMed] [Google Scholar]
- Hamilton DW (1975) Structure and function of the epithelium lining the ductuli efferentes, ductus epididymis and ductus deferens in the rats. In Greep RO, Astwood EB, eds. Handbook of Physiology, section 7, vol. 5. Washington, DC, American Physiological Society, 259–301
- Hara-Chikuma M, Verkman AS (2006) Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci 63:1386–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L, Barin K, Robaire B (1992) Structural differentiation of the epithelial cells of the testicular excurrent duct system of rats during postnatal development. Anat Rec 233:205–228 [DOI] [PubMed] [Google Scholar]
- Hermo L, Krzeczunowicz D, Ruz R (2004) Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl 25:494–505 [DOI] [PubMed] [Google Scholar]
- Hermo L, Oko R, Morales CR (1994) Secretion and endocytosis in the male reproductive tract: a role in sperm maturation. Int Rev Cytol 154:106–189 [PubMed] [Google Scholar]
- Hermo L, Robaire B (2002) Epididymal cell types and their functions. In Robaire B, Hinton BT, eds. The Epididymis: From Molecules to Clinical Practice. New York, Kluwer Academic/Plenum Publishers, 81–98
- Hess RA, Zhou Q, Nie R (2002) The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens. In Robaire B, Hinton BT, eds. The Epididymis: From Molecules to Clinical Practice. New York, Kluwer Academic/Plenum Publishers, 317–337
- Hinton BT, Turner TT (1988) Is the epididymis a kidney analogue? News Physiol Sci 3:28–31 [Google Scholar]
- Huang H-F, He R-H, Sun C-C, Zhang Y, Meng Q-X, Ma Y-Y (2006) Function of aquaporins in female and male reproductive systems. Hum Reprod Update 12:785–795 [DOI] [PubMed] [Google Scholar]
- Ishibashi K (2006a) Aquaporin subfamily with unusual NPA boxes. Biochim Biophys Acta 1758:989–993 [DOI] [PubMed] [Google Scholar]
- Ishibashi K (2006b) Aquaporin superfamily with unusual NPA boxes: S-aquaporins (superfamily, sip-like and subcellular-aquaporins). Cell Mol Biol (Noisy-le-grand) 52:20–27 [PubMed] [Google Scholar]
- Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, et al. (1997) Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem 272:20782–20786 [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Kuwahara M, Kageyama Y, Sasaki S, Suzuki M, Imai M (2000) Molecular cloning of a new aquaporin superfamily in mammals: AQPX1 and AQPX2. In Hohmann S, Nielsen S, eds. Molecular Biology and Physiology of Water and Solute Transport. New York, Kluwer Academic/Plenum Publishers, 123–126
- Johnson AL, Howards SS (1977) Hyperosmolality in intraluminal fluids from hamster testis and epididymis: a micropuncture study. Science 195:492–493 [DOI] [PubMed] [Google Scholar]
- King LS, Agre P (1996) Pathophysiology of the aquaporin water channels. Annu Rev Physiol 58:619–648 [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P (2004) From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 5:687–698 [DOI] [PubMed] [Google Scholar]
- King LS, Yasui M, Agre P (2000) Aquaporins in health and disease. Mol Med Today 6:60–65 [DOI] [PubMed] [Google Scholar]
- Laboux O, Dion N, Arana-Chavez VE, Ste-Marie LG, Nanci A (2004) Microwave irradiation of ethanol-fixed bone improves preservation, reduces processing time and allows both light and electron microscopy on a same sample. J Histochem Cytochem 52:1267–1275 [DOI] [PubMed] [Google Scholar]
- Levine N, Marsh DJ (1971) Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol 213:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nielsen S, Dai Y, Lazowski KW, Christensen EI, Tabak LA, Baum BJ (1994) Examination of rat salivary glands for the presence of the aquaporin CHIP. Pflugers Arch 428:455–460 [DOI] [PubMed] [Google Scholar]
- Liu K, Nagase H, Huang CG, Calamita G, Agre P (2006) Purification and functional characterization of aquaporin-8. Biol Cell 98:153–161 [DOI] [PubMed] [Google Scholar]
- Lykidis D, Van Noorden S, Armstrong A, Spencer-Dene B, Li J, Zhuang Z, Stamp GW (2007) Novel zinc-based fixative for high quality DNA, RNA and protein analysis. Nucleic Acids Res 35:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y, Matsuzaki T, Hara-Chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, et al. (2005) Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol 25:7770–7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y, Sakube Y, Sasaki S, Ishibashi K (2004) Molecular mechanisms and drug development in aquaporin water channel diseases: aquaporin superfamily (superaquaporins): expansion of aquaporins restricted to multicellular organisms. J Pharmacol Sci 96:276–279 [DOI] [PubMed] [Google Scholar]
- Nielsen S (2002) Renal aquaporins: an overview. BJU Int 90(suppl 3):1–6 [DOI] [PubMed] [Google Scholar]
- Nozaki K, Ishii D, Ishibashi K (2008) Intracellular aquaporins: clues for intracellular water transport? Pflugers Arch 456:701–707 [DOI] [PubMed] [Google Scholar]
- Page E, Winterfield J, Goings G, Bastawrous A, Upshaw-Earley J (1998) Water channel proteins in rat cardiac myocyte caveolae: osmolarity-dependent reversible internalization. Am J Physiol 274:H1988–2000 [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, et al. (2001) Aquaporin 9 expression along the male reproductive tract. Biol Reprod 65:384–393 [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Hermo L (1988) Efferent ducts, epididymis and vas deferens: structure, functions and their regulation. In: Knobil E, Neill J, eds. The Physiology of Reproduction, vol. 1. New York, Raven Press, 999–1080
- Robaire B, Hinton BT, Orgebin-Crist MC (2006) The epididymis. In Knobil E, Neill J, eds. Physiology of Reproduction. New York, Elsevier, 1071–1148
- Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, et al. (2007) Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA 104:3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom MS, Law RJ (2001) Membrane proteins: aquaporins: channels without ions. Curr Biol 11:R71–73 [DOI] [PubMed] [Google Scholar]
- Saparov SM, Liu K, Agre P, Pohl P (2007) Fast and selective ammonia transport by aquaporin-8. J Biol Chem 282:5296–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CM, Connolly DL, Tyson KL, Cary NR, Osbourn JK, Agre P, Weissberg PL (1999) Aquaporin-1 is expressed by vascular smooth muscle cells and mediates rapid water transport across vascular cell membranes. J Vasc Res 36:353–362 [DOI] [PubMed] [Google Scholar]
- Sidhaye V, Hoffert JD, King LS (2005) cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 280:3590–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohara E, Ueda O, Tachibe T, Hani T, Jishage K, Rai T, Sasaki S, et al. (2007) Morphologic and functional analysis of sperm and testes in Aquaporin 7 knockout mice. Fertil Steril 87:671–676 [DOI] [PubMed] [Google Scholar]
- Suzuki-Toyota F, Ishibashi K, Yuasa S (1999) Immunohistochemical localization of a water channel, aquaporin 7 (AQP7), in the rat testis. Cell Tissue Res 295:279–285 [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, et al. (1998) Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem 273:24737–24743 [DOI] [PubMed] [Google Scholar]
- Turner TT (1995) On the epididymis and its role in the development of the fertile ejaculate. J Androl 16:292–298 [PubMed] [Google Scholar]
- Turner TT, Cesarini DM (1983) The ability of the rat epididymis to concentrate spermatozoa. Responsiveness to aldosterone. J Androl 4:197–202 [DOI] [PubMed] [Google Scholar]
- van Os CH, Kamsteeg EJ, Marr N, Deen PM (2000) Physiological relevance of aquaporins: luxury or necessity? Pflugers Arch 440:513–520 [DOI] [PubMed] [Google Scholar]
- Verkman AS (2002) Physiological importance of aquaporin water channels. Ann Med 34:192–200 [PubMed] [Google Scholar]
- Verkman AS (2005) Novel roles of aquaporins revealed by phenotype analysis of knockout mice. Rev Physiol Biochem Pharmacol 155:31–55 [DOI] [PubMed] [Google Scholar]
- Verkman AS, Mitra AK (2000) Structure and function of aquaporin water channels. Am J Physiol Renal Physiol 278:F13–28 [DOI] [PubMed] [Google Scholar]
- Wang F, Feng X, Li Y, Yang H, Ma T (2006) Aquaporins as potential drug targets. Acta Pharmacol Sin 27:395–401 [DOI] [PubMed] [Google Scholar]
- Wester K, Asplund A, Bäckvall H, Micke P, Derveniece A, Hartmane I, Malmström PU, et al. (2003) Zinc-based fixative improves preservation of genomic DNA and proteins in histoprocessing of human tissues. Lab Invest 83:889–899 [DOI] [PubMed] [Google Scholar]
- Wintour EM (1997) Water channels and urea transporters. Clin Exp Pharmacol Physiol 24:1–9 [DOI] [PubMed] [Google Scholar]
- Yakata K, Hiroaki Y, Ishibashi K, Sohara E, Sasaki S, Mitsuoka K, Fujiyoshi Y (2007) Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim Biophys Acta 1768:688–693 [DOI] [PubMed] [Google Scholar]
- Yasui M (2004) Molecular mechanisms and drug development in aquaporin water channel diseases: structure and function of aquaporins. J Pharmacol Sci 96:260–263 [DOI] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG, Oberpenning F, Schulze H, Nieschlag E (1993) Changes in movement characteristics of human spermatozoa along the length of the epididymis. Biol Reprod 49:274–280 [DOI] [PubMed] [Google Scholar]
- Zardoya R (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97:397–414 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, et al. (2001) Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA 98:14132–14137 [DOI] [PMC free article] [PubMed] [Google Scholar]