Abstract
BACKGROUND
Post-transcriptional modification by SUMOylation is involved in numerous cellular processes including human spermatogenesis. For human male meiosis, we previously showed that the small ubiquitin-related modifier-1 (SUMO-1) protein localizes to chromatin axes in early pachytene spermatocytes, then to kinetochores as meiosis progresses. Here, we delineate possible functional roles based on subcellular localization for SUMO-1 and SUMO-2/3.
METHODS
Western and immunoprecipitation analyses were conducted on proteins isolated from the testis of two normal adult fertile men. Combinatorial immunofluorescence and chromosome-specific fluorescence in situ hybridization analyses were performed on male meiocytes obtained during testicular biopsy from four patients undergoing testicular sperm extraction for assisted reproduction technologies.
RESULTS
The synaptonemal complex (SC) and SC proteins (SCP)-1 and SCP2, but not SCP3, are SUMOylated by SUMO-1 during the pachytene substage. Likewise, two distinct localization patterns for SUMO-1 are identified: a linear pattern co-localized with autosomal SCs and isolated SUMO-1 near the centromeric heterochromatin of chromosomes 9 and 1. In contrast to SUMO-1, which is not detectable prior to pachytene in normal tissue, SUMO-2/3 is identified as early as leptotene and zygotene and in some, but not all, pachytene cells; no linear patterns were detected. Similar to SUMO-1, SUMO-2/3 localizes in two predominant subnuclear patterns: a single, dense signal near the centromere of human chromosome 9 and small, individual foci co-localized with autosomal centromeres.
CONCLUSIONS
Our data suggest that SUMO-1 may be involved in maintenance and/or protection of the autosomal SC. SUMO-2/3, though expressed similarly, may function separately and independently during pachytene in men.
Keywords: small ubiquitin-related modifier-1, men, meiosis, heterochromatin, chromosome
Introduction
The incidence of infertility among couples in North America and Europe ranges from 5% to 15% depending on the clinical definitions used; up to 50% have a male factor that contributes to infertility (Hull et al., 1985; Forti and Krausz, 1998). Male factors include testicular stem cell defects, abnormalities of meiosis and defects of sperm function. In men, meiosis begins with the onset of puberty and spermatogenesis. Meiosis is the specialized cellular division in which each diploid cell replicates its DNA once but undergoes two rounds of cell division to form four haploid sperm. Meiosis I is hallmarked by three unique events: pairing of duplicated, homologous chromosomes, formation of the synaptonemal complex (SC) and exchange of genetic material (recombination), all occurring during meiotic prophase.
New molecular mechanisms involved in the regulation of the meiotic process are being identified. One such novel process is SUMOylation, and the recent identification of the small ubiquitin-related modifier-1 (SUMO-1) protein during the pachytene substage of prophase suggests an involvement of SUMOylation in these events in men (Vigodner et al., 2006). Recent studies in budding yeast and mammals demonstrate the presence of SUMO proteins in meiotic cells, findings suggestive of specialized roles for SUMO and SUMOylation (Yan et al., 2003; Rogers et al., 2004; Vigodner and Morris, 2005; Yamaguchi et al., 2005; Hooker and Roeder, 2006; Sacher et al., 2006; Vigodner et al., 2006).
SUMOylation is a reversible post-translational modification involved in numerous essential processes. SUMOs are a family of eukaryotic proteins frequently associated with transcriptional regulation, protein–protein interactions, heterochromatin modifications and DNA repair (Sapetschnig et al., 2002; Vigodner and Morris, 2005; Di Bacco et al., 2006; Vigodner et al., 2006; Yurchenko et al., 2006). To date, there are four mammalian SUMO proteins, SUMO-1, -2, -3 and -4, which upon activation covalently bond to a lysine residue on a target protein through the action of specific activators and conjugating enzymes (Schwarz et al., 1998; Azuma et al., 2001, 2003; Tatham et al., 2001). Differential activation suggests both target and process-specific regulation (Sapetschnig et al., 2002). SUMO-1 shares 48% and 46% amino acid sequence identity with SUMO-2 and SUMO-3, respectively, which are members of a subfamily (SUMO-2/3) based on their 95% shared amino acid sequence and similar conjugation pathway (Saitoh and Hinchey, 2000).
Recent studies in budding yeast show that SUMOylation is associated with meiotic specific events—formation of the SC (Cheng et al., 2006; Hooker and Roeder, 2006) and homologous recombination (Sacher et al., 2006). The role(s) of SUMO(s) in mammalian meiosis remain undefined. SUMO-1 has been identified in both human and rodent spermatocytes, findings suggestive of a similar role in mammals (Yan et al., 2003; Rogers et al., 2004; Vigodner and Morris, 2005; Yamaguchi et al., 2005; Sacher et al., 2006; Vigodner et al., 2006). In rodents, SUMO-1 is associated exclusively with the sex body during pachytene similar to the association of γ-H2AX and BRCA1, associations postulated to function in meiotic sex chromosome inactivation necessary to silence unpaired chromosomes (Rogers et al., 2004; Turner et al., 2004; Vigodner and Morris, 2005).
Using immunofluorescence (IF) microscopy and chromosome-specific fluorescence in situ hybridization (FISH), we identified interaction(s) between individual SUMO proteins, SUMO-1 and SUMO-2/3, and the SC in over 1200 human spermatocytes. The present study further characterizes SUMO-1 when compared with SUMO-2/3 in human male meiosis by using a meiotic event, namely the formation of the SC, as a developmental guide. To our knowledge, our studies provide the first report of SUMO-2/3 expression in human spermatocytes.
Materials and Methods
Human testicular samples
Testicular samples were obtained, under ongoing Institutional Review Board approval and individual informed consent for the use of discarded tissue, from four individuals undergoing evaluation at Weill Cornell Medical Center for the treatment of azoospermia. Testicular tissue was procured by biopsy for the purpose of testicular sperm extraction (TESE) for ICSI from patients diagnosed with non-obstructive or obstructive azoospermia (Palermo et al., 1999; Schlegel, 1999). Biopsy samples of normal tissue were obtained from otherwise healthy individuals with normal spermatogenesis, but with reproductive tract obstruction because of congenital absence of the vas, or post-vasectomy.
Sample processing, IF and FISH
Cells obtained from testicular tissue were processed using an air-dried technique with the following modifications of a previously described protocol (Barlow and Hultén, 1996). Microscope slides were washed in 0.04% PhotoFlo solution (Eastman Kodak, Rochester, NY, USA) for 2 min, drained and air-dried. Once dried, slides were hydrated for at least 30 min at room temperature (RT) in 1× antibody dilution buffer [ADB; 1% normal donkey serum, 0.3% bovine serum albumin and 0.005% Triton X in phosphate-buffered saline (PBS); Sigma]. For IF, slides were overlaid with a primary anti-sera cocktail—combinations of anti-serum to centromeres (1:1000; Antibodies, Inc., Davis CA, USA), specific SC proteins, namely, SC protein (SCP)3 (1:100; a gift from Dr Terry Ashley, Yale University, CT, USA) or SCP1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and SUMO-1 (1:100; Affinity BioReagents, Golden, CO, USA), SUMO-2/3 (1:100; Cell Signaling Technology, Danvers, MA, USA) or MLH1, a mismatch repair protein (1:75; Calbiochem, San Diego, CA, USA) and coverslips applied for incubations overnight (37°C; humid chamber). Following the incubation of the primary antibodies, slides were washed for 20 min at RT in 1× ADB followed by a second wash in 1× ADB at 4°C for 5–16 h to prepare for the secondary antibodies. Slides were then incubated at 37°C for 90 min in a humid chamber with the secondary antibody cocktail—AMCA donkey anti-human immunoglobulin (Ig)G, Texas Red donkey anti-goat IgG and fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch Laboratories, Bar Harbor, ME, USA). Slides were washed 3× in PBS at RT for 10 min each, immediately mounted in Antifade (BioRad Laboratories, Hercules, CA, USA) and coverslip applied. To further confirm the specificity of the antisera, neutralization experiments were conducted as follows: 2 µg recombinant SUMO protein/µl of antibody was pre-incubated (45 min; RT) prior to hybridization. Slides were stored (4°C) until microscopic analysis.
Using a wide-field fluorescence Zeiss Axioplan 2 microscope (Carl Zeiss, Thornwood, NY, USA), spermatocytes were identified, captured for further analysis using a Hamamatsu Orca ER B/W digital camera (Hamamatsu, Bridgewater, NJ, USA) and coordinates noted for subsequent FISH analyses. All images were captured using a 100× objective and processed using MetaVue acquisition software (Molecular Devices, Sunnyvale, CA, USA).
For chromosomes 1 and 9 dual FISH analysis, previously immunolabeled and analyzed slides were hybridized with probes based on modifications of the manufacturer's protocol. Briefly, slides were refixed in 70% formamide (Ambion, Austin, TX, USA) in 2× standard saline citrate (SSC) at 73°C for 5 min and dehydrated at RT in serial ethanol dilutions of 70%, 85% and 100% ethanol for 1 min each. Slides were allowed to air dry. To each slide, 10 µl of probe mix (1 µl of CEP 1 and CEP 9 centromeric probes for respective chromosomes, 7 µl hybridization buffer and 1 µl H2O) was applied. Slides were incubated overnight in a humid chamber (37°C). Following hybridization, slides underwent several 5 min washes (73°C): first, 50% formamide in 2× SSC allowing coverslip removal; second, 1× SSC wash followed by 4× SSC/0.1% Tween-20. Next, slides were immediately overlaid with Antifade with 4,6-diamidino-2-phenylindole dihydrochloride (Sigma) counterstain and coverslip applied.
FISH-labeled cells identified in the prior pachytene analyses were re-located and re-captured using the same microscope settings and imaging software.
Statistical analysis
Over 100 cells from each sample were analyzed for SUMO-1 and SUMO-2/3. IF patterns were determined and data compiled; chi-square tests were performed using the online Interactive Chi-Square Tests (University of Kansas, Lawrence, KS, USA) to compare pattern frequencies between individuals (see Supplementary Data).
Protein extraction, immunoprecipitation and Western analysis
For protein and SUMOylation analyses using immunoprecipitation (IP) methods, total protein lysates were prepared from normal human testis, obtained from the whole testis of normal adult men (26 and 27 years of age; hT1; hT2, respectively). The protein concentration was determined by the Bradford assay (BioRad). Individual sets of protein lysates were used for IP experiments and Western analyses. The equally split sample (500 µg each) of each protein lysate was diluted in 500 µl of TPER extraction reagent (PIERCE, Rockford, IL, USA) with protease inhibitors at a final concentration of 1 mM dithiothreitol (DTT), 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride and 2 µg/ml aprotinin, pepstein, leupeptin (Sigma). To each set of protein lysate or no-protein-lysate sample, either 5 µl of anti-SUMO-1 (21C7; Zymed, Carlsbad, CA, USA) or 5 µl of anti-SCP1 (Novus, Littleton, CO, USA) antisera was added. The SUMO-1 epitope recognized by monoclonal antibody 21C7 is absent in SUMO-2/3 and the antibody detects only SUMO-1, as demonstrated previously in studies with these three human SUMO proteins (Matunis et al., 1996; Saitoh and Hinchey, 2000), providing further assurance of specificity. In parallel, the lysate and antibody mixtures were incubated overnight with gentle agitation (4°C). Specific antibodies were captured by incubation with 50 µl of EZView Red® protein A Affinity Gel (Sigma) (1.5 h at 4°C). The beads were then washed three times using the TPER reagent and centrifugation (30 s, 8200g). The pellets were then dissolved in 50 µl of NuPAGE® LDS sample buffer (Invitrogen, Carlsbad, CA, USA), with DTT (50 mM), and boiled for 5 min. The proteins in each immunoselected sample were subjected to separation by polyacrylamide gel electrophoresis (PAGE). Using an aliquot (25 µl) of each collected sample, the proteins were separated by PAGE using both 10% NuPAGE Bis–Tris and 3–8% NuPAGE–Tris-acetate gels (Invitrogen). The immunoselected and separated proteins were then transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA). For Western analysis, each membrane was sequentially probed using specific primary antibodies in 5% non-fat dry milk (BioRad)/1× Tris-buffered saline/1% Tween-20 (overnight, 4°C). For sequential immunoblotting, the absence of carry-over residual signals was confirmed before the particular membrane was re-probed.
For Western analyses, the primary antibodies used were SCP1 (T-17), SCP2 (K-13) (Santa Cruz, Santa Cruz, CA, USA) and SCP3 (BD Biosciences, Franklin Lakes, NJ, USA or Novus as indicated). Immunoblots were developed using horse-radish peroxidase-conjugated secondary antibody and ECL Plus® Western blotting reagents (Amersham Pharmacia Biotech, Buckinghamshire, UK) for detection of specific signals.
Results
Analysis of SUMOylation in human spermatocytes
More than 1200 spermatocytes were analyzed using the testicular biopsy materials from TESE for patients with defined testicular histology. The precise localization of SUMO-1 and SUMO-2/3 was determined in pachytene spermatocytes from four individuals, whose ages range from 28 to 46 years; each presented with a different clinical diagnosis (Table I). Patient A was diagnosed with obstructive azoospermia due to congenital bilateral absence of the vas deferens with qualitatively normal spermatogenesis. This biopsy material was used to represent a ‘control’ as this patient has qualitatively normal meiosis, documented by histology and sperm retrieval. No less than 100 spermatocytes were analyzed from each individual to determine intra- and inter-individual staining patterns among the present sample. A total of 587 cells were collected for SUMO-1 analysis and 780 for SUMO-2/3 analysis. In addition to our prior observations (Vigodner et al., 2006), three novel SUMO-1 staining patterns were identified.
Table I.
Patient sample demographics with SUMO-1 and SUMO-2/3 pattern distribution.
| ID | Age (years) | Spermatogenesis/diagnosisa | Pregnancy (ART) | SUMO-1 |
SUMO-2/3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linear n (%) | Focal n (%) | Dual n (%) | Centromeric n (%) | Focal n (%) | Scatter n (%) | None n (%) | ||||
| A | 34 | Normal; obstructedb | Yes | 21 (19) | 38 (35) | 50 (46) | 55 (24) | 119 (52) | 12 (5) | 43 (19) |
| B | 28 | Hypospermatogenesis | No | 61 (59) | 38 (36) | 5 (5) | 99 (48) | 34 (16) | 1 (0) | 74 (36) |
| C | 37 | Hypogonadotrophic hypogonadismc | No | 41 (23) | 39 (21) | 103 (56) | 78 (39) | 94 (47) | 22 (11) | 7 (3) |
| D | 46 | Normal; non-tumor tissued | No | 79 (45) | 57 (33) | 38 (22) | 56 (39) | 57 (40) | 5 (4) | 24 (17) |
aClinical findings including histopathology.
bCBAVD (obstructed); congenital bilateral absence of the vas deferens, absence of cystic fibrosis disease.
cKallmann syndrome.
dNormal spermatogenesis in tissue from patient with a seminoma.
ART, assisted reproduction technology.
SUMO-1 forms three different patterns during pachytene
Three meiosis-specific events are conserved across species: pairing of duplicated chromosomes, SC formation that tethers the paired homologues and homologous recombination, the physical exchange of DNA between homologous chromosomes, not sister chromatids. Previously, we localized SUMO-1 within human spermatocytes at pachytene (Vigodner et al., 2006). Herein, we demonstrate that specific SCPs, SCP1 and SCP2, are SUMOylated in the human testis. To further characterize its potential roles, SUMO-1 was visualized in conjugation with other markers of the pachytene stage and chromosomal structural components, SCPs and centromeres. For all individuals analyzed, there was a subset of pachytene cells that showed a linear pattern co-localized with the SC of all autosomal chromosomes (Fig. 1A–C). When present in its linear form, SUMO-1, is not typically present in the γ-H2AX and BRCA1-labeled sex body; nor is it associated with synaptic defects (e.g. gaps or splits) often displayed at the centromeric heterochromatin of human chromosomes 1 and 9 (Fig. 1) (Barlow and Hultén, 1996; Sun et al., 2005; Codina-Pascual et al., 2006). The SUMO-1 linear pattern was characteristic and maintained in spermatocytes even when SCP3 was not labeled concurrently (Fig. 1D).
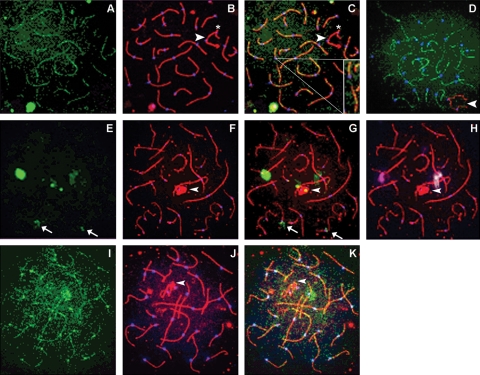
Figure 1:
During early pachytene, SUMO-1 is observed in three different patterns.
Human spermatocytes were analyzed for SUMO-1 (green), SC protein (SCP)3 (red; B and C, F–H, J and K) and centromeres (blue). (A) SUMO-1 forms light, dotted linear structures similar to those identified as lateral SC elements by SCP3. (B) SUMO-1 seen in linear structures in early human pachytene spermatocytes. Merging of both SUMO-1 and SCP3 (C) showed co-localization on autosomal chromosomes. SUMO-1 was co-localized with the SC only at synapsed regions; asynapsed SCP3 negative regions are also SUMO-1 negative (inset of individual autosomal chromosome in C). The paired sex body is also devoid of SUMO-1 (arrowhead), except at the pseudo-autosomal region, where there is a small region of SUMO-1 (asterisk). (D) Another early pachytene spermatocyte that SUMO-1 displays linear structures in the absence of SCP3. X and Y chromosomes are labeled by BRCA-1 (red). In a second subset of human pachytene spermatocytes, SUMO-1 is consistently observed as a large, dense focus at or near the centromere of chromosome 9. (E) SUMO-1 has one large, extremely bright signal, which when merged with the SCP3 and centromere signals (G) is found to associate at the centromeric region of an autosomal chromosome. In this cell, SUMO-1 also has three lighter, secondary signals that also associate near autosomal centromeres and two small foci that are co-localized with the sex body. Identification of human chromosomes 1 and 9 by chromosome-specific FISH analysis demonstrate that the largest and brightest SUMO-1 signal in E is always associated at or near the centromeric region of chromosome 9 (magenta FISH signal in H). The second FISH signal, that of human chromosome 1 (white; H) shows a co-localization of chromosome 1 with the largest secondary SUMO-1 focal association. Additionally, merging of the SUMO-1 image with SCs and centromeres showed that lighter secondary SUMO-1 signals were associated with unidentified human acrocentric chromosomes (arrow). In a fourth mid-pachytene human spermatocyte (I–K), a third distinct SUMO-1 pattern can be resolved: linear structures co-localized with synapsed SCs, a single focal association at chromosome 9 and co-localized centromeric signals on all autosomal chromosomes. All images, ×1000.
In a second subset of pachytene spermatocytes, SUMO-1 presented as a large, densely stained, cloud-like display at one-to-five nuclear areas (Fig. 1E–H). These focal SUMO-1 associations were typically located at or near centromeric heterochromatic regions, as determined with either SCP3 or SCP1 and centromeres. FISH analysis showed these to be associated most often with chromosome 9 (9qh), chromosome 1 (1qh) and, to a lesser extent, several acrocentric chromosomes, which were not further identified. However, SUMO-1 associations with chromosomes other than 9 or 1 were lighter and more diffuse.
In all but one sample, a large subset of pachytene cells displayed both morphologies (Fig. 1I–K). In such dual patterned cells, SUMO-1 was present in both its linear form coincident to the SC and focally at the centromeric heterochromatin of chromosomes 9 and 1.
In experiments with recombinant protein-neutralized antisera, the characteristic SUMO-1 patterns were abolished in focal and centromeric regions (data not shown).
The ratio of the three distinctive SUMO-1 patterns differs as pachytene progresses
Pachytene cells were substaged based on sex body morphology using the criterion that early pachytene display the X and Y chromosomes as two linear structures that become increasingly interwoven and knotted as pachytene progresses. Late pachytene cells characteristically have a very tightly knotted, easily identifiable sex body. Overall analyses of the data indicated that the incidence of the three SUMO-1 patterns changed with progression through pachytene. Combined data showed that at early pachytene, the incidence of linear-only structures, solely focal or dual patterns was ∼58%, 3% and 40%, respectively. As cells entered mid-pachytene, the ratios changed slightly for linear structures and dual patterns, decreasing to 48% and increasing to 49%, respectively, whereas solely focal associations remained unchanged (2.25%). In contrast, spermatocytes in late pachytene showed a marked reversal in SUMO-1 patterns. Strikingly, late pachytene cells showed solely focal associations in 54% of cells when compared with the low incidence (2–3%) in early or mid-pachytene. Linear-only structures decreased to 13%, a 45% decrease from early pachytene; spermatocytes with dual patterns also decreased (33%).
Characterizing the presence of SUMO-1
To determine a time course for the presence of SUMO-1 during meiotic prophase, leptotene, zygotene and diplotene cells based on the morphology of the SC were analyzed. SUMO-1 was not detected prior to the zygotene–pachytene transition (data not shown). Diplotene is the shortest prophase substage and spermatocytes at this stage are difficult to find, detectable in only 0.5% of prophase cells; we did not identify any cells at diplotene. Of the prophase cells analyzed, SUMO-1 was only detected in cells at the zygotene–pachytene transition and pachytene cells, those with complete synapsis of the autosomal chromosomes. Previously, this laboratory reported that SUMO-1 is often observed near or at the sex body during pachytene (Vigodner and Morris, 2005). Our current study confirms and expands on these findings to now include the timing of specific substages, notably late pachytene, after distinct formation of the sex body.
To determine whether linear SUMO-1 was a product of the formation of the SC or associated with the complete SC, SUMO-1 was characterized along with SCP1, a component of the mammalian central element. SUMO-1 did not follow a staining pattern similar to SCP1 during the formation of the SC, short linear structures that are polymerized during zygotene to form long, continuous linear structures at pachytene. Rather, SUMO-1 appeared co-localized with SCP1 only after synapsis was complete (data not shown).
For spermatocytes from Patients B and C, we assessed the recombination frequency using MLH1, a mismatch repair protein; normal range reported for MLH1 foci is between 46.2 ± 3.3 and 52.8 ± 4.8 foci (Lynn et al., 2002; Sun et al., 2005). Our samples were both well within the established norm; Patient B had a mean number of 52 foci and Patient C, a mean of 50 MLH foci per cell. This suggests that variable SUMOylation within pachytene cells does not reflect aberrant recombination frequency.
SC proteins are SUMOylated
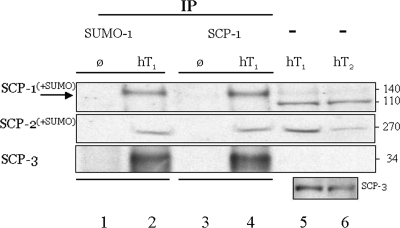
Using the protein lysates from each of the whole human testis obtained from two adult males with normal spermatogenesis, SUMOylation of the SC proteins was demonstrated using IP analyses with both anti-SUMO-1 and SCP1 antisera. We identified SUMOylated forms of SCP1 and SCP2, and in the completed tripartite SUMOylated SC structure (Fig. 2). Although SUMOylation by SUMO-1 was demonstrated for the SC complex and SCP1 and SCP2, in this study, we found no evidence for SUMOylation of SCP3 by SUMO-1.
Figure 2:
The human SC and proteins SCP1 and SCP2, but not SCP3, are SUMOylated in the testis of normal adult men.
Individual protein extracts were prepared from the whole testis of two normal adult men (hT1 and hT2); Ø, no-protein extract but IP experimental procedures in parallel; neat extract: -, no IP; hT1, normal human testis protein, 1 mg sample split for IP [500 µg with anti-SUMO-1 (Zymed) or anti-SCP1 (Novus) antisera]. Illustrated are the results following transfer of proteins electrophoresed using a 3–8% polyacrylamide gel. Human SCP1 (lanes 5 and 6: predicted/observed 114 kDa; ∼130 kDa observed, lanes 2 and 4); human SCP2 (predicted, 176 kDa; observed, ∼270 kDa, lanes 2, 4–6); human SCP3 (predicted/observed 34 kDa, lanes 2, 4–6). Lower inset: Western analysis, lanes 5 and 6; detection of SCP3 using Novus antisera in the non-IP samples hT1 and hT2, different exposure; companion lanes 2 and 4 data are not shown due to non-linear overexposure. Both β-actin and SCP-1 were equally detected in pre-IP samples (not shown).
SUMO-2/3 is present in human meiotic spermatocytes
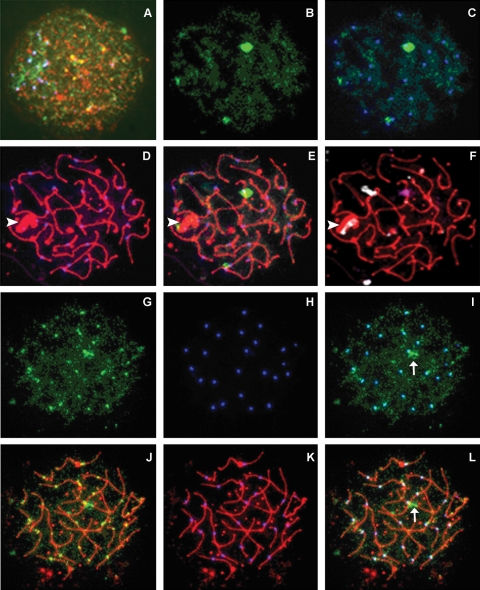
Human samples previously prepared for pachytene analysis were labeled for SCP3, centromeres and SUMO-2/3. In all of the samples analyzed, SUMO-2/3 was identified in some, but not all, pachytene cells. Additionally, in contrast to SUMO-1, which was not detectable prior to pachytene, SUMO-2/3 was observed in cells as early as leptotene and zygotene (Fig. 3A). Strikingly, no linear patterns were detected for SUMO-2/3. Similar to SUMO-1, SUMO-2/3 was localized in two predominant patterns: a single, dense cloud-like array near the centromere of a particular chromosome (Fig. 3B–F), similarly labeled focal associations, and individual foci co-localized with the autosomal centromeres (Fig. 3G–L). FISH analysis confirmed that analogous to the focal associations identified with SUMO-1, the dense focal associations of SUMO-2/3 were nearly always associated with the centromeric region of chromosome 9 (Fig. 3F). When combined, the data showed that focal associations were identified in 39% (n = 304/780) and centromeric associations in 37% (n = 288/780) of all analyzed nuclei. SUMO-2/3 was not detectable in 19% of spermatocytes (n = 148/780). The remainder of cells (5%; n = 40/780) was scored as having somewhat diffuse, ‘scattered’ punctuate SUMO-2/3 staining patterns.
Figure 3:
SUMO-2/3 is identified in human pachytene spermatocytes.
Meiotic spermatocytes were triple-labeled for SUMO-2/3 (green), SCP3 (red) and centromere (blue). SUMO-2/3 is detected in a scattered pattern in meiosis as early as leptotene (A). In some pachytene cells (B–F), SUMO-2/3 (alone in B, merged with centromeres in C, and merged with SCs and centromeres in E) appears as a bright, dense cloud at, or near, the centromere of human chromosome 9 (magenta FISH signal in F); smaller and lighter signals associate with the sex body (arrowhead) and the centromeric region of an unidentified chromosome. In a second pachytene cell (G–L), SUMO-2/3 forms small punctate foci similar to meiotic centromeres (G and J); merging the two images (I and L) shows complete co-localization of both signals at all centromeres. This cell also has a small, brightly stained focal association (arrow). SCs and centromeres are shown in D and K, respectively. All images, ×1000.
To our knowledge, these data are the first observations indicating that SUMO-2/3 is specifically involved in the meiotic process of human spermatogenesis.
Analysis of SUMO-1 and SUMO-2/3 showed that SUMOylation patterns vary between individuals with different clinical presentations, and a distinctively different SUMO-2/3 pattern was observed for Patient B compared with the others (see Supplementary Figure 1, and chi-square analyses in Supplementary Data).
Discussion
In this study, we further delineate the relationships between SUMOs and SUMOylation in human male meiosis. The distinct subcellular nuclear localization patterns identified previously for SUMO-1 (Vigodner et al., 2006) are further characterized and the first observations of SUMO-2/3 in human pachytene spermatocytes are demonstrated. We show that SCP1 and SCP2 are SUMOylated by SUMO-1 conjugation. Characterization of pachytene substages now clearly demonstrates linear patterns of SUMO-1 in early pachytene, a dual morphology during mid-pachytene, which may constitute a transitional stage and, at late pachytene, solely focal associations at 1qh and 9qh. In the testis of fertile adult men, SCP1, SCP2 and the complex are SUMOylated by SUMO-1. In this study, we did not detect SUMOylation of SCP3. SUMO-1 appears associated in the mature complex with SCP3 by the highly structured scaffold. Using the reported sequences for the three SCPs, and the SUMOplot™ Analysis Program (Abgent, http://www.abgent.com.cn/dir/sumoplot), multiple high probability SUMOylation sites are predicted. On the basis of such modeling, SCP1 (NP_003167.2) has nine potential SUMO sites and SCP2 (NP_055073.2) has eight potential sites. Given our findings, interestingly SCP3 (NP_710161) has only one potential site. These predictions and our results are therefore consistent with an important role for SUMOylation in the post-translational modification of the SC, especially one likely to involve both SCP1 and SCP2.
In all samples analyzed, the majority of spermatocytes had a dense SUMO-1 localization at or near the centromeric region of chromosome 9, whereas less frequently and abundantly at chromosome 1. Synaptic defects, incomplete synapsis, synaptic discontinuities and asynapsis are often identified in infertile male patients and are thought to be associated with reduced fertility (Judis et al., 2004; Sun et al., 2004; Guichaoua et al., 2005; Codina-Pascual et al., 2006). Human chromosome 9 has the largest autosomal region of centric heterochromatin of all human chromosomes; others are chromosomes 1 and 16 (Madon et al., 2005). The consistent SUMOylation of the centromeric heterochromatin of human chromosome 9 is noteworthy because of this unusual genomic structure. It has been previously shown that these regions are the last to synapse and may result in spermatogeneic arrest due to synaptic anomalies (Antonelli et al., 2000; Codina-Pascual et al., 2006). Gaps and synaptic discontinuities were often observed in our sample population; however, there was no apparent meiotic or spermatogeneic disruptions reflected in our SUMOylation data. SUMOylation near 9qh was observed in cells with or without synaptic gaps. We propose that the targeted SUMOylation of molecules here has a protective advantage for progression through pachytene. How the SUMOylated proteins, or SUMOs, precisely coordinate this response remains to be determined.
Analysis of both SUMO-1 and SUMO-2/3 demonstrates that SUMOylation patterns vary in individuals with particular clinical presentations (Table I and Supplementary Data, chi-square analyses). It remains unclear whether the SUMOylation patterns strictly correlate with a particular cause of infertility or represent, in part, variations on a biological theme.
Strikingly, a distinctively different SUMO-2/3 pattern was observed for Patient B in comparison with all others (see Supplementary Figure 1). Interestingly, this patient exhibited an apparent hypoSUMOylation pattern, remarkable for its conspicuous lack of SUMO-2/3, particularly at the centromeric heterochromatin of human chromosome 9. Of note, Patient B had severely defective sperm production with azoospermia and hypospermatogenesis on testicular histology. Although there is no single measure of defective meiosis, patients with severe hypospermatogenesis may be at increased risk for having disordered meiosis (Gonsalves et al., 2004). In this study, the other patients all show a fairly consistent proportion of cells with an easily identified SUMO-2/3 pattern, staining at all centromeres or focal associations at human chromosome 9 between 75% and 85% of all pachytene spermatocytes. The remainder showed either no detectable SUMO-2/3 staining or a scattered pattern with varying amounts. In contrast, Patient B displayed an overall decrease in the incidence of SUMO-2/3 stained cells; 64% showed SUMO-2/3 associated with the centromeres or focal association at 9qh. For pachytene spermatocytes that are SUMOylated, a considerably greater proportion of cells displayed a universal centromeric association, perhaps at the expense of focal association at 9qh, which showed an overall frequency of 13%; the range for the other samples was 29–40%. Of the other 36% of the cells not easily defined, <1% showed a scattered SUMO-2/3 pattern, with SUMO-2/3 undetectable in most cells.
When comparing the frequency of SUMO-1 morphologies (linear, focal, dual and other) among all pachytene spermatocytes from the four samples, there are striking apparent differences between them. Although there is no overall decrease in SUMOylation by SUMO-1 in Patient B and the focal association of SUMO-1 at the centromeric heterochromatin of chromosomes 9 and 1 appears within the normal range, there is a sizeable increase in the frequency of ‘other’ SUMO-1 morphologies compared with the other patients. This patient also shows a comparable decrease in the cells with the dual pattern. Though the immunodetection pattern for SUMO-1 appears individual-specific, the SUMOylation pattern of both SUMO-1 and SUMO-2/3 in pachytene spermatocytes from Patient B is suggestive of hypoSUMOylation, a finding that may correlate with the clinical observation of hypospermatogenesis.
Of further note is Patient C, diagnosed with Kallmann syndrome, whose germ cells appear to be hyperSUMOylated by SUMO-2/3 in comparison with those of the others evaluated with different diagnoses. Kallmann syndrome is an X-linked disorder associated with disruption in the hormone GnRH production, resulting in a lack of FSH and LH and hypogonadotrophic hypogonadism, conditions represented by both reduction and impairment in the fidelity of spermatogenesis (Diemer and Desjardins, 1999). Patients diagnosed with Kallmann syndrome are typically treated with testosterone therapy to induce puberty and secondary male sexual characteristics (Diemer and Desjardins, 1999). Patient C was subsequently treated with HCG and FSH hormone therapy to induce spermatogenesis prior to TESE (Raivio et al., 2007). This individual shows an overall increased SUMOylation by SUMO-2/3, specifically in the ‘scatter’ pattern (Supplementary Figure 1, panel II, C). Patient C shows a 10% increase in the total number of pachytene cells with identifiable SUMO-2/3 staining when compared with the control individual (Patient A).
In conclusion, we have found novel SUMOylation patterns within human male spermatocytes at the pachytene stage of meiosis. Our data are consistent with a role for SUMO-1 in maintenance of the SC scaffold. Moreover, focal associations at late pachytene directed to the centromeric heterochromatin for particular chromosomes are suggestive of a role in protection of chromosomes with synaptic irregularities. Separate and specific roles for SUMO-1 as cells progress through pachytene are indicated by our findings. Importantly, we show that in the normal human testis, the SC, and SCP1, SCP2 but not SCP3 are SUMOylated. Our current studies are the first to demonstrate that SUMOylation by SUMO-2/3 occurs in human spermatocytes. Taken together, these data suggest that SUMOylation patterns can be correlated with aberrant meiotic phenotypes in men, findings that require further investigation to establish causality in male infertility.
Supplementary Data
Supplementary Data are available at http://humrep.oxfordjournals.org/.
Funding
This work was supported by grant NIH R01HD-039024 (P.L.M.). Fellowship support (P.W.B.) provided by the F.M. Kirby Foundation.
Supplementary Material
Acknowledgements
We acknowledge expert assistance by Peggy King, R.N., Lyann Mitchell and Catherine Rapelje.
References
- Antonelli A, Gandini L, Petrinelli P, Marcucci L, Elli R, Lombardo F, Dondero F, Lenzi A. Chromosomal alterations and male infertility. J Endocrinol Invest. 2000;23:677–683. doi: 10.1007/BF03343793. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Tan SH, Cavenagh MM, Ainsztein AM, Saitoh H, Dasso M. Expression and regulation of the mammalian SUMO-1 E1 enzyme. FASEB J. 2001;15:1825–1827. doi: 10.1096/fj.00-0818fje. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Arnaoutov A, Dasso M. SUMO-2/3 regulates topoisomerase II in mitosis. J Cell Biol. 2003;163:477–487. doi: 10.1083/jcb.200304088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AL, Hultén MA. Combined immunocytogenetic and molecular cytogenetic analysis of meiosis I human spermatocytes. Chromosome Res. 1996;4:562–573. doi: 10.1007/BF02261719. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Lo YH, Liang SS, Ti SC, Lin FM, Yeh CH, Huang HY, Wang TF. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 2006;20:2067–2081. doi: 10.1101/gad.1430406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina-Pascual M, Navarro J, Oliver-Bonet M, Kraus J, Speicher MR, Arango O, Egozcue J, Benet J. Behaviour of human heterochromatic regions during the synapsis of homologous chromosomes. Hum Reprod. 2006;21:1490–1497. doi: 10.1093/humrep/del028. [DOI] [PubMed] [Google Scholar]
- Di Bacco A, Ouyang J, Lee HY, Catic A, Ploegh H, Gill G. The SUMO-specific protease SENP5 is required for cell division. Mol Cell Biol. 2006;26:4489–4498. doi: 10.1128/MCB.02301-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer T, Desjardins C. Developmental and genetic disorders in spermatogenesis. Hum Reprod Update. 1999;5:120–140. doi: 10.1093/humupd/5.2.120. [DOI] [PubMed] [Google Scholar]
- Forti G, Krausz C. Clinical review 100: evaluation and treatment of the infertile couple. J Clin Endocrinol Metab. 1998;83:4177–4188. doi: 10.1210/jcem.83.12.5296. [DOI] [PubMed] [Google Scholar]
- Gonsalves J, Sun F, Schlegel PN, Turek PJ, Hopps CV, Greene C, Martin RH, Pera RA. Defective recombination in infertile men. Hum Mol Genet. 2004;13:2875–2883. doi: 10.1093/hmg/ddh302. [DOI] [PubMed] [Google Scholar]
- Guichaoua MR, Perrin J, Metzler-Guillemain C, Saias-Magnan J, Giorgi R, Grillo JM. Meiotic anomalies in infertile men with severe spermatogenic defects. Hum Reprod. 2005;20:1897–1902. doi: 10.1093/humrep/deh868. [DOI] [PubMed] [Google Scholar]
- Hooker GW, Roeder GS. A role for SUMO in meiotic chromosome synapsis. Curr Biol. 2006;16:1238–1243. doi: 10.1016/j.cub.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judis L, Chan ER, Schwartz S, Seftel A, Hassold T. Meiosis I arrest and azoospermia in an infertile male explained by failure of formation of a component of the synaptonemal complex. Fertil Steril. 2004;81:205–209. doi: 10.1016/j.fertnstert.2003.05.021. [DOI] [PubMed] [Google Scholar]
- Lynn A, Koehler KE, Judis L, Chan ER, Cherry JP, Schwartz S, Seftel A, Hunt PA, Hassold TJ. Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science. 2002;296:2222–2225. doi: 10.1126/science.1071220. [DOI] [PubMed] [Google Scholar]
- Madon PF, Athalye AS, Parikh FR. Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod Biomed Online. 2005;11:726–732. doi: 10.1016/s1472-6483(10)61691-4. [DOI] [PubMed] [Google Scholar]
- Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo GD, Schlegel PN, Hariprashad JJ, Ergun B, Mielnik A, Zaninovic N, Veeck LL, Rosenwaks Z. Fertilization and pregnancy outcome with intracytoplasmic sperm injection for azoospermic men. Hum Reprod. 1999;14:741–748. doi: 10.1093/humrep/14.3.741. [DOI] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–873. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- Rogers RS, Inselman A, Handel MA, Matunis MJ. SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma. 2004;113:233–243. doi: 10.1007/s00412-004-0311-7. [DOI] [PubMed] [Google Scholar]
- Sacher M, Pfander B, Hoege C, Jentsch S. Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat Cell Biol. 2006;8:1284–1290. doi: 10.1038/ncb1488. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Sapetschnig A, Rischitor G, Braun H, Doll A, Schergaut M, Melchior F, Suske G. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 2002;21:5206–5215. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–135. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- Schwarz SE, Matuschewski K, Liakopoulos D, Scheffner M, Jentsch S. The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc Natl Acad Sci USA. 1998;95:560–564. doi: 10.1073/pnas.95.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Kozak G, Scott S, Trpkov K, Ko E, Mikhaail-Philips M, Bestor TH, Moens P, Martin RH. Meiotic defects in a man with non-obstructive azoospermia: case report. Hum Reprod. 2004;19:1770–1773. doi: 10.1093/humrep/deh335. [DOI] [PubMed] [Google Scholar]
- Sun F, Trpkov K, Rademaker A, Ko E, Martin RH. Variation in meiotic recombination frequencies among human males. Hum Genet. 2005;116:172–178. doi: 10.1007/s00439-004-1215-6. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem. 2001;276:35368–35374. doi: 10.1074/jbc.M104214200. [DOI] [PubMed] [Google Scholar]
- Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14:2135–2142. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Vigodner M, Morris PL. Testicular expression of small ubiquitin-related modifier-1 (SUMO-1) supports multiple roles in spermatogenesis: silencing of sex chromosomes in spermatocytes, spermatid microtubule nucleation, and nuclear reshaping. Dev Biol. 2005;282:480–492. doi: 10.1016/j.ydbio.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Vigodner M, Ishikawa T, Schlegel PN, Morris PL. SUMO-1, human male germ cell development, and the androgen receptor in the testis of men with normal and abnormal spermatogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1022–E1033. doi: 10.1152/ajpendo.00527.2005. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sharma P, Athanasiou M, Kumar A, Yamada S, Kuehn MR. Mutation of SENP1/SuPr-2 reveals an essential role for desumoylation in mouse development. Mol Cell Biol. 2005;25:5171–5182. doi: 10.1128/MCB.25.12.5171-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Santti H, Janne OA, Palvimo JJ, Toppari J. Expression of the E3 SUMO-1 ligases PIASx and PIAS1 during spermatogenesis in the rat. Gene Expr Patterns. 2003;3:301–308. doi: 10.1016/s1567-133x(03)00045-0. [DOI] [PubMed] [Google Scholar]
- Yurchenko V, Xue Z, Sadofsky MJ. SUMO modification of human XRCC4 regulates its localization and function in DNA double-strand break repair. Mol Cell Biol. 2006;26:1786–1794. doi: 10.1128/MCB.26.5.1786-1794.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.