Abstract
Purpose
To examine the relationship between the prevalence of open-angle glaucoma (OAG) and intraocular pressure (IOP) and the impact of central corneal thickness (CCT) on this relationship.
Design
Population based cross-sectional study.
Methods
The study cohort consisted of 5970 participants from the Los Angeles Latino Eye Study (LALES) with no history of glaucoma treatment and with complete ophthalmic examination data. The relationship between the prevalence of OAG and IOP was contrasted across persons with CCT designated as thin, normal or thick.
Results
Prevalence of OAG was exponentially related to IOP. When stratified by CCT, persons with thin CCT had a significantly higher prevalence of OAG than did those with normal or thick CCT’s at all levels of IOP. Adjusting each IOP individually for CCT did not impact significantly the relationship between the prevalence of OAG and IOP.
Conclusions
These findings suggest that adjusting for the impact of CCT on IOP by correction algorithms is not necessary in a population analysis of glaucoma prevalence; CCT and other associated corneal properties, however, are important independent risk factors for the prevalence of OAG.
INTRODUCTION
Elevation of intraocular pressure (IOP) is no longer considered a key element in the definition and diagnosis of open-angle glaucoma1 (OAG), yet it remains the only treatable risk factor and is known to be associated with presence and progression of the disease.2-5 Although IOP can be misleading on an individual case basis, large population-based surveys of eye disease have maintained the link between IOP and OAG. Specifically, the overall prevalence of OAG in a population increases with higher IOP; in fact, some populations have shown an IOP level above which the prevalence of OAG increases exponentially.6-9 This has led to the clinical practice of treating ocular hypertension when it exceeds a certain level.
Recent studies on central corneal thickness (CCT) and its impact on applanation tonometry have shown that CCT does affect the accuracy of the IOP reading, with thinner corneas giving a falsely low reading while thicker corneas yield a falsely high reading.10,11 This has prompted the development of “correction factors” and algorithms that attempt to adjust the applanation IOP based on deviation from a mean or normal CCT.
In the Los Angeles Latino Eye Study (LALES), we have reported the prevalence of OAG in Latinos to be 4.74% (95% CI: 4.22%-5.30%).12 In the study described herein, we examined the relationship between IOP and the prevalence of OAG in Latinos and the impact of CCT on this relationship. Our intent was not to analyze CCT as a screening tool for OAG, but, rather, to determine if stratifying or adjusting for CCT had an independent impact on the relationship between the prevalence of OAG and the measured IOP. Specifically, we explored two hypotheses: 1) compared to the normal CCT group, the thin CCT group would have a steeper curve on the prevalence of OAG-IOP graphs, with OAG prevalence rising more sharply at lower IOP: 2) the thick CCT group would have the flattest OAG prevalence-IOP curve, and corrected IOP curves (using existing correction factors based on CCT) would begin their exponential rise later (at higher IOPs) and the rise of this curve would be steeper than the uncorrected curve. These analyses would allow us to further elucidate the nature of the relationship between IOP, CCT and the prevalence of OAG in a population-based sample, highlighting in particular the role of CCT as an independent risk factor for the presence of OAG.
MATERIALS AND METHODS
The study population consisted of subjects from the Los Angeles Latino Eye Study (LALES), a population-based prevalence study of eye disease among Latinos aged 40 years and older in Los Angeles (LA) County. Demographic and socioeconomic characteristics of Latinos in the six census tracts of La Puente, California, were shown to be representative of the Latino population in LA County and in the United States as a whole.13 The study received Institutional Review Board approval, and all study procedures adhered to the principles for research on human subjects as stipulated by the Declaration of Helsinki. All eligible residents (Latino, age >40) underwent a detailed, standardized eye examination, including visual acuity testing, IOP measurement with Goldmann applanation tonometry, visual field testing (Humphrey Visual Field Analyzer II, SITA standard 24-2 [Carl Zeiss, Dublin, CA]), simultaneous stereoscopic optic disc photography, optical coherence tomography (OCT) imaging and frequency doubling technology (FDT) perimetry.
A two-step process was used to diagnose OAG, and has been described previously in greater detail.12 First, the clinical history was obtained; this included a history of or treatment for glaucoma, family history of glaucoma, and treatment for other ocular diseases such as cataract, diabetic retinopathy and age-related maculopathy. In addition, a detailed clinical evaluation of visual acuity, IOP, and CCT was done, as were gonioscopy and examination of the anterior and posterior segments of the eye—all of which were performed on a single clinic visit. The second step involved two glaucoma specialists, who reviewed all of the data as well as the optic disc photographs and visual field examination results before making a diagnosis. The specialists independently graded the optic disc and visual field information for each eye, then arrived at a diagnosis of normal, glaucoma suspect, or OAG based on standardized criteria, independent of IOP data. The latter were used only after a diagnosis of OAG had been made, and then only to differentiate between normal and ocular hypertensive individuals. If the two specialists were in agreement, their diagnosis was assigned to that specific eye. If there was a disagreement, a third glaucoma specialist reviewed the data, and agreement of 2 of the 3 specialists was used to assign the diagnosis. The OCT and FDT data were not used in the diagnosis of OAG.
IOP was measured with the Goldmann applanation tonometer using the average of three readings obtained by a certified ophthalmic technician. The CCT was measured with an ultrasound pachymeter (DGH, Exton, Pennsylvania), and was based on the average of three consecutive measurements. Subjects were excluded from this analysis if measurements of IOP, CCT, visual fields or optic nerve photography were inadequate. Individuals with significant corneal pathology such as dystrophy, edema or scar were excluded, as were those currently on IOP lowering therapy or with a history of glaucoma surgery. A total of 6130 participants completed the clinical eye examination and glaucoma evaluation, of whom 158 were excluded for one or more of the above-mentioned reasons.
One eye of each participant was selected based on the following criteria. If the participant had only one eye diagnosed with OAG, then that eye was selected. If both eyes were glaucomatous or non-glaucomatous, the eye with the worse mean deviation on Humphrey visual field testing was selected.
The correction algorithms were obtained from previous studies on CCT and its impact on Goldmann applanation tonometry. The most conservative impact was reported by Whitacre and Hassanein10, who found a 1 mmHg difference in IOP per 50 micron change in CCT. An intermediate correction factor of 2.5 mmHg per 50 microns was proposed by Pillunat and colleagues as a result of a cannulation study of 125 patients who underwent cataract surgery with manometric water column and applanation tonometry measurements. (Pillunat LE, et al. IOVS 2003;44:ARVO E-Abstract 4237) The same factor was adopted by Shih and coworkers14 in their assessment of the impact of IOP adjustment for CCT on the clinical management of glaucoma patients. The final algorithm carries a correction factor of 3.5 mmHg per 50 microns of CCT, and is based on the work by Ehlers and colleagues,15,16 who found a value of 3.57 mmHg per 50 microns, and the meta-analysis performed by Doughty and Zaman,17 which showed a deviation of 3.33 mmHg per 50 microns in linear regression analysis. Of note, the starting points of CCT used as the basis for correction calculations differs from study to study. For example, in Ehler’s study and Orssengo and Pye’s model of the cornea, 520 microns was used, while in the cannulation study by Pillunat the figure was calculated as 550 microns. We therefore used the value determined by the meta-analysis by Doughty and Zaman (545), as well as our population mean (550). These correction factors add or subtract the specified amount from the IOP value according to the linear formula:
Accordingly, a plot of unadjusted IOP and its relationship to OAG prevalence was calculated for the LALES population. The curve was analyzed in the following manner to determine the IOP level at which occurred the greatest turning point in increasing prevalence for OAG. First, the un-weighted Lowess estimation curve for raw data was used, as it best captures local trends and allows for identification of the turning point or points at which the slope value increases sharply. Next, the tangent slope to the LOWESS curve at each of its points was calculated [m = (yi+1 − yi)/(xi+1 − xi)]. The slope measure accounts for the variability in both dependent and independent variables, and thus most accurately captures the amount by which a difference exists in OAG prevalence rates for consecutive IOP values. Finally, changes in the slope values were compared for each pair of consecutive points by measuring the increase in consecutive slope values and the difference between consecutive slope values. The IOP point at which these 2 values were greatest was considered to be the turning point in the increasing prevalence of OAG.
The first analysis involved plotting unadjusted Goldmann IOP and OAG prevalence while stratifying patients into sub-groups based on CCT. The CCT values that corresponded to thin, normal and thick were: ≤510 microns, 511-580 microns, and >580 microns. The curves for the three different CCT groups were compared statistically by a test for difference between slopes following log transformation. Next, to determine how much of this difference could be explained by the impact of the CCT, the IOPs for the thin and thick CCT groups were recalculated using the most conservative correction algorithm, and the prevalence curves were replotted. Odds ratio estimates for OAG prevalence as a function of IOP were then calculated in the three CCT tertiles (CCT ≤534 microns, 534-564 microns, >564 microns). Tertiles were used due to the small numbers in the various subgroups. For example, there were very few subjects with a CCT <510 in the highest IOP group, and, similarly, there were few subjects with a CCT >580 who were in the lowest IOP group.
In the second analysis there was no CCT stratification, but each IOP was individually adjusted according to the three correction algorithms, and the population curve was recalculated. Since the data plots approximated exponential curves, they were transformed into linear data by log transformation in order to facilitate comparison. These curves were then compared using the test for differences between slopes following log transformation.
RESULTS
A total of 6130 participants completed the glaucoma evaluation. After exclusion of those with missing data (n=73) and those with a history of glaucoma treatment (n=89; note, 2 had both), a total of 5970 individuals were included in the present analysis.
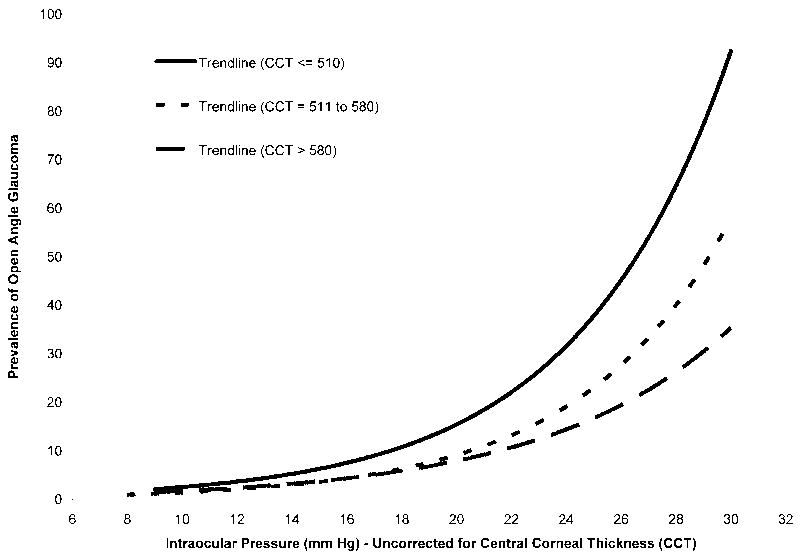
The age and gender, as well as IOP and CCT measurements of the study population are shown in Table 1. Figure 1 shows the relationship of the prevalence of OAG to IOP as stratified by the three CCT groups. The thin CCT group (≤510 microns) showed the greatest increase in OAG prevalence as a function of IOP. The normal group (511-580 microns) showed an intermediate increase, whereas the group with the thickest CCT (>580 microns) showed the least increase. When the thick and thin groups were adjusted for the impact of CCT on IOP, the prevalence curves shifted towards the normal CCT curve (data not shown). A comparison of slopes after log transformation showed the following differences: thin CCT compared to normal CCT, P <.05; thick CCT compared to normal CCT, P =.07; and thin CCT compared to thick CCT, P =.005.
Table 1.
Demographics and Clinical Data of Participants in the Los Angeles Latino Eye Study (n = 5970)
| Age (years) | |
|---|---|
| 40 – 49 | 2323 (38.9%) |
| 50 – 59 | 1814 (30.4%) |
| 60 – 69 | 1146 (19.2%) |
| 70 – 79 | 553 (9.3%) |
| 80+ | 134 (2.2%) |
| Gender | |
| Male | 2489 (41.7 %) |
| Female | 3481 (58.3 %) |
| Intraocular Pressure (mmHg) | |
| Mean (SD) | 14.5 (3.2) |
| <15 mmHg | 3232 (54.1%) |
| 15 - 20 | 2527 (42.3%) |
| 21 – 25 | 181 (3.0%) |
| >25 | 30 (0.5%) |
| ≥21 | 211 (3.5 %) |
| Central Corneal Thickness (microns) | |
| Mean (SD) | 550.0 (3.5) |
| ≤ 510 | 719 (12.0 %) |
| 511 – 580 | 4196 (70.3 %) |
| > 580 | 1055 (17.7 %) |
Figure 1.
Relationship between the prevalence of open-angle glaucoma and uncorrected intraocular pressure (IOP) stratified by central corneal thickness (CCT) in microns in the Los Angeles Latino Eye Study.
A calculation of odds ratios of OAG prevalence for IOP groups (<15, 15-20, 21-25, ≥26 mmHg) in each of the CCT groups is shown in Table 2. Although there is overlap between the 95% confidence intervals for the odds ratios, the largest difference is seen in the highest IOP group, with the thin CCT group having the highest, normal CCT having intermediate, and thick CCT showing the lowest odds ratios for prevalence of OAG. An additional analysis of CCT as a continuous variable (instead of stratified) included in the model found no statistical significance. Similarly, an analysis of an interaction term between CCT and IOP as continuous variables was not significant.
Table 2.
Odds Ratio Estimates of the Prevalence of Glaucoma for Intraocular Pressure (IOP) Groups Stratified by Central Corneal Thickness (CCT) Groups in the Los Angeles Latino Eye Study
| Intraocular Pressure (mmHg) | Odds Ratio (95% CI) |
|---|---|
| Central Corneal Thickness (≤ 534 microns) | |
| <15 | 1.00 |
| 15 – 20 | 2.02 (1.24 – 3.29) |
| 21 – 25 | 7.17 (3.30 – 15.60) |
| ≥ 26 | 74.59 (17.71 – 314.08) |
| Central Corneal Thickness (534 to 564 microns) | |
| <15 | 1.00 |
| 15 – 20 | 2.22 (1.33 – 3.71) |
| 21 – 25 | 10.88 (4.73 – 25.02) |
| ≥26 | 44.71 (10.55 – 189.41) |
| Central Corneal Thickness ( > 564 microns) | |
| <15 | 1.00 |
| 15 – 20 | 2.84 (1.63 – 4.95) |
| 21 – 25 | 8.01 (3.24 – 19.82) |
| ≥26 | 38.13 (9.99 – 145.66) |
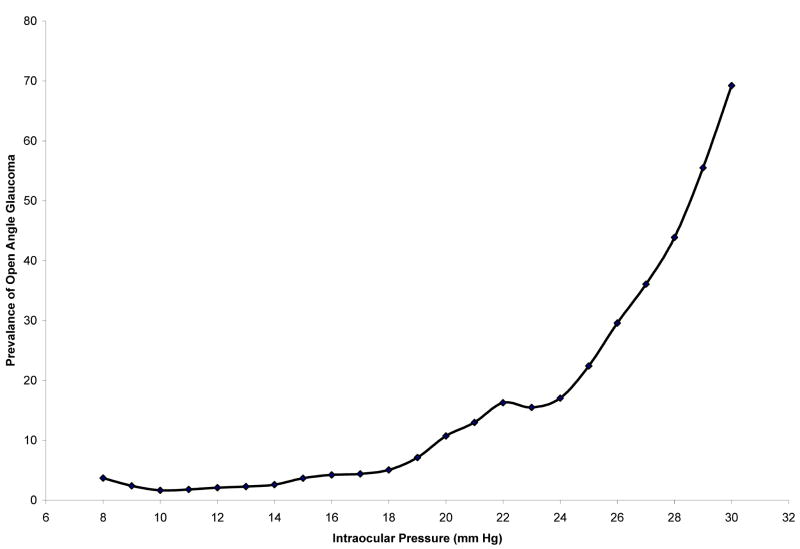
Figure 2 shows the Lowess curve for OAG prevalence-IOP relationship determined by unadjusted Goldmann tonometry readings. Using the tangent slope strategy to identify turning points, an IOP range of 19 to 20 mmHg was identified as the turning point where there was the greatest increase in OAG prevalence.
Figure 2.
Lowess curve for relationship between prevalence of open-angle glaucoma and intraocular pressure (IOP) using unadjusted Goldmann tonometry data in the Los Angeles Latino Eye Study. Since we are interested in the IOP levels for which we observe an increase in prevalence of OAG, we take into account positive slope values for determining turning points.
Table 3 shows the prevalence of OAG for uncorrected and corrected IOP levels, using the three correction algorithms. There is a statistically significant trend for an increase in the prevalence of OAG with increase of IOP for all groups (P < .0001). All four curves begin an exponential increase in OAG prevalence at an IOP of approximately 19 to 20 mmHg, as determined by slope calculations. At this point, all of the adjusted IOP curves rise more steeply than does the unadjusted IOP curve, but there is no discernible difference between the three adjusted curves. The R-square value for the uncorrected IOP curve was 0.89, while values for the three curves with adjusted IOP were nearly identical, with R-square = 0.94. There was no statistically significant difference between the uncorrected or corrected curves by slope comparison after log transformation. Using the population mean of CCT = 550 microns, there was a slight shift in the curves, but the outcome was not significantly different.
Table 3.
Prevalence of Open-angle Glaucoma Stratified by Intraocular Pressure (IOP) Levels and Different CCT-related Correction Algorithms for IOP in the Los Angeles Latino Eye Study
| Correction Algorithm | Prevalence of Open-Angle Glaucoma at various IOP levels | Trend Analysis | ||||
|---|---|---|---|---|---|---|
| 15 mmHg | 18 mmHg | 21 mmHg | 25 mmHg | 30 mmHg | ||
| Uncorrected | 6.5 % | 13.5 % | 20.5 % | 29.8 % | 41.5 % | p<0.0001 |
| C1 | 7.0 % | 14.1 % | 21.2 % | 30.7 % | 42.5 % | p<0.0001 |
| C2 | 6.7 % | 13.9 % | 21.2 % | 31.0 % | 43.1 % | p<0.0001 |
| C3 | 6.7 % | 14.3 % | 21.9 % | 32.0 % | 44.6 % | p<0.0001 |
C1 = Correction algorithm #1; 1 mmHg per 50 micron change in CCT (central corneal thickness)
C2 = Correction algorithm #2; 2.5 mmHg per 50 microns change in CCT
C3 = Correction algorithm #3; 3.5 mmHg per 50 microns change in CCT
DISCUSSION
Although an elevated IOP is not essential for the diagnosis of glaucoma, its importance in the pathogenesis of the disease is supported by evidence from both interventional and epidemiologic studies. Specifically, lowering of the IOP has been shown to reduce the risk of development or progression of OAG in patients with ocular hypertension, early glaucoma, and normal tension glaucoma,2-4 and lower IOP is associated with less progression in advanced glaucoma.5 Population-based studies have also shown a clear relationship between the prevalence of OAG and the level of IOP.6-9
The “gold standard” for IOP measurement is Goldmann applanation tonometry, in which a measured force is used to indent the cornea a standardized surface area. Based on the Imbert-Fick principle, pressure inside of the eye is proportional to the force required for indentation. One source of error, however, is resistance of the cornea itself to indentation. Accordingly, applanation is actually a measurement of the true IOP plus resistance by the cornea, and the corneal resistance can be affected by thickness, hydration, elasticity and, perhaps, as yet unknown factors. Corneal thickness has been shown to affect IOP readings, with thin corneas resulting in a falsely low IOP, and thick corneas resulting in a falsely high IOP.18,19
The importance of CCT was highlighted by the Ocular Hypertension Treatment Study, in which it was found to be a significant risk factor, independent of IOP, for the development of glaucomatous damage.3 Although CCT has been associated with the severity of glaucomatous damage,20 its impact as a risk for OAG is not known.
To our knowledge, our study is the first population-based analysis of OAG prevalence and its relationship to IOP with respect to CCT. We analyzed the impact of CCT in two ways: first, by dividing the population based on CCT and observing the difference between OAG prevalence and IOP before and after adjusting for the impact of CCT on IOP within these groups, and, second, by examining the impact of adjusting IOP for the entire population based on various CCT “correction” algorithms.
One possible bias in our study was the exclusion of participants currently receiving medical treatment or with a history of laser or surgical therapy for glaucoma. This may have eliminated some individuals with a high baseline IOP that was noted prior to the study and may have skewed the prevalence towards lower IOP. However, the aim of the study was to examine the relationship between baseline, untreated IOP and OAG prevalence. Inclusion of these patients would quite likely have skewed the prevalence of OAG towards a lower IOP by including glaucoma patients with medically or surgically lowered IOP. However, without reliable data on the pretreatment baseline IOP of these individuals, their exclusion was considered to be necessary.
After the population was divided into thin, normal and thick CCT measurements, a clear difference was seen in the relationship between OAG prevalence and IOP. The group with the thin CCT had the greatest risk of having OAG at a given IOP, risk of those with normal CCT was intermediate, and risk for those with a thick CCT was lowest. Furthermore, when IOP was adjusted for the thin and thick CCT groups using the most conservative algorithm, the curves shifted towards the normal, albeit not completely. This suggests that the impact of IOP measurement is partially responsible for the difference seen in OAG prevalence and IOP between thin, normal and thick CCT, but that there is an independent risk related to CCT itself. It is possible, however, that this independent risk is due to other corneal properties associated with CCT.
We found that adjusting each IOP value within the population had a small but measurable impact on the prevalence of the OAG-IOP curve, with each correction algorithm shifting the curve more steeply towards the exponential. Interestingly, both adjusted and unadjusted curves began their exponential increase in OAG prevalence at an IOP of approximately 19-20 mmHg, which coincided with two standard deviations above the mean IOP. However, this difference is not sufficient to warrant an adjustment in IOP in epidemiologic studies. It should be noted also that none of the correction algorithms used have been independently validated, and they likely represent an oversimplification of a complex relationship between corneal properties and applanation tonometry. The relationship between IOP and CCT is not likely to be linear, and CCT may not be as important as are other corneal factors, such as hydration or rigidity.
The results of the study reported herein support the relationship between IOP, CCT and OAG prevalence in the Latino population. Further studies validating this relationship in other populations should be determined.
Acknowledgments
Support: National Institutes of Health Grants: NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
The Los Angeles Latino Eye Study Group, University of Southern California, Los Angeles, CA: Rohit Varma, MD, MPH; Sylvia H. Paz, MS; Stanley P. Azen, PhD; Lupe Cisneros, COA; Elizabeth Corona; Carolina Cuestas, OD; Denise R. Globe, PhD; Sora Hahn, MD; Mei-Ying Lai, MS; George Martinez; Susan Preston-Martin, PhD; Ronald E. Smith, MD; LaVina Tetrow, Mina Torres, MS; Natalia Uribe, OD; Jennifer Wong, MPH; Joanne Wu, MPH; Myrna Zuniga.
Battelle Survey Research Center, St. Louis, MO: Sonia Chico, BS; Lisa John, MSW; Michael Preciado, BA; Karen Tucker, MA.
Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI: Ronald Klein, MD, MPH.
Footnotes
Financial Disclosure: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
Statement about Conformity: The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Academy of Ophthalmology. Preferred Practice Patterns in the Management of Glaucoma and Ocular Hypertension. San Francisco, CA: AAO Press; 2003. [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 3.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 5.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 6.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, Connell AMS, Schachat AP, Hyman L The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 9.Klein BEK, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 10.Whitacre M, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–596. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 11.Feltgen N, Leifert D, Funk J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br J Ophthalmol. 2001;85:85–87. doi: 10.1136/bjo.85.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–1131. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Shih CY, Graff Zivin JS, Trokel SL, Tsai JC. Clinical significance of central corneal thickness in the management of glaucoma. Arch Ophthalmol. 2004;122:1270–1275. doi: 10.1001/archopht.122.9.1270. [DOI] [PubMed] [Google Scholar]
- 15.Ehlers N, Hansen FK. Central corneal thickness in low-tension glaucoma. Acta Ophthalmol (Copenh) 1974;52:740–746. doi: 10.1111/j.1755-3768.1974.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 16.Ehlers N, Hansen FK, Aasved H. Biometric correlations of corneal thickness. Acta Ophthalmol (Copenh) 1975;53:652–659. doi: 10.1111/j.1755-3768.1975.tb01784.x. [DOI] [PubMed] [Google Scholar]
- 17.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 18.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Opthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 19.Francis BA, Hsieh A, Lai MY, et al. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. 2007;114:20–26. doi: 10.1016/j.ophtha.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 20.Herndon LW, Weizer JS, Stinnett SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004;122:17–21. doi: 10.1001/archopht.122.1.17. [DOI] [PubMed] [Google Scholar]