Abstract
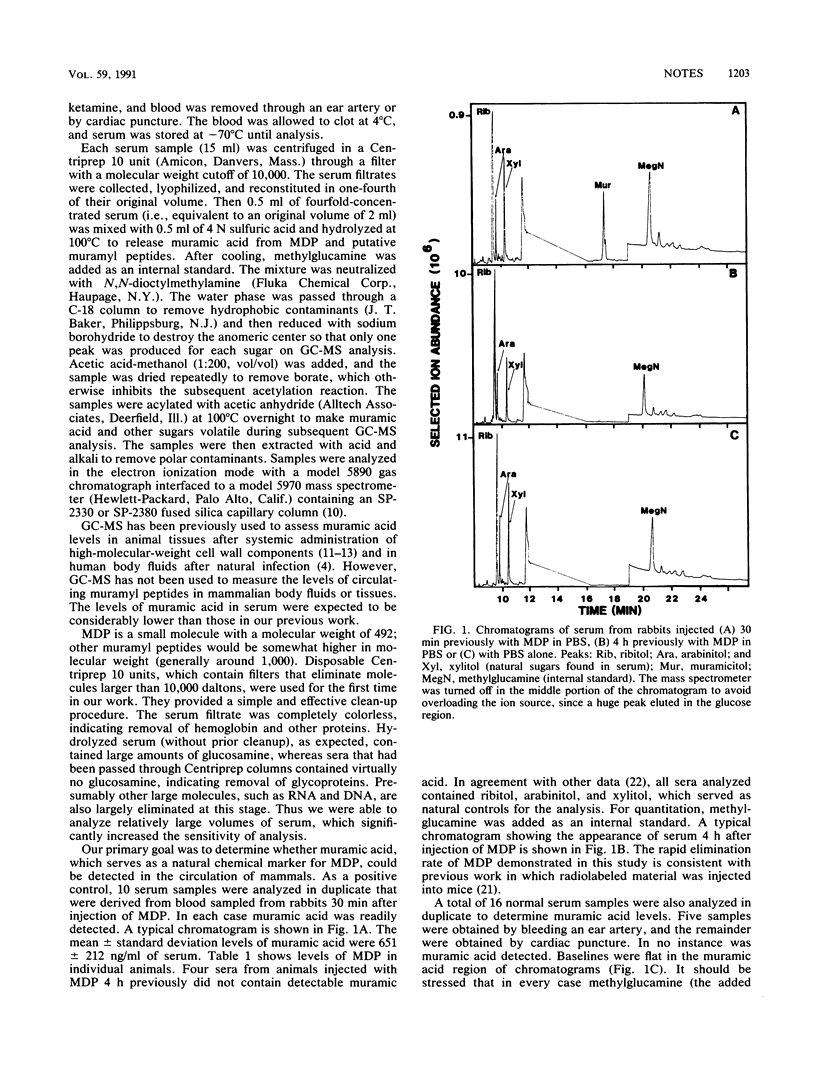
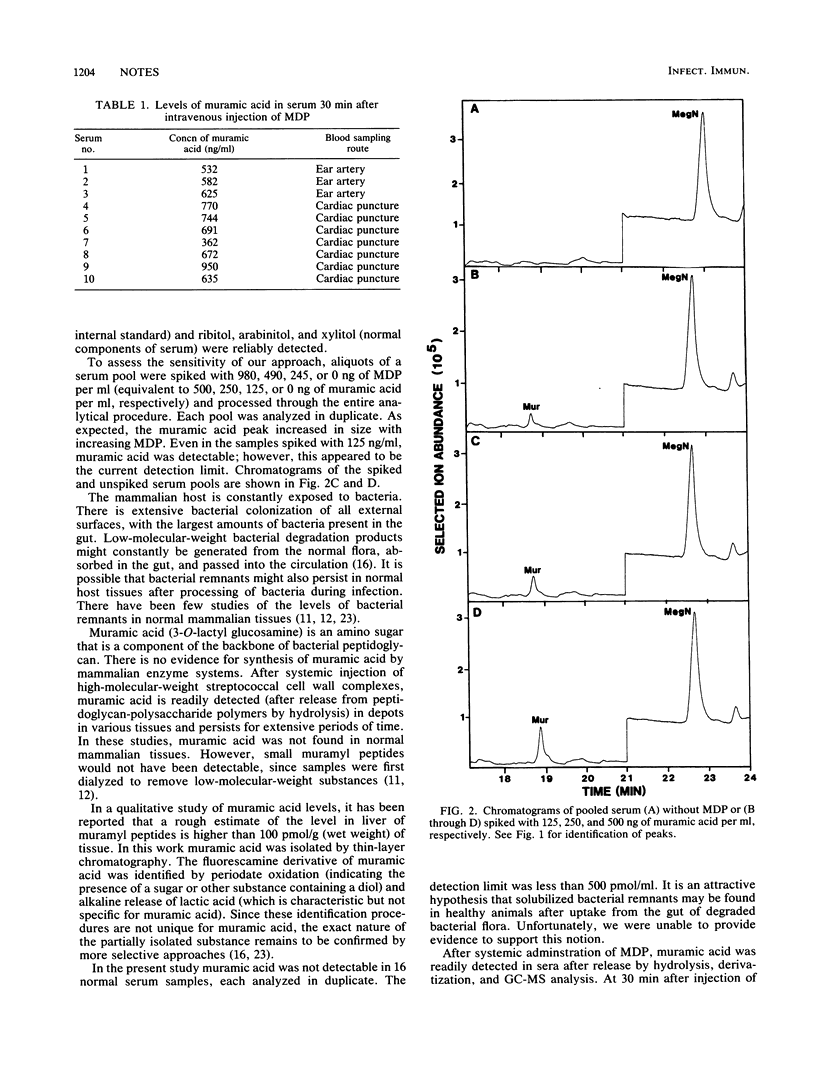
Although it is clear that muramyl peptides are involved in sleep associated with bacterial infection, their role in normal physiological sleep is less certain. It has been speculated that "natural" muramyl peptides, derived from degraded gut flora, may pass into the bloodstream, where they play a role in normal sleep (M. Karnovsky, Fed. Proc. 45:2556-2560, 1986). Muramic acid serves as a chemical marker for muramyl peptides, since it is not synthesized by mammals. After injection of synthetic muramyl dipeptide in rabbits, muramic acid was readily detected (after release by acid hydrolysis) in the circulation; however, levels rapidly decreased. This was an important positive control in assessing circulating levels of natural muramyl peptides. Muramic acid was not found in normal serum (detection limit, approximately 500 pmol/ml), demonstrating the absence of appreciable amounts of circulating natural muramyl peptides. At this time we are unable to provide supportive evidence for Karnovsky's hypothesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler L., Hudson A. M. Pharmacokinetics and metabolism of muramyl dipeptide and nor-muramyl dipeptide [3H-labelled] in the mouse. Int J Immunopharmacol. 1984;6(2):133–139. doi: 10.1016/0192-0561(84)90008-0. [DOI] [PubMed] [Google Scholar]
- Chedid L., Carelli C., Audibert F. Recent developments concerning muramyl dipeptide, a synthetic immunoregulating molecule. J Reticuloendothel Soc. 1979 Dec;26(Suppl):631–641. [PubMed] [Google Scholar]
- Chetty C., Klapper D. G., Schwab J. H. Soluble peptidoglycan-polysaccharide fragments of the bacterial cell wall induce acute inflammation. Infect Immun. 1982 Dec;38(3):1010–1019. doi: 10.1128/iai.38.3.1010-1019.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensson B., Gilbart J., Fox A., Morgan S. L. Mass spectrometric quantitation of muramic acid, a bacterial cell wall component, in septic synovial fluids. Arthritis Rheum. 1989 Oct;32(10):1268–1272. doi: 10.1002/anr.1780321012. [DOI] [PubMed] [Google Scholar]
- Cookson B. T., Cho H. L., Herwaldt L. A., Goldman W. E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989 Jul;57(7):2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. Role of bacterial debris in inflammatory diseases of the joint and eye. APMIS. 1990 Nov;98(11):957–968. doi: 10.1111/j.1699-0463.1990.tb05021.x. [DOI] [PubMed] [Google Scholar]
- Fox A., Schwab J. H., Cochran T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect Immun. 1980 Aug;29(2):526–531. doi: 10.1128/iai.29.2.526-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbart J., Fox A. Elimination of group A streptococcal cell walls from mammalian tissues. Infect Immun. 1987 Jun;55(6):1526–1528. doi: 10.1128/iai.55.6.1526-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J., Fox A. Degradation of muramyl dipeptide by mammalian serum. Infect Immun. 1985 Oct;50(1):320–321. doi: 10.1128/iai.50.1.320-321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L., Rosenthal R. S., Martin S. A., Cady A. B., Obal F., Jr, Guinand M., Krueger J. M. Somnogenic activity of O-acetylated and dimeric muramyl peptides. Infect Immun. 1989 Sep;57(9):2726–2732. doi: 10.1128/iai.57.9.2726-2732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L. Muramyl peptides in mammalian tissues and their effects at the cellular level. Fed Proc. 1986 Oct;45(11):2556–2560. [PubMed] [Google Scholar]
- Kufoy E. A., Fox K., Fox A., Parks C., Pakalnis V. A. Modulation of the blood-aqueous barrier by gram positive and gram negative bacterial cell wall components in the rat and rabbit. Exp Eye Res. 1990 Feb;50(2):189–195. doi: 10.1016/0014-4835(90)90230-r. [DOI] [PubMed] [Google Scholar]
- Ladesić B., Tomasić J., Kveder S., Hrsak I. The metabolic fate of 14C-labeled immunoadjuvant peptidoglycan monomer. II. In vitro studies. Biochim Biophys Acta. 1981 Nov 18;678(1):12–17. doi: 10.1016/0304-4165(81)90042-8. [DOI] [PubMed] [Google Scholar]
- Masek K. Immunopharmacology of muramyl peptides. Fed Proc. 1986 Oct;45(11):2549–2551. [PubMed] [Google Scholar]
- Mollner S., Braun V. Murein hydrolase (N-acetyl-muramyl-L-alanine amidase) in human serum. Arch Microbiol. 1984 Dec;140(2-3):171–177. doi: 10.1007/BF00454921. [DOI] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Yapo A., Petit J. F., Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1(1):35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- Roboz J., Kappatos D. C., Holland J. F. Role of individual serum pentitol concentrations in the diagnosis of disseminated visceral candidiasis. Eur J Clin Microbiol. 1987 Dec;6(6):708–714. doi: 10.1007/BF02013083. [DOI] [PubMed] [Google Scholar]
- Sen Z., Karnovsky M. L. Qualitative detection of muramic acid in normal mammalian tissues. Infect Immun. 1984 Mar;43(3):937–941. doi: 10.1128/iai.43.3.937-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasić J., Ladesić B., Valinger Z., Hrsak I. The metabolic fate of 14C-labeled peptidoglycan monomer in mice. I. Identification of the monomer and the corresponding pentapeptide in urine. Biochim Biophys Acta. 1980 Apr 17;629(1):77–82. doi: 10.1016/0304-4165(80)90266-4. [DOI] [PubMed] [Google Scholar]
- Ueda K., Morgan S. L., Fox A., Gilbart J., Sonesson A., Larsson L., Odham G. D-alanine as a chemical marker for the determination of streptococcal cell wall levels in mammalian tissues by gas chromatography/negative ion chemical ionization mass spectrometry. Anal Chem. 1989 Feb 1;61(3):265–270. doi: 10.1021/ac00178a015. [DOI] [PubMed] [Google Scholar]
- Zídek Z., Maśek K., Jiricka Z. Arthritogenic activity of a synthetic immunoadjuvant, muramyl dipeptide. Infect Immun. 1982 Feb;35(2):674–679. doi: 10.1128/iai.35.2.674-679.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



