Abstract
Aryl hydrocarbon receptor (AhR) activation by 2,3,7,8-tetrachlorodibenzio-p-dioxin (TCDD) leads to immune suppression associated with the induction of regulatory T cells (Treg) expressing the transcription factor Foxp3. The immunological mechanisms of suppression are not well understood however dendritic cells (DC) are considered a key target for AhR-mediated immune suppression. Here we show that activation of AhR by TCDD induces DC indoleamine-2,3-dioxygenase 1 (IDO1) and indoleamine 2,3-dioxygenase-like protein (IDO2). Induction of IDO1 and IDO2 was also found in lung and spleen associated with an increase of the Treg marker Foxp3 in spleen of TCDD-treated C57BL/6 mice, which is suppressed by inhibition of IDO. These data indicate that AhR-activation is an important signaling pathway for IDO expression and suggest a critical role of IDO in the mechanism leading to the generation of Treg that mediates the immune suppression through activation of AhR.
Keywords: AhR, DC, CD86, Foxp3, IDO1, IDO2, TCDD
Introduction
Activation of the AhR through environmental pollutants like dioxin is well known to induce profound suppression of immune responses especially T cell dependent responses which is the primary toxicity associated with the exposure to dioxin [1]. Emerging from recent studies it becomes clear that the AhR can regulate the generation of Treg or pro-inflammatory T cells producing IL-17 (TH17). Activation of the AhR by TCDD induces Treg whereas ligation of AhR by 6-formylindolo[3,2-b]carbazole (FICZ) leads to differentiation of TH17 cell which produces interleukin (IL)-22 [2,3]. FICZ is a tryptophan-derived photoproduct that is thought to be an endogenous ligand with high affinity for the AhR [4]. It has long been recognized that derivates of tryptophan can activate AhR. In contrast to FICZ, the tryptophan-derived AhR endogenous ligand 2-(1'H-indole-3'-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) suppressed autoimmune encephalomyelitis (EAE) in mice like TCDD [2]. TCDD is a prototype of a class of environmental contaminants with high affinity to the AhR. AhR activation by TCDD has broad effects on the immune system and the AhR-dependent induction of a T-cell population with characteristics of Treg has been described earlier [5]. Interestingly, induction of Treg after activation of AhR by an anti-inflammatory compound (VAF347) has been found to be mediated through DCs [6]. Recent studies of T cell differentiation regulated by AhR proposes that activation of AhR dependent on its activating ligand can induce immunity or tolerance.
More recently DCs have been recognized as potential target for TCDD. DCs are the most potent antigen-presenting cells (APC) and are key regulators of immunity or tolerance. One of the most potent ways in which DCs can create immunosuppression is by activating Treg cells [7]. Studies by Munn et al. [8] show, that the expression of IDO, an enzyme that degrades the essential amino acid tryptophan, is a major mechanism of peripheral tolerance in subsets of DCs. IDO catalyzes the initial and rate-limiting step in the degradation of tryptophan along the kynurenine pathway [9].
Materials and methods
Reagents and Antibodies
Alpha-methyl-dl-tryptophan, L-Kynurenine, L-tryptophan, and 1-methyltryptophan (1-MT) were obtained from SIGMA. FICZ was purchased from BIOMOL. Monoclonal IDO1 antibody (Chemicon-Millipore) was used for Western blot analyses. Human GM-CSF (R&D Systems) and IL-4 (Biosource) were used for DC differentiation.
DC culture system
Human monocytic cell line U937 was obtained from ATTC and maintained in RPMI 1640 medium. To generate DCs, U937 cells were cultured for 3 days in growth medium supplemented with GM-CSF (20 ng/ml) and IL-4 (40 ng/ml) as previously described [8]. Under these conditions, cells were cultured in presence of AhR agonists TCDD, FICZ or AhR antagonist MNF. As a positive control to induce IDO in DCs, cells were cotreated with INFγ (100 U/ml) during DC differentiation.
Mice
C57BL/6J mice were purchased from Jackson Laboratory. AhR-deficient mice were generated and kindly provided by Christopher Bradfield. 1-MT, a pharmacological inhibitor of IDO was dissolved in water and adjusted to pH 9.9 before use. Mice were given 1-MT in the drinking water (1 mg/ml) one day before administration of TCDD or FICZ and continuing until mice were sacrificed.
Flow cytometric analysis
Prior to staining U937 cells were treated 3 days with medium containing GM-CSF (20 µg/ml) and IL-4 (40 U/ml) and supplemented with TCDD or DMSO. After treatment cells were double labeled with anti HLA-DR PE, biotinylated anti-CD86 (eBioscience) followed by streptavidin APC. Dead cells were excluded by propidium iodide. Data were acquired on a LSR II cytometer (Becton-Dickinson). Post acquisition data analysis was performed using Flowjo software (Treestar). Individual histograms depict a minimum of 7,000 PI negative events.
Induction and Assay of IDO Activity
Cells were harvested by centrifugation and washed twice with phosphate buffered saline, frozen at −70°C. Cell pellets and spleen samples were lyophilized overnight. The powdered cell and tissue residues were resuspended in PBS and, after centrifugation, the supernatants were assayed for IDO activity by the method of Takikawa et al. [10] with some modifications.
Quantitative real-time reverse transcription-polymerase chain reaction (RTPCR) analysis
For preparation of total RNA the tissues were homogenized first in TRIzol using a TissueLyser (Valencia, CA). The RNA was extracted with chloroform and further purified with a high pure RNA isolation kit. cDNA synthesis was carried out as previously described [11]. Quantitative detection of mRNA level was performed with a LightCycler Instrument (Roche) using the Fast Real-Time SYBR Green PCR Kit (Qiagen) according to the manufacturer's instructions. The primers for each gene were designed using OLIGO primer analysis software, provided by Steve Rosen and Whitehead Institute/MIT Center for Genome Research. Primer sequences are available upon request. The intra-assay variability was < 7%.
Statistics
All data were obtained from at least three independent experiments performed in duplicate, and the results are given as the mean ± the standard error of the mean. To demonstrate statistical significance, the variables were examined for one-sided Student's t test. The level of significance was p<0.05.
Results
Effect of AhR-activation on DC differentiation and function
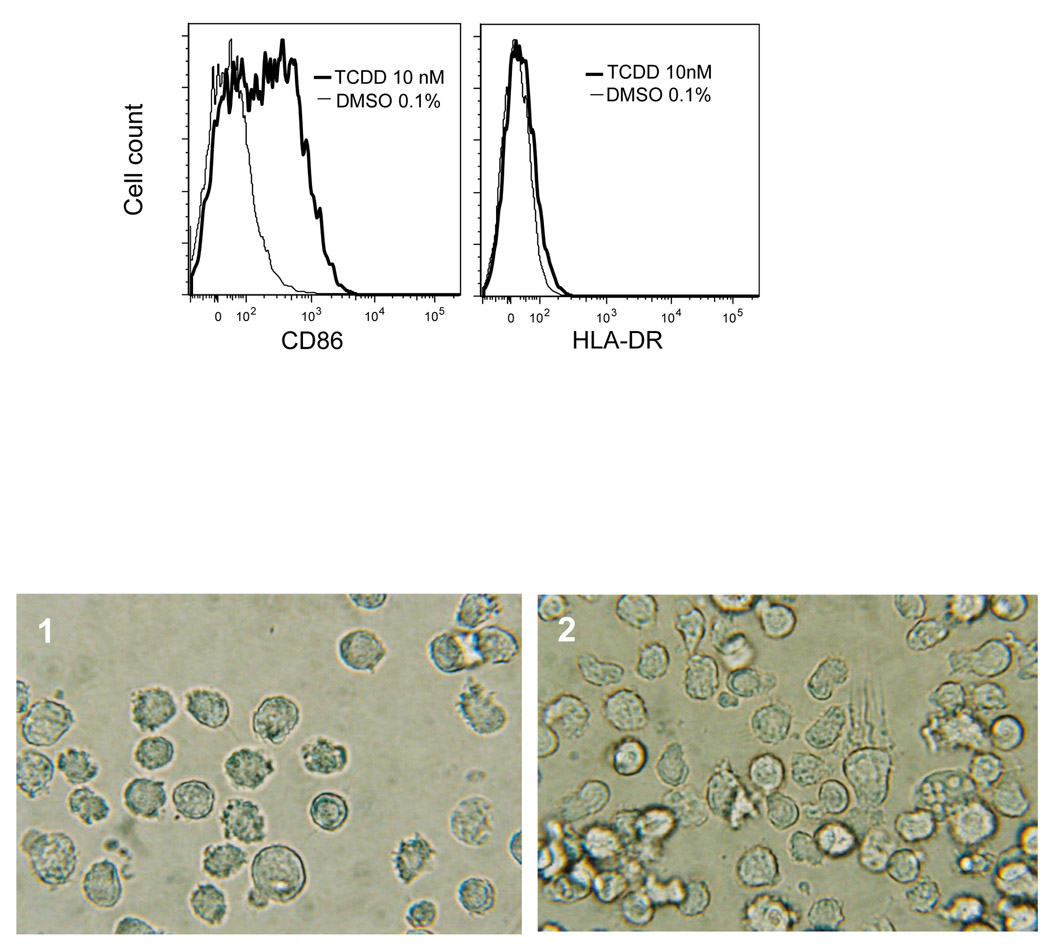
Table 1 shows the expression level of phenotypic surface markers of naive U937 and differentiated DCs in absence or presence of 3'-methoxy-4'-nitroflavone (MNF), TCDD, or FICZ. In naive U937 cells, surface marker specific for DC such as CD1a, CD54 (ICAM-1), and CD86 are relatively low expressed. Cells treated with GM-CSF/IL-4 augmented expression of all surface markers most significantly CD54 and CD86. Expression of CD86 and CD1a was increased by TCDD, and FICZ, whereas MNF significantly suppressed the GM-CSF/IL-4-induced expression of CD86 and CD1a. The elevated expression of CD86 and a slight increase of the human major histocompatibility complex (MHC) class II (HLA-DR) on the surface of TCDD-treated U937 DC was confirmed by flow cytometric analysis (Fig. 1A).
Table 1.
Activation-like changes in DC derived from human monocytic U937 naïve cells after AhR activation by TCDD and FICZ. The expression of surface markers CD1a, CD54, and CD86 and chemokines DC-CK1, DC-STAMP and IL-8 were analyzed by real time PCR. The results are expressed as mean ± SD of at least three independent experiments.
| U937 cells | CD1a | CD54 | CD86 | DC-CK1 | DC-STAMP | IL-8 |
|---|---|---|---|---|---|---|
| Naïve | 0.7 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.3 | 0.7 ± 0.4 | 0.6 ± 0.3 | 1.3 ± 0.4 |
| DC Ctrl | 3.5 ± 0.4 | 10.3 ± 0.2 | 17.3 ± 1.1 | 25.6 ± 2.2 | 3.4 ± 0.4 | 37.0 ± 4.1 |
| DC+MNF | 2.5 ± 0.3* | 10.5 ± 0.7 | 10.4* ± 0.7 | 31.8 ± 4.4** | 1.6 ± 0.1* | 21.0 ± 3.2* |
| DC+TCDD | 10.9 ± 0.5** | 10.6 ± 0.9 | 85.3 ± 4.1** | 4.8 ± 1.0* | 8.1 ± 1.3** | 470.2 ± 10.4** |
| DC+FICZ | 9.7 ± 0.4** | 9.6 ± 0.5 | 89.3 ± 2.2** | 3.2 ± 0.3* | 7.5 ± 1.0** | 250.2 ± 9.2** |
p < 0.005 significantly lower than DC Ctrl
p < 0.005 significantly higher than DC Ctrl.
Figure 1. AhR activation by TCDD increases DC differentiation.
(A) Prior to staining U937 cells were treated 3 days with GM-CSF and IL-4 and supplemented with TCDD (heavy lines), or DMSO (thin lines). After treatment cells were labeled with anti-CD86 or anti HLA-DR followed by streptavidin APC. Dead cells were excluded by propidium iodide. (B) Phase contrast micrograph of unlabeled U937 derived DCs. Cells were cultured with GM-CSF and IL-4 for 3 days (1). To activate AhR cells were treated with 10 nM TCDD during DC differentiation (2). X 400.
Next we tested the effect of MNF, TCDD and FICZ on the expression of the dendritic-cell-derived C-C chemokine (DC-CK1), the dendritic-cell-specific transmembrane protein (DC-STAMP), and Interleukin 8 (IL-8). TCDD increased IL-8 expression about 20-fold in U937 cells after stimulation with GM-CSF/IL-4. DC-STAMP was less (2-fold) but also significantly increased by TCDD (Table 1). In contrast, the upregulation of DC-CK1 in differentiated DC control cells was clearly blocked in presence of TCDD. As in the case of DC surface markers, MNF has the contrary effect compared to TCDD by increasing DC-CK1, but decreasing IL-8 and DC-STAMP. Morphologically, the naive U937 cells show spherical and non-adherent aspects, which are slightly changed into an immature DC phenotype by the stimulation with GMCSF/ IL-4. On the other hand, in presence of TCDD, U937 derived DC display morphologic changes into more elongated and stellate cells typical for dendritic cells (Fig. 1B).
Effect of TCDD and FICZ on mediators of active immunosuppression
Here we analyzed the effect of TCDD and FICZ on various potent mediators of active suppression including Fas ligand (FASL), arginase (ARG), programmed cell death ligand (PDL), and IDO1. The expression of FASL was slightly increased in DC differentiated cells in presence of TCDD, whereas ARG2, PDL1 and PDL2 showed no significant change by TCDD (Supplementary Table S1). In contrast IDO1 as well as IDO2 were clearly induced in AhR-activated DC (Fig. 2A and B). IDO1 and IDO2 were very low expressed in DC control cells and in some cases not detectable.
Figure 2. Induction of IDO1 and IDO2 by TCDD in DC is AhR-dependent.
(A) IDO1 and (B) IDO2 expression in monocyte-derived U937 DC after treatment with 100 U/ml INFγ, 100 nM FICZ, or 10 nM TCDD in presence or absence of the AhR antagonist MNF for 3 days. (C) INFγ and TCDD induce IDO enzyme activity in U937-derived DC. Using a colorimetric assay, we measured IDO activity in FICZ-, TCDD- and INFγ- treated DCs. Without INFγ or TCDD, IDO activity was not detectable (n.d.). The same test conditions were performed in presence of the IDO inhibitor 1-MT.
AhR-dependent induction of IDO1 and IDO2 by TCDD but not by INFγ
To examine the induction of IDO1 and IDO2 through activation of the AhR we tested the effect of the prototype ligand TCDD and the tryptophan-derived photoproduct FICZ. TCDD causes significant increases of IDO1 and IDO2 mRNA. Treatment with 100 U/ml INFγ lead to about 500-fold higher mRNA level of IDO1. Treatment with 100 nM FICZ lead only to a 6-fold increase of IDO1 (Fig. 2A). In contrast, IDO2 was only induced by TCDD but not by FICZ or INFγ (Fig. 2B).
To determine, if activation of the AhR is required to induce IDO1 and IDO2, we examined the effect of TCDD and INFγ in DC under cotreatment with the AhR antagonist MNF. Cotreatment with MNF clearly blocked induction of IDO1 and IDO2 by TCDD. On the other hand, induction of IDO1 by INFγ was even more pronounced in presence of MNF compared to cells treated with INFγ alone (Fig. 2A).
In order to verify that induction of IDO mRNA is translated into functional protein we measured enzyme activity of IDO. Without INFγ or TCDD no IDO activity was detectable (Fig. 2C). However, differentiation of DC in presence of TCDD or INFγ for 72 h, produced detectable IDO activity. FICZ did not activate IDO enzymatic activity. The same test conditions were performed in presence of the IDO inhibitor 1-MT, which completely blocked TCDD- as well as INFγ-induced enzymatic activity.
Induction of IDO1 and IDO2 by TCDD in spleen and lung of C57BL/6J mice is AhR-dependent
Control animals exhibited the highest level of IDO1 in lung and spleen (Fig. 3A). Compared to IDO1, the expression of IDO2 is highest in liver and kidney followed by thymus and spleen (Fig. 3B) which is in line with previous studies [12]. Both, IDO1 and IDO2 mRNA expression is most significantly upregulated in spleen and to a lower extent in lung 7 days after a single injection of TCDD (Fig.3A and Supplementary Fig. 1A). Similar increase of IDO1 and IDO2 mRNA in spleen was found after 1 day of exposure to TCDD (Supplementary Fig. S1B). The induced mRNA expression of IDO1 and IDO2 was associated with increased IDO enzymatic activity and IDO1 protein level in spleen of C57BL/6 wild-type mice at day 7 after injection of TCDD (Fig. 3B and C). As shown in DCs, the enzymatic activity of IDO was blocked by the pharmacological inhibitor 1-MT. In order to examine the involvement of the AhR in the TCDD-mediated induction of IDO1 and IDO2, we further analyzed the expression of IDO in AhR-deficient mice. The results show, that treatment with TCDD had no significant effect on IDO1 or IDO2 mRNA expression in spleen, lung, or other tissues of AhR-deficient mice (Supplementary Fig. S2A and B) supporting our data from in vitro that the AhR is required to induce IDO1 and IDO2 by TCDD.
Figure 3. Induction of IDO1 and IDO2 in lung and spleen of C57BL/6 mice in response to TCDD but not FICZ.
(A) IDO1 expression in female wild type C57BL/6 mice. Mice were injected i.p. with a single dose of 15 µg/kg TCDD or 50 µg/kg FICZ. Control animals received the solvent vehicle. After 10 days total RNA from various tissues was subjected to real time PCR. The values are given as relative units. (B) Induction of IDO enzyme activity by AhR activation in spleen of TCDD-treated BL6 wt mice. BL/6 wt mice were treated with a single dose of 15 µg/kg TCDD or 50 µg/kg FICZ. In addition, TCDD- and FICZ-treated mice received the IDO inhibitor 1-MT. * p ≤ 0.01 vs group control. (C) Induction of IDO1 protein in spleen by TCDD. Wild type C57BL/6 animals were treated with a single dose of 15 µg/kg TCDD or 50 µg/kg FICZ for 10 days. Spleen was removed for Western blot analysis of IDO1 and β-actin.
Suppression of the TCDD-induced Treg marker Foxp3 by inhibition of IDO
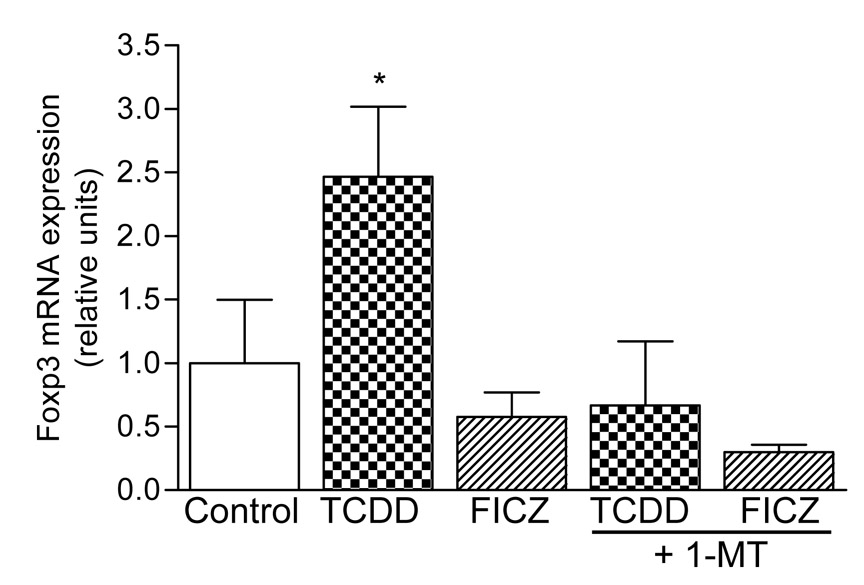
AhR activation by TCDD leads to a significant increase of the transcription factor Foxp3 at day 7 in spleen of C57BL/6 mice (Fig. 4) which indicates the increased differentiation of Treg cells as reported recently [2]. No significant induction of Foxp3 was observed at day 1 or day 3 in spleen of C57BL/6 mice (data not shown). To test if the TCDD-induced expression of Foxp3 is mediated through the increased activity of IDO in wild-type mice, animals were treated with 1-MT, a pharmacological inhibitor of IDO. The results show that 1-MT blocked the TCDD-induced Foxp3 expression in spleen. Foxp3 was not elevated by treatment with the tryptophan derivate FICZ (Fig. 4). No TCDD-mediated increase of Foxp3 was observed in AhR-deficient mice (Supplementary Fig. 3) confirming the AhR-dependent increase of Foxp3 by TCDD as shown by Quintana et al. [2].
Figure 4. The induction of the Treg cell marker Foxp3 in spleen by TCDD is suppressed through inhibition of IDO.
C57BL/6 wt mice were injected with a single dose of 15 µg/kg TCDD or 50 µg/kg FICZ. In addition, TCDD- and FICZ-treated mice received the IDO inhibitor 1-MT. * p ≤ 0.01 vs group control.
Discussion
Here we report that activation of the AhR by the prototypic AhR agonist TCDD, and the tryptophan-derived photoproduct FICZ in human U937 monocyte-derived DCs induced the expression of the CD86 and increased the expression of the surface marker CD1a which may initiate DC precursors to produce mature APCs. In addition, the DC-STAMP and IL-8 were significantly elevated, whereas upregulation of DC-CK1 was blocked during DC differentiation in presence of TCDD or FICZ. It is noteworthy, that the expression of the surface markers and chemokines during DC differentiation were inhibited in presence of the AhR antagonist MNF. These data suggest a critical role of the AhR in the regulation of these surface markers and chemokines and indicate that endogenous AhR ligands or activators might participate in DC differentiation and function. Interestingly, IL-4 has been shown to activate AhR in B cells [13]. The activation-like changes in AhR-activated DCs were associated with morphological changes into more elongated and stellate cells compared to the untreated DCs. DC-expressed chemokines play an important role in the interaction between DCs and T cells and the induction of immune responses. The main T cell population attracted by DC-CK1 and IL-8 consists of naïve resting T cells. Thus, the block of DC-CK1 through activation of AhR may explain a less active DC in terms of attracting naïve T cells, but it does not explain the potential role of DCs to promote tolerance and to exert the immune suppressive effects after AhR activation by TCDD or ITE.
To explore whether activation of AhR is also critical to induce DCs which can promote tolerance and thus immune suppression we analyzed the effect of TCDD on various potent mediators of active suppression in immune responses. IDO1 was clearly induced in AhR-activated DCs by TCDD. In addition, we found that TCDD induces the expression of the recently discovered gene IDO2. IDO2 is closely related to IDO1 and is expressed in mice and humans. The corresponding genes have a similar genomic structure and enzymatic activity [12]. Measuring the enzymatic activity of IDO confirmed the functional expression of IDO after treatment with TCDD or interferon-γ (INFγ); INFγ is a well known endogenous stimulator of IDO1. On the other hand, activation of AhR by FICZ in vitro did not activate enzymatic activity of IDO. Interestingly, expression of IDO2 was decreased by INFγ in U937-derived DC compared to control, which confirms previous reports [8, 12]. Cotreatment with the AhR antagonist MNF clearly blocked induction of IDO1 and IDO2 by TCDD but not the induction of IDO1 by INFγ. The results are underlining the different mechanisms of both inducers (INFγ and TCDD) as well as the different regulation of the IDO1 and IDO2 genes. Recently it has been demonstrated that the noncanonical NF-κB pathway is also critical for the induction of IDO1 through ligation of CD40 for instance [14]. Therefore it is not unlikely, that TCDD-mediated induction of IDO1 not only requires activation of AhR, but also involves RelB. RelB is a critical component of the noncanonical NF-κB pathway and has been recently shown to interact with AhR to induce IL-8 for instance [11,15]. Since the discovery of IDO2 is so recent, not many data are available regarding the regulation of the gene. However, two putative XREs have been identified at -2,781 and -2,810 bp upstream the start site of exon 1 of IDO2 (unpublished data).
Next we verified findings received from U937-derived DCs in vitro in the C57BL/6 animal model. IDO1 and IDO2 mRNA expression are most significantly upregulated in spleen and to a lower extent in lung in TCDD-treated mice. Analysis of IDO1 and IDO2 in AhR-deficient mice supports our data from in vitro that the AhR is required to induce IDO1 and IDO2 by TCDD.
Several studies have demonstrated that the immunoregulatory pathway of tryptophan catabolism, initiated by IDO, represents a mechanism of peripheral tolerance. IDO expressing DCs are regarded as regulatory DCs specialized to cause tolerance through its regulatory effect on T cells [8]. The most impressive data, demonstrating the critical role of IDO to induce tolerance was shown by Munn et al. [16]. They found that pharmacological inhibition of IDO leads to rejection of all fetuses in mice. Numerous studies followed, which confirmed that IDO expression in DCs or macrophages is responsible to suppress T cell responses and induce tolerance [17].
Depending on its ligand, activation of AhR by TCDD or FICZ may result in Treg expressing Foxp3 or TH17 T cells, respectively [2,3]. Our results show the increase of Foxp3 expression by TCDD in spleen, whereas Foxp3 was not elevated in AhR deficient mice. In contrast to TCDD, FICZ did not activate IDO which could explain that AhR activation by FICZ does not induce Foxp3 and development of Treg. IDO is a critical factor for development of Treg, and our results show, that IDO inhibition blocked the TCDD-induced increase of Foxp3, suggesting that IDO is responsible for TCDD’s effect on the development of Treg and consequently its suppressive action on immune responses.
The ligand-specific AhR-mediated expression of IDO1/IDO2 and induction Treg differentiation indicate that AhR ligands can be critical factors in the development of autoimmune disease, infection or cancer. Local suppression of tumor-specific immune responses is thought to be a hallmark of all successful tumors. Mounting evidence suggest that the IDO pathway might be a driving factor during cancer progression to provide immune escape [18]. Our data warrant further studies to investigate the possible role of IDO induced by AhR activating ligands such as dioxins for the tumor promoting activity of these compounds.
Supplementary Material
Acknowledgements
We thank C. Bradfield for providing us with the AhR-deficient mouse strain; J. Abel for providing MNF and H. Woldai and D. Alvizar-Trujillo for preparation of animal tissues. This work was supported by research grants R01-ES05233 from the National Institute of Environmental Health Sciences and a Grant-in-Aid of the American Heart Association.
Abbreviations
- AhR
aryl hydrocarbon receptor
- FICZ
6-formylindolo[3,2-b]carbazole
- IDO1
indoleamine-2,3-dioxygenase 1, IDO2, indoleamine 2,3-dioxygenase-like protein
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- Treg
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int. Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 2.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche E, Schäfer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hübenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Fürst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. U S A. 2007;21:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005;7:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 6.Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschlager M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft specific tolerance through direct- and DC-mediated effects on regulatory T cells. Blood. 2008 Jun 11; doi: 10.1182/blood-2007-08-109843. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Mahnke K, Ring S, Johnson TS, Schallenberg S, Schönfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-H3 expression and antigen presentation. Eur. J. Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 8.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 9.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338:12–19. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 1988;263:2041–2048. [PubMed] [Google Scholar]
- 11.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007;12:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;1:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka G, Kanaji S, Hirano A, Arima K, Shinagawa A, Goda C, Yasunaga S, Ikizawa K, Yanagihara Y, Kubo M, Kuriyama-Fujii Y, Sugita Y, Inokuchi A, Izuhara K. Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int. Immunol. 2005;6:797–805. doi: 10.1093/intimm/dxh260. [DOI] [PubMed] [Google Scholar]
- 14.Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML, Tak PP, de Jong EC. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 15.Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 17.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 18.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.