Abstract
BACKGROUND
Estrogen receptor related beta (ERRβ, ESRRB/NR3B2) is an orphan receptor that shares significant sequence homology with estrogen receptors ERα and ERβ. ERR family members are reported to exhibit constitutive transcriptional activity; however, little is known about the biological function of ERRβ. In an attempt to delineate its role, we examined expression of ERRβ in normal human endometrium, a tissue that undergoes cyclic remodelling under the influence of estrogen and progesterone.
METHODS
Well-characterized endometrial tissue (n = 31), including full-thickness biopsies, was obtained from women with regular menstrual cycles. RT–PCR was used to measure mRNA encoding ERRβ, the peroxisome proliferator activated receptor gamma coactivators (PGC)-1α and β and to determine whether ERRβ splice variant mRNAs were expressed. ERRβ was immunolocalized using both single and double antibody immunohistochemistry.
RESULTS
Total ERRβ mRNA appeared higher in proliferative phase samples but results did not reach significance. Transcripts corresponding to the long- and short-splice variants of ERRβ as well as PGC1α and β were detected but ERRβΔ10 was absent. ERRβ protein was localized to cell nuclei within multiple endometrial cell types including the glands, stroma, endothelium and immune cells, including uterine natural killer (uNK) cells and macrophages. Fluorescent immunohistochemistry revealed that some cells co-expressed ERRβ and ERα or ERβ, for example, endothelial and uNK cells were ERRβ+/ERβ+.
CONCLUSIONS
ERRβ mRNA and protein are expressed in healthy human endometrium. Further studies are warranted to characterize the functional impact of ERRβ on endometrial biology.
Keywords: endometrium, estrogen receptor, uterine natural killer cell, macrophage, peroxisome proliferator-activated receptor gamma coactivator
Introduction
Nuclear receptors (NR) act as ligand-activated transcription factors and affect tissue homeostasis in response to a range of signals, including steroid hormones and various endogenous and exogenous molecules (Aranda and Pascual, 2001); some NR superfamily members are reported to act in the absence of a cognate ligand. These orphan receptors include the NR3B subfamily, named estrogen receptor related (ERR) owing to their sequence homology to the estrogen receptors (ERα/ESR1 and ERβ/ESR2) (Giguere, 1999; Giguere, 2002). Three ERR genes have been cloned (ERRα/ESRRA, β/ESRRB and γ/ESRRG). ERRs are reported to constitutively modulate transcription via estrogen response elements (ERE) or steroidogenic factor-1 response elements (SFRE/ERRE) in the regulatory regions of target genes (Giguere, 2002). Initial research was aimed at establishing whether ERRs can stimulate the expression of genes in estrogen responsive tissues. For instance, it was reported that the osteopontin and pS2 gene promoters could be activated either by ERRα or by ERRβ in a ligand-independent manner via interactions with ERE sequences (Vanacker, et al., 1999; Lu, et al., 2001). Conversely, reporter gene assays also suggested that ligand-activated ERα (but not ERβ) can activate the osteopontin promoter via an ERRE sequence (Vanacker, et al., 1999). These studies pointed towards an interplay between ERs and ERRs in modulating gene expression of the same target genes.
In common with ERα and ERβ, ERRs can be activated through post-translational modifications including phosphorylation (Driggers and Segars, 2002), which may be induced by growth factors such as epidermal growth factor (Barry and Giguere, 2005). In addition, their function is also regulated by the NR coactivators peroxisome proliferator-activated receptor gamma coactivators 1α and 1β (PGC1α/PPARGC1A and PGC1β/PPARGC1B). These proteins are widely expressed, and they are thought to play a key role in the regulation of energy metabolism (Kamei, et al., 2003; Puigserver and Spiegelman, 2003). Cell-based studies have shown that PGC1α enhances ERRα expression and ERRα transcriptional activity via direct interaction between the coactivator and the NR (Schreiber et al., 2003). Finally, these coregulators are themselves post-translationally regulated in response to stimuli such as dietary signals or infection (Puigserver and Spiegelman, 2003), adding an additional layer of control to transcriptional regulation of ERR target genes and further flexibility to the phenotypic adaptation of cells to their environment.
The human endometrium undergoes cyclic remodelling under the influence of sequential exposure to the ovarian steroids estradiol and progesterone (Jabbour et al., 2006). As each cycle progresses, component cells of the endometrium serially proliferate and differentiate in preparation for implantation of the conceptus; in the absence of pregnancy, the upper functional layer is shed (menses). A complex series of biological processes is involved in these pivotal reproductive events, including the regulation of cell division, the metabolism of several biochemical mediators and the inflammatory response. Although the precise molecular and cellular mechanisms by which steroid hormones promote uterine receptivity are still the subject of intensive investigation, it is generally accepted that estradiol and progesterone, acting via their cognate receptors, ensure a pattern of gene expression that facilitates implantation and the early stages of pregnancy. Dysfunctional regulation of these events may result in subfertility and various reproductive tract pathologies, including implantation failure and aberrations of menstrual bleeding (Jabbour et al., 2006; Talbi, et al., 2006; Aghajanova, et al., 2008).
The processes that regulate endometrial function need to be controlled both temporally and spatially, thereby making the study of transcriptional regulators paramount in our understanding of endometrial biology. The pattern of expression of ER subtypes in the endometrium has been reported previously (Critchley et al., 2001, 2002). We have also documented the pattern of expression of NR in the uterine natural killer cells (uNK), which represent a major fraction of the endometrial immune cell population, especially in the luteal phase (Moffett and Loke, 2006) and demonstrated that these cells express ERβ and glucocorticoid receptor (GR) but not ERα or the progesterone receptor (PR) (Henderson et al., 2003). As an extension of these investigations and because ERRs and ERs appear to be functionally connected, we initiated a study to determine if ERRβ was also expressed in the endometrium. While this work was being carried out, a paper was published describing three ERRβ splice variants: a long form consisting of 11 exons, a short form lacking exons 10 and 11 and a form missing exon 10 (ERRβΔ10) (Zhou et al., 2006). It was reported that none of these variants was expressed in three human samples described as ‘uterus’ (Zhou et al., 2006). In the present paper, we report that both ERRβ short- and long-form mRNAs are expressed in the normal human endometrium, that total transcript levels do not appear to vary significantly over the span of the endometrial cycle, although there appeared to a trend for them to be higher during the proliferative phase, and that the protein can be detected in the nuclei of multiple cells types, including immune and endothelial cells.
Materials and Methods
Sample collection
Endometrial biopsies were collected at different stages of the menstrual cycle with either an endometrial suction curette (Pipelle, Laboratoire CCD, Paris, France) or a full-thickness sample (surface epithelium to endometrial–myometrial junction) from women attending the gynaecological services at the Royal Infirmary, Edinburgh, UK. All women from whom endometrial tissue was collected provided written informed consent for biopsy collection, and there was institutional ethical approval. Subjects were of reproductive age (median 40 years; range 30–48 years) and all described regular menstrual cycles (25–35 days length). Endometrial tissue was collected from women at the time of hysterectomy, laparoscopic sterilization or hysteroscopy. At the time of recruitment, no subject was known to have endometriosis or submucus fibroids. No subject had taken a sex steroid hormonal preparation during the 3 months prior to biopsy collection. Endometrial tissue was fixed in 4% neutral buffered formalin overnight at 4°C before being routinely wax embedded for immunohistochemical assessment. In addition, endometrial tissue was either snap frozen in liquid nitrogen or placed in RNA Later (Ambion) overnight at 4°C for subsequent RNA extraction. Histological dating of the samples was performed according to the criteria of Noyes (Noyes et al., 1950). Serum samples collected at the time of endometrial biopsy were used for determination of circulating estradiol and progesterone concentrations by radioimmunoassay (Table I). These were consistent with the patient’s reported last menstrual period and a histological dating assessment that was undertaken by an expert histologist (Critchley et al., 2001, 2002). Numbers of samples used for RNA extraction were menstrual n = 5, proliferative n = 8, early secretory n = 7, mid-secretory n = 4 and late secretory n = 7. For immunohistochemistry, 3–5 independent samples were examined at each stage.
Table I.
Hormone profile of patients during the menstrual cycle (mean ± SE).
| (n) | Estradiol (pmol/l) | Progesterone (nmol/l) |
|---|---|---|
| Menstrual (5) | 145 ± 24 | 2.8 ± 0.8 |
| Proliferative (8) | 454 ± 107 | 3.4 ± 0.6 |
| Early secretory (7) | 488 ± 69 | 89 ± 9 |
| Mid-secretory (4) | 871 ± 374 | 79 ± 16 |
| Late secretory (7) | 455 ± 129 | 17 ± 11 |
Gene expression analysis
RNA was extracted using an RNeasy kit (QIAGEN); expression levels of mRNAs were determined by Taqman™ quantitative RT–PCR (qRT–PCR). Assay on demand™ primer/probe sets specific for ERRβ (Hs01584021_m1: detects a region common to all splice variants) and ERα (Hs00174860_m1) were from Applied Biosystems; data were normalized by measuring 18S ribosomal RNA (assay 4308329) in the same reactions. Transcript abundance was expressed as a ratio against a standard comparator sample run with all plates that consisted of complementary DNA made from an ERα-positive endometrial adenocarcinoma Ishikawa cell line (Nishida et al., 1985).
The sequences of primers specific for the amplification of ERRβ short-form (hERR2f1328 and hERR2r1690), long-form (hERRB2f1565 and hERRB2r1833) and ERRβΔ10 (hERRB2f1607 and hERRB2r2151) have been published (Zhou et al., 2006). Messenger RNA was detected using a PCR-based method (Zhou et al., 2006) with minor modifications as follows: RNA extracted from samples was reverse transcribed using oligo-dT primers and analysed by touchdown PCR. The conditions were as follows: 5 min at 94°C, then 10 cycles for 30 s at 94°C, 30 s at 60°C, with the temperature decreasing by 0.5°C every cycle, 45 s at 72°C, followed by 25 cycles for 30 s at 94°C, 30 s at 55°C, 45 s at 72°C and a final extension step for 10 min at 72°C.
For the detection of the NR coactivators, primers were designed against PGC1α using GenBank sequence NM_013261 (PGC1α forward: 5′-GCG CTG ACA GAT GGA GAC GT-3′ and PGC1α reverse: 5′-TCT GTG GGT TTG GTG TGA GG-3′) and PGC1β using sequence NM_133263 (PGC1β forward: 5′-TGG AGA GCC CCT GTG AGA GT-3′ and PGC1β reverse: 5′-TCG CTC TGG GTG CTT CTT TG-3′). A standard PCR protocol was applied for 30 cycles and using the annealing temperature of 59°C. The reactions yielded products of size 347 and 350 bp for PGC1α and PGC1β, respectively. Following amplification, products were visualized on 1.5% (w/v) agarose gels, purified and sequenced. In parallel reactions where reverse transcriptase was omitted, no amplicons were detected. Control reactions containing primers directed against glyceraldehyde-3-phosphate dehydrogenase (GAPDH forward: 5′-CTG CAC CAC CAA CTG CTT AGC-3′; GAPDH reverse: 5′-ATG CCA GTG AGC TTC CCG TTC-3′) were carried out using an annealing temperature of 58°C and 30 cycles of a standard PCR protocol yielding a 204 bp product. Reverse transcription and PCR were carried out using Omniscript and HotStart Taq polymerase (Qiagen) following the manufacturer’s instruction.
Immunohistochemistry
Tissue samples were fixed in 4% neutral buffered formalin and embedded in paraffin wax. A list of all the antibodies and reagents used in this study can be found in Table II. To confirm the specificity of the anti-ERRβ antibody, a blocking peptide (LS-P7128, LifeSpan Biosciences) was pre-incubated overnight with an aliquot of the antibody (10 µg peptide per microgram antibody) and immunohistochemistry performed as below.
Table II.
List of antibodies and reagents used for immunohistochemistry.
| Antibody | Abbrv | Source | Product no. | Working dilution | Incubation time |
|---|---|---|---|---|---|
| ERRβ | ERRβ | Abcam | ab12986 | 1:500/1:200* | Overnight at 4°C |
| ERβ1 (clone PPG5/10) | ERβ1 | Serotec | MCA19745 | 1:250 | Overnight at 4°C |
| ERα (clone 6F-11) | ERα | Novocastra | NCL-ER-6F11/2 | 1:20 | Overnight at 4°C |
| CD68 (clone KP 1) | CD68 | Dako | M0814 | 1:50 | Overnight at 4°C |
| CD56 (clone 123C3) | CD56 | Zymed Laboratories | 18-0152 | 1:50 | Overnight at 4°C |
| CD45 (clone 2B11+PD7/26) | CD45 | Dako | M0701 | 1:50 | Overnight at 4°C |
| Goat anti rabbit biotinylated | GARB | Dako | E0432 | 1:500 | 30 min |
| Goat anti-rabbit peroxidase | GARP | Dako | P0448 | 1:200 | 30 min |
| Goat anti-mouse Alexa Fluor 488 | GAM 488 | Molecular Probes | A-11029 | 1:200 | 60 min |
| Streptavidin Alexa Fluor 546 | Streptavidin 546 | Molecular Probes | S-11225 | 1:200 | 60 min |
| Tyramide fluorescein | Tyramide fluorescein | Perkin Elmer Life Sciences | NEL 744 | 1;50 | 10 min |
| To Pro | To Pro | Molecular Probes | T3605 | 1:1000 | 10 min |
| DAPI | DAPI | Sigma | D9542 | 1:1000 | 10 min |
*Primary antibody was used at the higher concentration in fluorescent immunohistochemical procedures.
ERRβ, estrogen receptor related beta; DAPI: 4’,6-diamidino-2-phenylindole.
Single antibody staining
Slide-mounted 5 µm sections were deparaffinized, rehydrated and subjected to heat-induced antigen retrieval in a pressure cooker containing 0.01 M citrate buffer. The sections were then incubated with 3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase. All incubations are carried out at room temperature unless otherwise stated and were carried out in Tris-buffered saline (TBS; 50 mM Tris pH 7.4, 0.85% saline). Slides were blocked for 30 min in normal goat serum (NGS, Biosera) diluted 1:4 in TBS containing 5% bovine serum albumin, and an avidin-biotin block was performed as per manufacturer’s instructions, using reagents from Vector (Peterborough, UK). Rabbit anti-ERRβ was diluted 1:500 in NGS/TBS/BSA and incubated on sections overnight at 4°C; after washes in TBS, sections were incubated with GARB (goat anti-rabbit biotinylated) diluted 1:500 in NGS/TBS/BSA for 30 min. After further washes in TBS, sections were incubated in Streptavidin-horse-radish peroxidase for 30 min, washed in TBS (twice for 5 min each time) and bound antibodies were visualized by incubation with 3,3’-diaminobenzidine tetra-hydrochloride (liquid DAB+, product no. K346811 from DAKO).
Double antibody fluorescent immunohistochemistry
Slides were subjected to antigen retrieval as described above. The procedures for the double fluorescent immunohistochemistry are presented in Table III; all detections were performed sequentially. After blocking, washes between antibody incubations were performed twice for 5 min each using phosphate-buffered saline instead of TBS.
Table III.
Summary of protocols used for fluorescent colocalization.
| ERRβ/ERα | ERRβ/ERβ1 | ERRβ/CD68 | ERRβ/CD56 | ERRβ/CD45 |
|---|---|---|---|---|
| Citrate retrieve | Citrate retrieve | Citrate retrieve | Citrate retrieve | Citrate retrieve |
| NGS block | Methanol/peroxide block | NGS block | NGS block | NGS block |
| Avidin block | NGS block | Avidin block | Avidin block | Avidin block |
| Biotin block | Avidin block | Biotin block | Biotin block | Biotin block |
| ERRβ 1:200 | Biotin block | CD68 1:50 | CD56 1:50 | CD45 1:50 |
| GARB | ERRβ 1:200 | GAM488 | GAM488 | GAM488 |
| Streptavidin 546 | GARB | NGS block | NGS block | NGS block |
| NGS block | Streptavidin 546 | ERRβ 1:200 | ERRβ 1:200 | ERRβ 1:200 |
| ERα 1:20 | NGS block | GARB | GARB | GARB |
| GAM 488 | ERβ1 1:250 | Streptavidin 546 | Streptavidin 546 | Streptavidin 546 |
| To-Pro | GAMP | DAPI | DAPI | DAPI |
| Tyr fluorescein | ||||
| GAM 488 | ||||
| To-Pro |
NGS, normal goat serum.
Slides were examined using a Zeiss LSM Meta-confocal microscope fitted with a motorized stage. For the study of the full-thickness tissue (encompassing the functionalis and basalis layers of the endometrium and the myometrium), a tiled montage 1 frame wide by 8–10 frames in depth was acquired. Once settings were optimized for the brightest staining section, all further images were taken at the same settings to allow comparison.
Statistical analysis
Statistical analysis was carried out with Prism (GraphPad). Data normality was assessed with a Kolmogorov–Smirnov test and the analysis of variance or the non-parametric equivalent (Kruskal–Wallis test) with a 5% level of statistical significance.
Results
Expression of ERRβ mRNA(s) in normal human endometrium
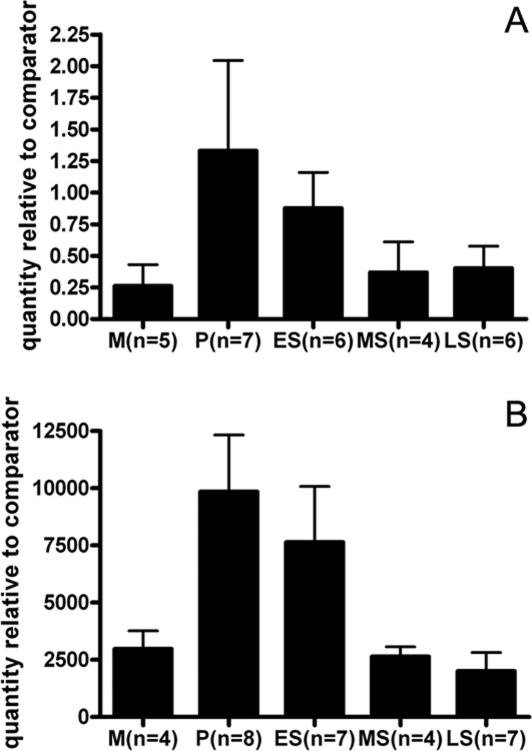
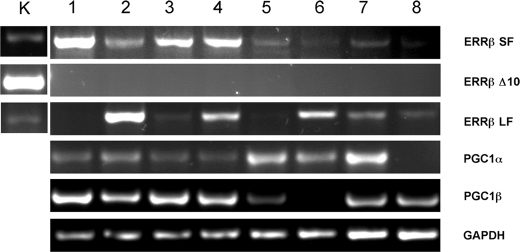
Expression of ERRβ mRNA was detected in the human endometrium throughout the menstrual cycle using qRT–PCR. There were no significant differences between samples obtained at different stages of the cycle (P = 0.679), although there was a suggestion that the levels were higher in the proliferative and early secretory phases (Fig. 1A). In line with expectations (Henderson et al., 2003) when ERα mRNA was measured in the same set of samples, highest levels of expression were detected in proliferative and early secretory phases (P = 0.0052) (Fig. 1B). The existence of ERRβ splice variant mRNAs in normal cycling endometrium was investigated with a PCR-based assay. In the proliferative phase, mRNAs corresponding to both the short and long forms of ERRβ were detected in three of four samples, in the remaining sample only the ERRβ short form was detected (Fig. 2, lane 1). The same pattern was seen in RNA from tissues sampled at the mid-secretory phase, and mRNA corresponding to the ERRβΔ10 form was never detected; although this method is only semi-quantitative, transcript abundance appeared higher in samples from the proliferative phase. All three transcripts were expressed in RNA prepared from human kidney, which was used as a positive control (Fig. 2K). Expression of mRNA encoding PGC1α was detected in all proliferative phase samples and three of four samples from the mid-secretory phase. PGC1β mRNA was present in all four proliferative phase samples and in three out of four samples from the mid-secretory phase (Fig. 2).
Figure 1:
Detection of ERRβ mRNAs in endometrial tissue using qRT–PCR.
Expression of ERRβ (A) and ERα (B) mRNAs in human endometrial samples recovered during the normal cycle. RNA was extracted from pipelle biopsies taken from patients at different stages of the cycle, mRNA was evaluated using qRT–PCR. Data are expressed relative to an internal control and was compared using a one-way analysis of variance for ERα (P = 0.0052) or a Kruskal–Wallis test for ERRβ (P = 0.679). Data are mean ± SE. M, menstrual; P, proliferative; ES, early secretory; MS, mid-secretory; LS, late secretory.
Figure 2:
Evidence that both long and short forms of ERRβ and the nuclear receptor coactivators PGC1α and PGC1β are present in normal endometrium.
RT–PCR analysis of RNA from kidney (K), proliferative (lanes 1–4) and mid-secretory (lane 5–8) phase endometrium. The abbreviations on the right-hand side indentify DNA amplied with primers specific for the following: ERRβ short form (SF), ERRβΔ10 (Δ10), ERRβ long form (LF), PGC1α, PGC1β and GAPDH. The experiment was repeated three times and similar results were obtained on each occasion.
Expression of ERRβ protein
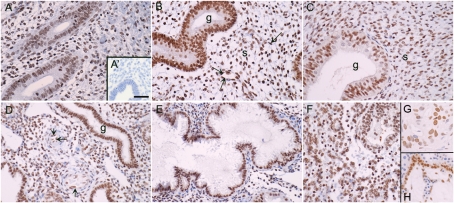
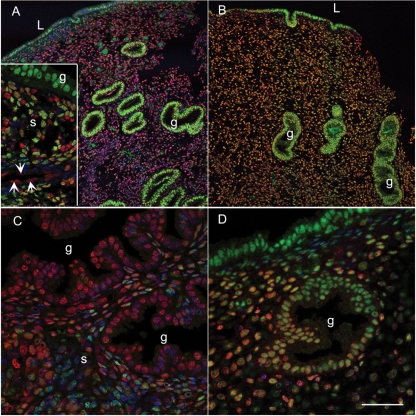
Western blotting of nuclear proteins from Ishikawa cells infected with a virus expressing the short form of ERRβ resulted in binding of antibody to a protein of the expected size (∼45 kDa), which was not detected when the membrane was probed with pre-absorbed antibody (not shown). ERRβ protein was immunolocalized to multiple cell types within the endometrium using an antibody directed against a sequence that is present in both the long and short forms of the protein (Fig. 3A–F). Specificity was confirmed by incubation of antibody with the immunising peptide (Fig. 3A′, inset) and positive nuclear staining was demonstrated in breast cancer tissue (Fig. 3G) and the cytotrophoblast cells within term placenta (Fig. 3H). Immunopositive staining for ERRβ was detected in the nuclei of cells within the glandular epithelium (g in Fig. 3B–D), the stroma as well as in the endothelial cells of blood vessels (Fig. 3D, arrows). There was no obvious stage-dependent change in the intensity of immunoexpression using this method of immunohistochemistry.
Figure 3:
ERRβ protein is expressed in human endometrium throughout the cycle.
Endometrial samples were dated as being from the following stages of the menstrual cycle; (A) Early proliferative, (B) late proliferative, (C) early secretory, (D) mid-secretory, (E) late secretory, (F) menstrual. (G and H) Immunopositive staining of cell nuclei in breast cancer and first trimester placenta, respectively (positive controls). The arrows point towards the endothelial cells of the spiral arterioles. The inset (A′) shows a section incubated with antibody pre-absorbed with the blocking peptide. Magnifications all ×20, bar in panel A′ is 50 microns and applies to all other images.
ERRβ was co-expressed with ERα or ERβ in some cell types within the endometrium
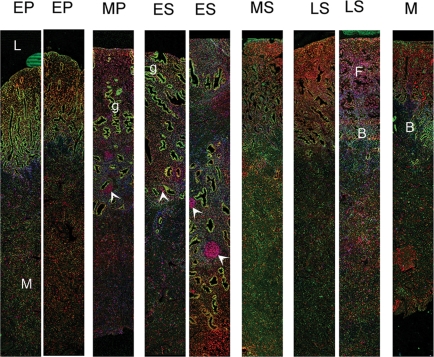
Fluorescent immunohistochemistry using full-thickness endometrial biopsies revealed that expression of ERRβ was different to that of either ERα (Fig. 4) or ERβ (Supplementary Fig. S1). For example, during the proliferative and early secretory phases, the intense immunoexpression of ERα in the glandular epithelium of the functional layer masked the immunostaining of ERRβ (Figs 4 and 5A), whereas ERRβ appeared to be expressed in a higher proportion of the stromal cells and was readily detected in ERα-negative endothelial cells (Fig. 5A inset, arrows). Expression of ERRβ was maintained in the epithelial cells within the functional layer in the late secretory phase when ERα was no longer detectable (Fig. 5C); expression of ERα in the basal compartment was maintained throughout the cycle (Fig. 4). In full-thickness samples obtained from the mid-proliferative and early secretory phases, groups of cells that were ERRβ positive/ERα negative were present within the basal compartment (Fig. 4, arrowheads). At all stages of the cycle ERRβ and ERβ were co-expressed in multiple cell types in both the stromal and epithelial cell compartments of the functional layer (Fig. 5B and D yellow nuclei). Endothelial cells were immunopositive for both ERRβ and ERβ although ERβ immunopositive staining was intense in the myometrial layer, whereas expression of ERRβ was low/negative (Supplementary Fig. 1, unpublished data).
Figure 4:
Full thickness endometrial biopsies taken throughout the menstrual cycle reveal differences in the expression of ERα and ERRβ proteins.
ERRβ (red) was detected in cell nuclei in the functional layer (F) closest to the lumen (L) of the uterus. Tissues were dated as originating during the following phases of the cycle: EP, early proliferative; MP, mid-proliferative; ES, early secretory; MS, mid-secretory; LS, late secretory; M menstrual. Immunopositive staining for ERα (green) was particularly intense in cells lining the glands(g) during MP and ES phases. Note that groups of ERRβ positive cells (arrowheads) within the basal layer of the endometrium. The positions of the basal (B) and myometrial (M) layers are indicated.
Figure 5:
Co-expression of ERα, ERβ and ERRβ proteins in functional layer of the endometrium during the normal menstrual cycle.
Co-localization of ERRβ (red) with ERα (A and C) or ERβ (B and D) (both green); counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (blue), yellow indicates colocalization. (A) Section from mid-proliferative endometrium with intense immunostaining for ERα in the glandular epithelium (g); inset shows high-power magnification to highlight mixed cell population in the stroma with cells including endothelial cells lining blood vessels (arrows) that express ERRβ but not ERα (red nuclei); (B) mid-secretory endometrium (same sample as in A) with prominent expression of ERβ1 in glandular epithelium and cells lining the lumen (L); (C) section from late secretory endometrium (code 2232) with reduced expression of ERα revealing expression of ERRβ as red nuclei; (D) ERRβ and ERβ in late secretory endometrium (2232), note overlapping pattern of expression (yellow nuclei). Panels A and B ×10 and C and D ×40, scale bar = 50 microns and applies to panels C and D.
ERRβ was expressed in immune cell populations within the normal endometrium
Leukocyte populations within the endometrial stromal cell compartment vary during the menstrual cycle and include macrophages, neutrophils and uNK cells. Immunopositive staining for ERRβ was detected in cell nuclei of immune cell populations identified by double fluorescent immunohistochemistry as being leukocytes (CD45 positive, Fig. 6A and B), uNK cells (CD56 positive, Fig. 6C and D) and macrophages (CD68 positive, Fig. 6E and F). Furthermore, in the endometrial samples where spatial orientation of the tissue was maintained, we observed large groups of ERRβ positive cells that did not appear to be ERα or ERβ positive (Fig. 4). We speculate that these cells are immune cell aggregates that are known to occur in the basal layer of the human endometrium (Marshall and Jones, 1988).
Figure 6:
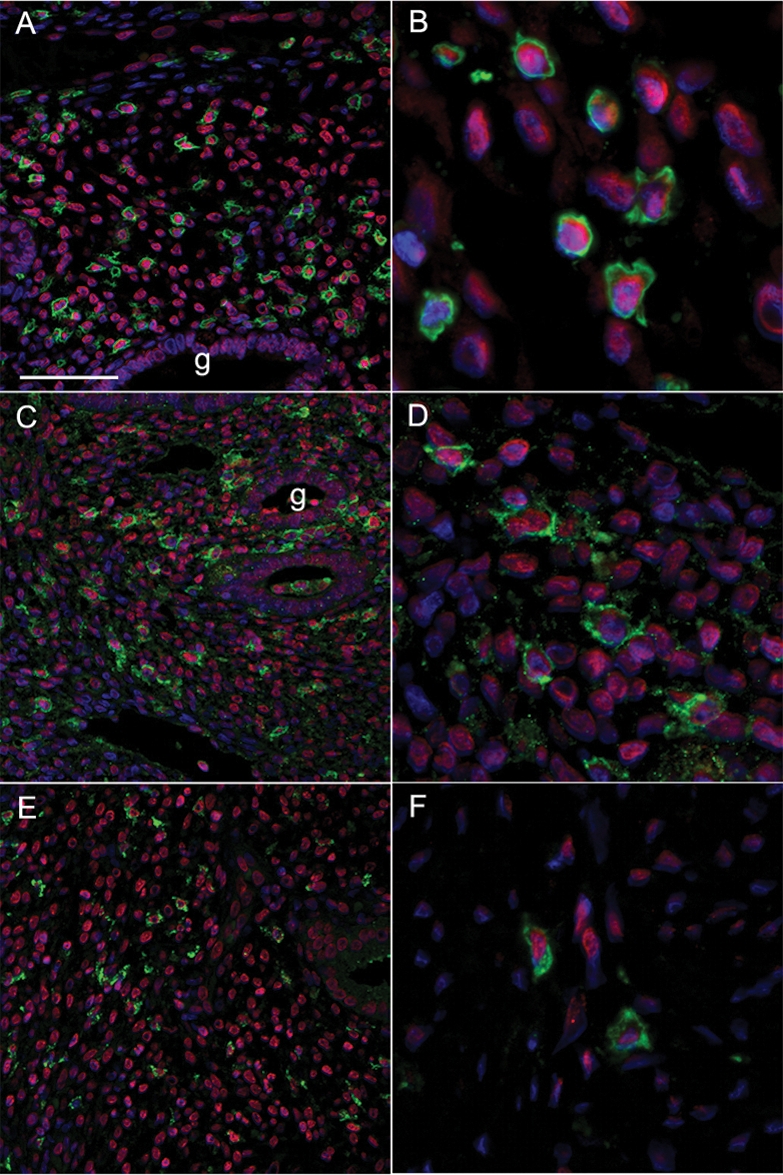
ERRβ protein is expressed in immune cell populations within the human endometrium.
Co-localization of ERRβ (red nuclei) with surface markers for selected immune cell populations (all green), counterstain for cell nuclei was DAPI (blue). (A and B) CD45 leukocyte common antigen (green); (C and D) CD56 (green), uNK cells; (E and F) CD68 (green), uterine macrophages. Magnification panels A, C and E all ×20, scale bar 100 microns; panels B, D and F are cropped high-power views from the same samples.
Discussion
In this study, we have demonstrated for the first time that mRNAs encoding ERRβ long and short forms, but not ERRβΔ10, are expressed in human endometrium at all stages of the cycle. The function of NR is modulated by receptor coactivators, it was therefore important that we were also able to demonstrate expression of mRNAs for PGC1α and β. ERRβ protein was detected in immune cells including macrophages and uNK cells as well as in endothelial cells where it was co-expressed with ERβ.
The total amount of ERRβ mRNA was low compared with that for ERα and, together with a certain amount of inter-individual variability, this may explain why a previous study (Zhou et al., 2006) failed to detect expression in uterine RNA samples of commercial origin, using identical PCR cycling conditions. Although the results did not reach significance using both semi-quantitative and qRT–PCR, there was a trend for ERRβ mRNAs to be higher in samples from the proliferative phase. In preliminary experiments, we have failed to detect any consistent change in expression following treatment of epithelial and stromal endometrial cell lines with either estradiol or progestagen; however, bioinformatic analysis has revealed the presence of putative PR binding sites in the 5′ region of the ERRβ gene (unpublished observation). In ERRα knockout mice, kidney ERRβ mRNA levels were reduced compared with those in wild-type littermates, suggesting that the amount of ERRα might influence expression of ERRβ in this tissue (Luo et al., 2003). Data showing that expression of ERRα is up-regulated by estradiol treatment in the mouse uterus (Shigeta et al., 1997), the HEC-1 human endometrial adenocarcinoma cell line and MCF-7 breast cancer cells (Liu et al., 2003) have been published. These authors reported that the estrogenic response was mediated by a 34 bp DNA element containing multiple steroid hormone response element half-sites that are conserved between the human and mouse ERRα gene promoters (Liu et al., 2003). However, when we carried out sequence alignment analysis of 10 kb in the 5′ region of ERRα and β, we failed to detect this response element in the ERRβ promoter and further studies are needed to establish the role played, if any, by steroid hormones in regulating ERRβ mRNA expression in vivo.
In contrast to a previous paper that reported that ERRβ was immunolocalized within the cytoplasmic compartment [Gao et al., 2006, we consistently detected the protein in the nuclear compartment, a finding that is in agreement with nuclear localization of fluorescent protein tagged constructs (Zhou et al., 2006) and our unpublished data]. The commercial antibody we used was directed against a peptide present in both long and short forms of ERRβ, and although the results of our RT–PCR studies would suggest that both forms were expressed in the same samples, this would need to be elucidated using a new antibody specific to the C-terminal domain of the ERRβ long-form protein.
NR coactivators have an important impact on NR signalling, through remodelling of the local chromatin environment and recruitment of the transcription machinery (McKenna et al., 1999). Other reports have described the expression of NR regulatory proteins within the endometrium, including SRC1 (steroid receptor coactivator 1), and the corepressors NCoR (nuclear receptor corepressor) and SMRT (silencing mediator of retinoid and thyroid) (Shiozawa et al., 2003). Dysregulation of NR coactivators has been reported to occur in the endometrium of women with polycystic ovarian syndrome (Gregory et al., 2002). Although the PGC-1 family of NR coactivators is reported to interact with several different NR, they appear to be critical regulators of ERR protein activity and are considered to act as ‘protein ligands’ for this class of orphan receptor (Huss et al., 2002; Kamei et al., 2003). PGC1α binds to ERRα via a specific leucine-rich domain that is distinct from the region involved in binding to other NR, including ERα (Huss et al., 2002). Results from in vitro transactivation assays suggest that ERRβ can also be regulated by PGC1β (Kamei et al., 2003). In silico comparisons between ERRα and ERRβ protein sequences (our unpublished observation) reveal that the region associated with binding to PGC1α is conserved between the proteins.
The endometrium contains a diverse population of immune cells that play a vital role in maintaining a balance between protecting the tissue from pathogenic attacks and tolerating the allogeneic sperm and trophoblast cells (Lea and Sandra, 2007). In the present study, we have demonstrated that ERRβ can be immunolocalized to uNK cells, macrophages and leukocytes within the functional layer of the endometrium and to aggregates of cells within the basal compartment that we also believe to be immune cells. uNK cells have a unique phenotype (CD56 bright, CD16− and CD3−), distinguishing them from peripheral blood NK cells (CD56 dim, CD16 bright and CD3−). They are the major immune cell population in the late secretory phase and early pregnancy, and they play a key role in implantation and early placentation (Moffett and Loke, 2006; Lea and Sandra, 2007). The cyclical change in uNK cell number in the endometrium suggests that this cell type may be regulated by changes in the amounts of sex steroid hormones. We have previously demonstrated that uNK express ERβ and GR but not ERα (Henderson et al., 2003) and recently discovered that they also express ERRα (unpublished observations). The function of uterine macrophages is less clearly defined, but we speculate that they may be involved in clearing extracellular components from degraded cells (Repnik et al., 2008). Studies in mice lacking ERRα demonstrate that it is required for induction of mitochondrial reactive oxygen species production in macrophages, a response that was also dependent upon PGC-1β (Sonoda et al., 2007). We believe that our data are the first to demonstrate expression of ERRβ in immune cells within endometrium and further studies are therefore required to determine the significance of this result.
Since ERRβ and the ERR coactivators PGC1α and β are all expressed in the human endometrium, it is appropriate to consider how the protein might influence endometrial function. On the basis of the available literature on the function(s) of ERRs, we speculate that ERRβ might have an impact on ER signalling, influence expression of previously identified ERR target genes and/or influence cell differentiation. The evidence for each of these functions is considered below.
In the current study, we found that immunoexpression of ERRβ in normal endometrium occurred in multiple cell populations within the normal endometrium. Although some cells appeared to express ERRβ alone (e.g. immune aggregates), in other cell types, the receptor was co-expressed with ERα (e.g. epithelial cells in the proliferative phase) or ERβ (e.g. endothelial cells, uNK cells). We have also detected expression of ERRβ in stage 1 endometrial cancers (unpublished observations), and it is notable that expression of ERRα also occurs in endometrial cancers where it has been suggested that it may be linked to dysregulation of estrogen signalling (Watanabe et al., 2006). Experiments support the idea that ERRα and ERRβ proteins both have the ability to bind to identical ERE and ERRE sequences (Vanacker et al., 1999). Genes expressed in the human endometrium that contain promoters reported to be regulated by ERRα include lactotransferin (Yang et al., 1996), thyroid hormone receptor α (Vanacker et al., 1998a,b), osteopontin (Vanacker et al., 1998a,b), aromatase (CYP19) (Yang et al., 1998) and monoamine oxidase (Zhan et al., 2004); therefore, our data showing expression of ERRβ raises the possibility that this protein may also influence expression of the same set of genes.
There is an emerging consensus that ERRs play a key role in regulating genes involved in energy homeostasis, including fatty acid metabolism (Sladek et al., 1997). For example, transgenic mice lacking ERRα have a reduced fat mass and are resistant to diet-induced obesity (Luo et al., 2003). To date, there is no data to support a similar role for ERRβ and this therefore requires further study. Finally, a number of recent papers report a role for ERRβ with the regulation of stem cell differentiation (Ivanova et al., 2006; Loh et al., 2006). In murine embryonic stem cells, ERRβ is not only a direct target of the stem cell regulator Oct4, but also acts as a transcription factor regulating Oct4 (Zhou et al., 2007). Studies in ERRβ knock-out animals have revealed problems with differentiation of trophoblast, and viable knock-out animals can only be generated by aggregating mutant embryos with wild-type cells which can then develop a functional placenta (Luo et al., 1997). Additionally, ERRβ is expressed in primordial germ cells during embryonic life and gene deficiency leads to a reduction in the number of differentiated germ cells (Mitsunaga et al., 2004); therefore, we propose that ERRβ may also have an impact on endometrial cell differentiation.
In summary, we have demonstrated for the first time that ERRβ short and long splice variant mRNAs are both expressed in the human endometrium. ERRβ protein is expressed within the cell nuclei of epithelial, stromal, immune and endothelial cells. Expression of the protein partially overlaps with that of ERα and ERβ. We speculate that ERRβ may play a role in endometrial cell fate determination and in regulating factors important for endometrial function and receptivity, including osteopontin. Clearly, further studies are required to uncover the full impact of expression of ERRβ in endometrial biology and investigate whether there are differences in the functional effects of the splice variant isoforms.
Funding
Studies were supported by MRC Human Reproductive Sciences Unit funding to PTKS (U1276.00.002.00005.01) and a MRC programme grant to HODC (G0500047).
Supplementary Material
Acknowledgements
We thank Gillian Cowan for help with the immunohistochemistry, Frances Collins and Karen Kerr for expert technical assistance, James Price (Qiagen UK) for helpful discussion, Dr Alistair Williams for histological assessment of endometrial biopsies and our research nurses Catherine Murray and Sharon McPherson, for patient recruitment.
References
- Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19:204–211. doi: 10.1016/j.semcdb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- Barry JB, Giguere V. Epidermal growth factor-induced signaling in breast cancer cells results in selective target gene activation by orphan nuclear receptor estrogen-related receptor alpha. Cancer Res. 2005;65:6120–6129. doi: 10.1158/0008-5472.CAN-05-0922. [DOI] [PubMed] [Google Scholar]
- Critchley HOD, Brenner RM, Henderson TA, Williams K, Nayak NR, Slayden OD, Millar MR, Saunders PTK. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86:1370–1378. doi: 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Henderson TA, Kelly RW, Scobie GS, Evans LR, Groome NP, Saunders PT. Wild-type estrogen receptor (ERbeta1) and the splice variant (ERbetacx/beta2) are both expressed within the human endometrium throughout the normal menstrual cycle. J Clin Endocrinol Metab. 2002;87:5265–5273. doi: 10.1210/jc.2002-020502. [DOI] [PubMed] [Google Scholar]
- Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Sun P, Wang J, Zhao D, Wei L. Expression of estrogen receptor-related receptor isoforms and clinical significance in endometrial adenocarcinoma. Int J Gynecol Cancer. 2006;16:827–833. doi: 10.1111/j.1525-1438.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, Fritz MA, Lessey BA. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87:2960–2966. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci USA. 2003;100:12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea RG, Sandra O. Immunoendocrine aspects of endometrial function and implantation. Reproduction. 2007;134:389–404. doi: 10.1530/REP-07-0167. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, Teng CT. Estrogen stimulates estrogen-related receptor alpha gene expression through conserved hormone response elements. Endocrinology. 2003;144:4894–4904. doi: 10.1210/en.2003-0432. [DOI] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Lu D, Kiriyama Y, Lee KY, Giguere V. Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res. 2001;61:6755–6761. [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol. 2003;23:7947–7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Jones DB. An immunohistochemical study of lymphoid tissue in human endometrium. Int J Gynecol Pathol. 1988;7:225–235. doi: 10.1097/00004347-198809000-00003. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor co-regulators: cellular and molecular biology. Endocrine Reviews. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Mitsunaga K, Araki K, Mizusaki H, Morohashi K, Haruna K, Nakagata N, Giguere V, Yamamura K, Abe K. Loss of PGC-specific expression of the orphan nuclear receptor ERR-beta results in reduction of germ cell number in mouse embryos. Mech Dev. 2004;121:237–246. doi: 10.1016/j.mod.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nippon Sanka Fujinka Gakkai Zasshi. 1985;37:1103–1111. [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Ferti Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Repnik U, Tilburgs T, Roelen DL, van der Mast BJ, Kanhai HH, Scherjon S, Claas FH. Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta. 2008;29:405–412. doi: 10.1016/j.placenta.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Shigeta H, Zuo W, Yang N, DiAugustine R, Teng CT. The mouse estrogen receptor-related orphan receptor alpha 1: molecular cloning and estrogen responsiveness. J Mol Endocrinol. 1997;19:299–309. doi: 10.1677/jme.0.0190299. [DOI] [PubMed] [Google Scholar]
- Shiozawa T, Shih HC, Miyamoto T, Feng YZ, Uchikawa J, Itoh K, Konishi I. Cyclic changes in the expression of steroid receptor coactivators and corepressors in the normal human endometrium. J Clin Endocrinol Metab. 2003;88:871–878. doi: 10.1210/jc.2002-020946. [DOI] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguere V. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997;17:5400–5409. doi: 10.1128/mcb.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Laganiere J, Mehl IR, Barish GD, Chong LW, Li X, Scheffler IE, Mock DC, Bataille AR, Robert F, et al. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Vanacker JM, Bonnelye E, Delmarre C, Laudet V. Activation of the thyroid hormone receptor alpha gene promoter by the orphan nuclear receptor ERR alpha. Oncogene. 1998;a 17:2429–2435. doi: 10.1038/sj.onc.1202167. [DOI] [PubMed] [Google Scholar]
- Vanacker JM, Delmarre C, Guo X, Laudet V. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ. 1998;b 9:1007–1014. [PubMed] [Google Scholar]
- Vanacker JM, Bonnelye E, Chopin-Delannoy S, Delmarre C, Cavailles V, Laudet V. Transcriptional activities of the orphan nuclear receptor ERR alpha (estrogen receptor-related receptor-alpha) Mol Endocrinol. 1999;13:764–773. doi: 10.1210/mend.13.5.0281. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Kinoshita Y, Hosokawa K, Mori T, Yamaguchi T, Honjo H. Function of estrogen-related receptor alpha in human endometrial cancer. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2005-1990. [DOI] [PubMed] [Google Scholar]
- Yang N, Shigeta H, Shi H, Teng CT. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem. 1996;271:5795–5804. doi: 10.1074/jbc.271.10.5795. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhou D, Chen S. Modulation of aromatase expression in the breast tissue by ERR alpha-1 orphan receptor. Cancer Res. 1998;58:5695–5700. [PubMed] [Google Scholar]
- Zhan X, Dravid G, Ye Z, Hammond H, Shamblott M, Gearhart J, Cheng L. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004;364:163–171. doi: 10.1016/S0140-6736(04)16629-4. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu Z, Wu J, Liu JH, Hyder SM, Antoniou E, Lubahn DB. Identification and characterization of two novel splicing isoforms of human estrogen-related receptor beta. J Clin Endocrinol Metab. 2006;91:569–579. doi: 10.1210/jc.2004-1957. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chipperfield H, Melton DA, Wong WH. A gene regulatory network in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:16438–16443. doi: 10.1073/pnas.0701014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.