Abstract
Cryosurgical treatment of solid cancer can be greatly assisted by further translation of our finding that a cytokine adjuvant TNF-α can achieve complete cancer destruction out to the intra-operatively imaged iceball edge (−0.5°C), over the current clinical recommendation of reaching temperatures < −40°C. The present study investigates the cellular and tissue level dose-dependency and molecular mechanisms of TNF-α induced enhancement in cryosurgical cancer destruction. Microvascular endothelial-MVEC and human prostate cancer-LNCaP Pro 5 (LNCaP) cells were frozen as monolayers in the presence of TNF-α. Normal skin and LNCaP tumor grown in a nude mouse model were also frozen at different TNF-α doses. Molecular mechanisms were investigated by using specific inhibitors to block NF-κB-mediated inflammatory or caspase-mediated apoptosis pathways. The amount of cryoinjury increased in a dose-dependent manner with TNF-α both in vitro and in vivo. MVECs were found to be more cryosensitive than LNCaP cells in both the presence and absence of TNF-α The augmentation in vivo was significantly greater than in vitro with complete cell death up to the iceball edge in tumor tissue at local TNF-α doses greater than 200 ng. The inhibition assays showed contrasting results with caspase-mediated apoptosis the dominant mechanism in MVECs in vitro and NF-κB-mediated inflammatory mechanisms within the microvasculature dominant in vivo. These results suggest the involvement of endothelial-mediated injury and inflammation as the critical mechanisms in cryoinjury and suggest the use of vascular-targeting molecules such as TNF-α to enhance tumor killing and achieve the clinical goal of complete cell death within an iceball.
Keywords: cryosurgery, LNCaP, MVEC, TNF-α, prostate cancer, inflammation, apoptosis, necrosis
Introduction
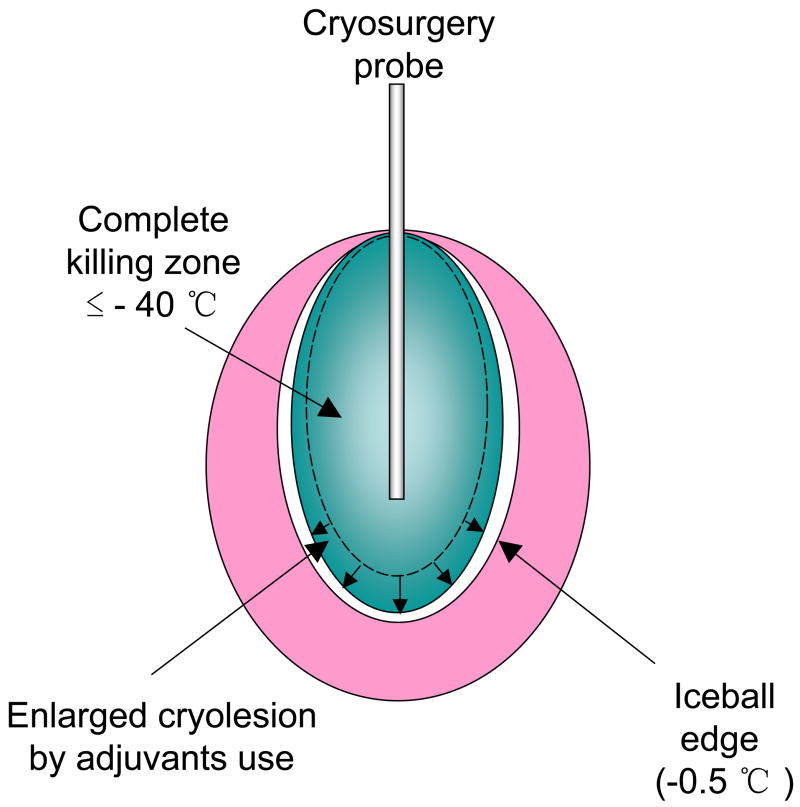
Cryosurgery is a surgical technique that utilizes extreme cold temperatures to treat diseased tissues such as tumors in the body. The finding that clinical imaging [Ultrasound (US), Computed Tomography (CT) and Magnetic Resonance (MR)] can be used to monitor the growth of an iceball in vivo has made cryosurgery an important minimally invasive thermal therapeutic modality (1, 2). Despite advantages including ease of operation, low morbidity and low cost, the use of cryosurgery is limited by its inability to destroy the entire tissue within an iceball as reflected in local recurrence of cancer after freezing (3). The clinical guideline to ensure complete cell death by this technique alone is −40°C, which limits the control and predictability of the procedure (2, 3) (Fig. 1). Thus, whereas the edge of the iceball (−0.5°C) can be visualized using US, CT or MR, the means to enhance or predict the “kill zone” within the iceball are urgently needed. Recent research in cryosurgery has focused on the use of molecular adjuvants to increase tissue cryosensitivity at the periphery of an iceball (0 to −40°C) (Fig. 1), which would otherwise remain viable (4–8). It was shown recently for the first time in an in vivo prostate cancer model, that a cytokine, TNF-α, could enhance cryosensitivity and achieve tissue destruction up to the edge of an iceball (−0.5°C) (8). This study focuses on understanding the dose-dependency and mechanisms of TNF-α induced cryosurgical augmentation at both the cellular (in vitro) and tissue level (in vivo). The results are very significant as they suggest the involvement of contrasting injury mechanisms in vitro as compared to in vivo. Several theories have been put forth as to the mechanisms of tissue injury by freezing alone and grouped mainly under direct cellular effects and vascular effects (9). Direct cell injury during freezing can occur by intracellular ice formation (IIF) at higher cooling rates near the cryoprobe or solution effects injury at low cooling rates present at the periphery of the iceball. While IIF directly damages cells by mechanical interaction with large stable ice crystals, solution effects causes cellular dehydration with an increase in both intracellular and extracellular solute concentrations leading to destabilization of the cell membrane (9–11). Post-thaw analysis of injury suggests that both apoptosis and necrosis can occur (7, 10). A second theory regarding the mechanism of damage at the tissue level is vascular injury due to the shutdown of microvasculature after freezing and the resultant ischemic necrosis (12–14). Damage to the endothelium, ischemia-reperfusion injury, inflammation and the resultant loss of microcirculatory support is considered critical in defining the edge of the cryolesion (12, 14). At molecular level, studies have shown the activation of NF-κB and the expression of cell adhesion molecules such as selectins, ICAM and VCAM after endothelial insult, leading to an enhanced inflammatory response (15). Accentuation of either cellular or vascular mechanisms of injury is a goal in adjuvant-enhanced cryosurgery.
Fig. 1.
Improvements of the outcome and image guidance of cryosurgery by use of adjuvants. Adjuvants enlarge the cryolesion within the iceball, and create an overlap of killing zone and the iceball edge.
Adjuvants aiming to accentuate established cryoinjury mechanisms can be grouped into several categories: (1) thermophysical adjuvants such as antifreeze protein (AFP) and chemicals (i.e. salts and some amino acids), which work directly on cells, (2) chemotherapeutics such as peplomycin, adriamycin, 5-fluoracil, bleomycin and navelbine, which are established cytotoxic drugs and (3) cytokines or vascular based agents (4, 6, 7, 16, 17). While all of these approaches have shown some accentuation of injury at temperatures >−40°C, particularly in vitro, none have been able to show an overlap of the iceball edge with the edge of the cryolesion in vivo.
Recent research in our laboratory has focused on the accentuation of vascular injury during freezing using a vascular targeting cytokine, TNF-α (5, 8). For the first time it was shown that with the proper dose and delivery of TNF-α, it is possible to achieve an overlap of the kill zone and the iceball edge, allowing imaging feedback of injury and attain control of the procedure (8). TNF-α is a well-known cytokine for its role in inflammation, immunity and for its anti-tumor properties (18–20). At the cellular level, TNF-α can lead to direct cell death by apoptosis or induce inflammation by the activation of NF-κB, which is important, particularly in tissues, due to the presence of immune cells (19, 21). It now remains to be determined which one (or both) of these pathways are active at the cellular and tissue levels in enhancing cryosurgical injury.
The aims of this study are to investigate and compare both in vitro and in vivo: 1) the effect of TNF-α dose on cryoinjury and; 2) the mechanisms of TNF-α induced accentuation in cryoinjury. Cryoinjury was assessed in vitro using viability and DNA fragmentation assays and in vivo by measuring perfusion defects and histology. Specific inhibitors were used to target caspase-mediated apoptosis and NF-κB-mediated inflammatory pathways to investigate the molecular mechanisms involved in TNF-α enhanced cryoinjury. The results demonstrate a direct dose-dependency of TNF-α on cryoinjury both in vitro and in vivo but contrasting cell injury mechanisms responsible for the observed augmentation.
Material and Methods
In Vitro
Cell Culture
Both LNCaP Pro 5 (LNCaP) cells and MVECs released form newborn human foreskin (29) were grown as adherent monolayers in 25cm2 T-flasks as previously described. All treatments were performed when flasks were 80% confluent.
TNF-α and Inhibitor Treatments
Recombinant human TNF-α (A gift from Cytimmune Sciences, Rockville, MD) was diluted with Dulbecco’s phosphate buffered saline (DPBS) (BioWhittaker Inc, Walkersville, MD), and reconstituted to a final concentration (1 ng/ml, 10 ng/ml, 100 ng/ml, 1000 ng/ml) with fresh media. Pan-caspase inhibitor Z-VAD-FMK (R&D Systems, Minneapolis, MN) and NF-κB inhibitor BAY 11-7085 (EMD Biosciences, La Jolla, CA) were dissolved in DMSO and diluted to final concentrations of 100 μM and 10 μM respectively in fresh media before application. The cells were incubated with media containing TNF-α for 4 hours. The inhibitors were present in the media until injury assay.
Freeze/Thaw (FT) of Monolayer Cultures
Cell monolayers in 25cm2 flasks were frozen in an ethanol bath maintained at constant temperatures (−5ºC or −10ºC), and spontaneously nucleated when the temperature reached −1°C. LNCaP cells were frozen until the culture temperature reached −10ºC and then held on a copper block maintained at −10ºC for 5 minutes. MVECs were frozen till they reached −5ºC without any hold time. Cells were allowed to thaw passively at room temperature for 15 minutes and then incubated at 37°C until injury assessment.
Injury Measurement in Monolayer Cultures
Cell Counting and Viability Assay
Cells were mixed with 9 μM of Hoechst 33342 (Sigma-Aldrich, St. Louis, MO) and 7 μM of propidium iodide (PI) (Sigma-Aldrich, St. Louis, MO) and assessed at 200X by fluorescent microscopy (Olympus BX-50, Tokyo, Japan). At least seven representative fields and a total of 200–300 cells/sample were counted in each monolayer culture. Cell viability was calculated at several time points (0hr, 24 hr, 48hr, 72hr) after the treatments.
DNA Fragmentation Assay
Cells were collected and fixed with 70% ethanol at 4°C overnight. After centrifugation, cells were washed twice with 1ml DPBS. One ml of staining solution (containing 3.8 mM sodium citrate and 50 μg/ml PI in DPBS) and 50 μl of 10 μg/ml RNAse A (Worthington Biochemicals, Lakewood, N.J.) was added to the pellets. The pellets were stored in dark for 1 hour at 37°C and kept covered until further analysis. The PI fluorescence was measured using about 1×104 cells in a FACSCalibur E4513 flow cytometer. The fraction of the cells in various cell cycle stages was estimated from DNA content of the cells (22).
In Vivo
DSFC Implantation
The dorsal skin fold chamber (DSFC) allows intravital two dimensional, controlled growth and visualization of tumors as described previously (23, 24). This model was adapted by our lab to perform controlled freezing and has since been used in several cryosurgery studies (5, 8, 23, 25). Briefly, the dorsal skin of each nude mouse was sandwiched between two anodized aluminum frames with 10 mm diameter viewing windows, separated by a distance of 450 μm, maintained by spacers on the screws (23, 24). The epidermis was removed from the viewing side along with excess fascia to permit better visualization of the microvasculature.
Tumor Implantation
One-two million LNCaP cells were suspended in 30 μl of matrigel matrix (matrigel diluted 3:1 in serum free media; BD Biosciences, Bedford, MA) and inoculated into the DSFC chamber window on both day 0 and day 4 of implantation. The experiments were performed on day 12 following DSFC implantation, when the tumor covered the entire chamber (5, 8).
TNF-α and Inhibitor Treatments
On the day of the study, the glass window was removed and soluble TNF-α (2 ng, 200 ng, 500 ng or 1 μg) dissolved in 30 μl saline was applied topically in the DSFC. The glass window was replaced after 15 minutes and cryosurgical treatment was performed 4 hours later. Specific NF-κB inhibitors BAY 11-7085 and andrographolide were dissolved in DMSO at concentrations of 10 mg/ml and 15 mg/ml respectively. BAY 11-7085 at a dose of 0.4 mg/kg was applied topically in the DSFC 15 minutes before TNF-αapplication, while andrographolide was administered twice, intraperitoneally (i.p.) at a dose of 30 mg/kg each at 2 hours and 15 minutes before TNF-α application (26–29). Pan-caspase inhibitor Q-VD-OPH (MP Biomedicals, Aurora, OH) was administered i.p at a dose of 50 mg/kg, 15 minutes before TNF-α application (26–29).
Freeze/Thaw (FT) of the DSFC
The normal skin control (i.e non-tumor subcutaneous tissue within the DSFC) was frozen 3 days after chamber implantation to allow the tissue to recover from surgery. The tumor implanted DSFCs were allowed 12 days to grow in the DSFC prior to cryosurgery.
The freezing procedure in the DSFC has been described in detail previously (8). Briefly, a 1 mm diameter brass fin fitted to a 5 mm cryoprobe (Endocare, CA) was inserted in the center of the DSFC and allowed to attain a temperature of −100°C for 55 seconds, followed by a passive thaw at room temperature. The temperature was monitored throughout the procedure by the use of thermocouples placed at 2, 3 and 4 mm radius from the center respectively and also by an infrared (IR) camera (FLIR, Boston, MA). The thermocouple measured temperatures and IR measured temperatures correlated well with each other and were also validated by a quasi steady state mathematical model, as shown in our previous publications (5, 8, 23).
Injury Measurement in the DSFC
The effects of TNF-α and/or cryosurgery on vascular damage were visualized using a 10 mg/ml 70 kD fluorescein isothiocyanate (FITC) - labeled dextran intravenously by tail vein injection at 3 days post-treatment (5, 8, 25). Stasis was defined as the lack of blood flow (i.e perfusion defects) as evidenced by the lack of fluorescence signal within a vessel. The chamber was traversed radially in the four perpendicular directions (N, S, E, W) and radial locations of stasis (i.e. perfusion defects) noted using a micrometer scale fixed on the stage with the chamber center as the origin. Temperatures at the four measured radii of stasis were then extrapolated from the respective IR image taken after cryosurgery and averaged to obtain the temperature threshold (defined as minimum temperature for complete cell death) of injury for that particular animal (8). Animals were sacrificed after vascular imaging and entire tissue inside the chamber fixed in 10% neutral-buffered formalin and processed for histology (8).
Statistical Analysis
Comparison of the effect of different treatment conditions on the cells was performed using one-way ANOVA followed by Bonferroni’s multiple comparison test or a t-test. Each group consisted of at least n of 3.
Results
Cell Injury in Vitro
Viability
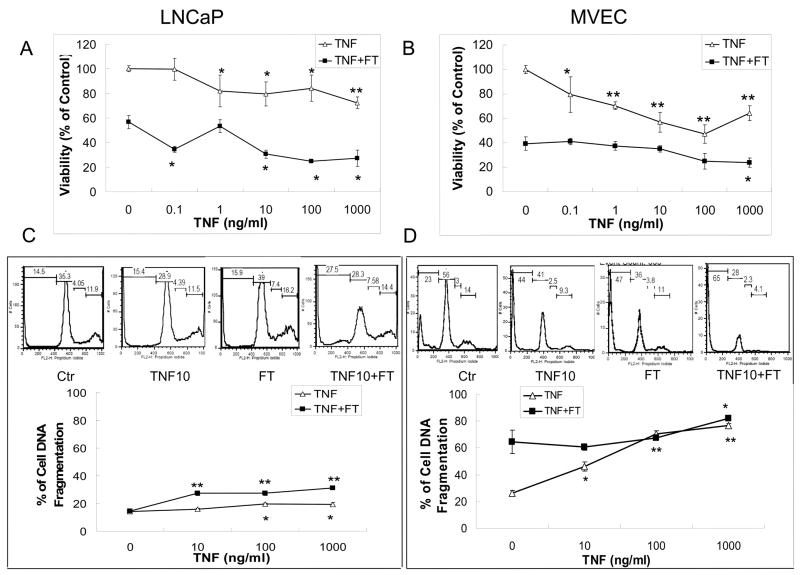
MVECs were found to be more sensitive than LNCaPs to cryoinjury at similar FT conditions (data not shown). Therefore, the freezing protocols were adjusted to produce similar viability (approximately 50%) in both the cell lines thereby allowing comparison after TNF-αaddition, and also to simulate the thermal conditions present near the edge of the iceball. The effect of TNF-α induced augmentation of cryoinjury was observed in MVECs as early as 24 hours after treatment, and a similar trend continued at 48hr and 72hr (data not shown). In LNCaP cells, a significant effect due to combined treatment was observed at 48hr after treatment (data not shown). Therefore, the 24 and 48-hour time points were chosen for MVECs and LNCaP cells respectively to study the dose–dependency and mechanisms of TNF-α induced augmentation in cryoinjury.
A direct dose dependency on cell viability was observed in the cells after the application of TNF-α(0.1 ng –1000 ng/ml), before FT. A 4-hour treatment of TNF-α at 1000 ng/ml dose reduced the cell viability to 64.2 ± 6.1% and 72.3 ± 4.8% for MVECs and LNCaP cells respectively (Fig. 2A and 2B). FT alone reduced the cell viability in MVECs to 39.3 ± 5.5% and in LNCaP cells to 56.7 ± 5.5%. Pre-treatment of cells with 1000ng/ml TNF-α for 4 hours before FT showed a significant augmentation in cell injury when compared to FT alone with viability reducing to 23.7 ± 3.8% and 27.2 ± 6.6% (p < 0.05) for MVECs and LNCaP cells respectively.
Fig. 2.
In vitro effect of TNF-α concentration on the viability of (A) LNCaP monolayers with freezing to −10°C, 5 min hold time, and (B) MVEC monolayers with freezing to −5°C, 0 min hold time. Cells were treated with TNF-α at concentrations of 0.1, 1, 10, 100, 1000 ng/ml for 4 hours prior to freezing. In vitro effect of TNF-α concentration on DNA fragmentation of (C) LNCaP monolayers with freezing to −10°C, 5 min hold time, and (D) MVEC monolayers with freezing to −5°C, 0 min hold time. Cells were treated with TNF-α at concentrations of 10, 100, 1000 ng/ml for 4 hours prior to freezing. Viability and DNA fragmentation were assessed at 48 hours (LNCaP cells) or 24 hours (MVECs) after freeze/thaw (FT). Values presented are the means ± standard error mean from 3 independent experiments, and control indicates untreated cells. Significant effects of TNF-α compared to control and TNF-α + FT compared to FT alone (*, p<0.05; **, p<0.001).
DNA Fragmentation
DNA fragmentation analysis was performed to provide evidence, which is supplemental to viability analysis of TNF-α enhanced cryoinjury. The data from DNA fragmentation assay is shown in Fig. 2C and 2D. PI intensity (x-axis) corresponds with DNA content of sub G1, G1, S and G2/M phase cells. In this assay, sub G1 phase cells were cells with reduced DNA content due to either apoptosis or necrosis. Typical histograms of DNA content per 104 cells in LNCaP cells and MVECs are shown at different TNF-α doses. In addition, % of sub G1 cells was calculated to compare percentage of DNA fragmented cells after each treatment. TNF-α at a dose of 1000 ng/ml had a minimal effect on LNCaP cells (5% enhancement), but dramatically augmented the DNA damage in MVECs to 76.6 ± 1.6 % from 26.2 ± 2.1% observed in control cells (p < 0.001). Freezing alone showed a DNA fragmentation of 14.5 ± 1.6% and 64.6 ± 8.9% for LNCaP cells and MVECs respectively. On pretreatment with 1000 ng/ml of TNF-α before freezing, the DNA fragmentation increased by 16.7% in LNCaP cells and 17.5% in MVECs (p < 0.05) when compared to FT alone. The trends in the results suggest that the drop in the viability as seen in Figs 2A and 2B is likely due to the presence of cells in sub G1 state (i.e apoptotic or necrotic).
Tissue Injury in Vivo
Injury in the DSFC was assessed by intravital fluorescence of perfusion and post-mortem histology (data not shown). All control unfrozen normal skin and tumor DSFCs demonstrated blood flow throughout the chamber as visualized by FITC-dextran fluorescence. Sham treatment displayed patent vasculature beyond the probe insertion site at 0.5 mm radius. The chambers frozen with or without TNF-α intervention displayed a central static region surrounded by perfused tissue in the rest of the chamber. There was increased permeability (not quantified) at the edge of the injury evident as blurriness due to the leakage of dye. White blood cell rolling and adhesion, indicative of enhanced inflammation, was observed just before cryotreatment in all TNF-α pre-treated animals as previously noted (5, 30). Post treatment analysis of histology showed a centrally necrotic region surrounded by a transition region, which was comprised of both viable and dead cells, inflammatory cells and thrombosed, dilated vessels as previously described by Hoffman et al., 2001 [36]. Surrounding the transition region, normal tissue morphology (untreated) could be seen. We have previously shown using a fluorescent dye DiOC7, that the boundary of blood perfusion coincided very well with the edge of necrosis on H & E stained slides (8). Thus, the average temperature measured at the edge of stasis in the DSFC by vascular imaging represents the temperature threshold for tissue necrosis by cryosurgery for that particular animal.
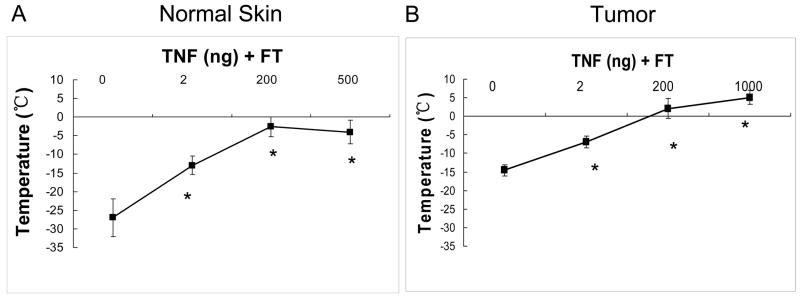
Fig. 3 shows the temperature threshold of necrosis as measured at the edge of stasis for different groups in both normal skin and tumor. The temperature threshold increased with the addition of TNF-α in a dose dependent manner for both normal skin and tumor as seen in Fig. 3. In normal skin it increased significantly from −27.7 ± 5.0ºC for cryo (FT) only to −13.0 ± 2.5ºC, −2.6 ± 2.7ºC and −4.0 ± 3.1ºC with pre-treated doses of 2, 200 and 500 ng TNF-α respectively (Fig. 3A). As expected, tumor was found to be more sensitive to cryoinjury when compared to normal skin with a temperature threshold of −14.5 ± 1.5ºC by FT alone treatment (Fig. 3B). With pre-treated doses of 2, 200 and 1000 ng TNF-α, the temperature threshold increased considerably (p < 0.01) to −7.0 ± 1.6ºC, 2.1 ± 2.7ºC and 5.1 ± 1.9ºC respectively (Fig. 3B). Thus, at a total dose of 200 ng or greater, it was possible to obtain an overlap between the kill zone and the iceball edge in the tumor tissue.
Fig. 3.
In vivo effect of TNF-α dosage in normal skin (A) and LNCaP pro 5 tumor (B) grown in a dorsal skin fold chamber. Tissues were incubated with TNF-α for 4 hours prior to freezing. The minimum temperature required for complete cell death was measured at the edge of perfusion defect from the infra-red image taken during the treatment. Values presented are mean ± standard deviation from 5–8 animals in treated groups were statistically different thaneach group. All TNF- α untreated freeze alone groups (*, p < 0.01).
Apoptosis and NF-κB Inhibition In Vitro
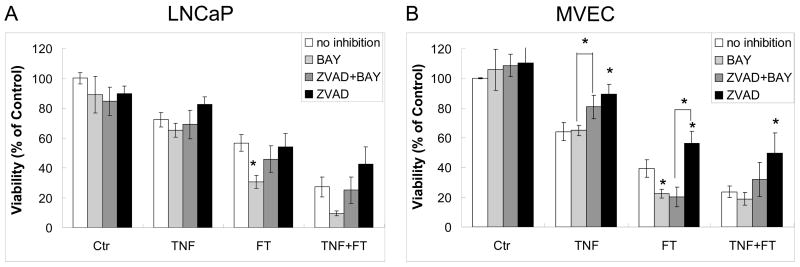
Caspase-mediated apoptosis and NF-κB-mediated inflammatory response are the two well-documented mechanisms of TNF-α induced injury of cells in vitro (31, 32). To elucidate the molecular mechanisms involved in TNF-α enhanced cryoinjury, pan-caspase inhibitor Z-VAD-FMK (ZVAD) and NF-κB inhibitor BAY 11-7085 (BAY) were added before TNF-α pre-treatment. As shown in Fig. 4A, inhibition of apoptosis with ZVAD had no significant effect on LNCaP viability in any of the treatments (TNF-α, FT, or TNF-α + FT). However, inhibition of NF-κB with BAY decreased LNCaP viability by 26.1% after FT (p < 0.05). Contrastingly in MVECs (Fig. 4B), ZVAD had a survival effect rescuing 25.3% cells after TNF-α treatment (p < 0.05), 17.2% after FT (p < 0.05), and 26.0% after TNF-α + FT (p < 0.05). Inhibition with BAY reduced MVEC viability by 16.9% after FT (p < 0.05). The combined treatment of ZVAD + BAY showed some rescue compared with BAY alone in MVECs with statistical significance only in TNF-α treatment group (p < 0.05), and significantly reduced MVEC viability compared with ZVAD alone in FT treatment group (p < 0.05). These results indicate an active NF-κB pathway after FT in both MVECs and LNCaP cells and active caspases in MVECs in the presence of TNF-α.
Fig. 4.
In vitro effect of using NF-κB inhibitor BAY 11-7085 (BAY) and caspase inhibitor Z-VAD-FMK (ZVAD) and on (A) LNCaP monolayers with freezing to −10°C, 5 min hold time, and (B) MVEC monolayers with freezing to −5°C, 0 min hold time. TNF-α treatment was at 1000 ng/ml for 4 hours prior to freezing. 100 μM ZVAD and 10 μM BAY were present in the medida until viability assay. Viability was assessed at 48 hours (LNCaP cells) or 24 hours (MVECs) after freeze/thaw (FT). Values presented are the means ± standard error mean from 3 independent experiments, and control indicates untreated cells. Significant effect of inhibitors compared with no inhibitor (*, p < 0.05). Significant effects of BAY compared with ZVAD+BAY, and ZVAD compared with ZVAD+BAY were shown in brackets (*, p < 0.05).
Apoptosis and NF-κB Inhibition In Vivo
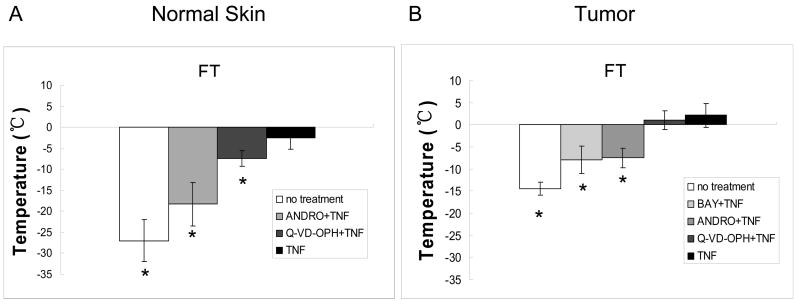
The inhibition experiments in vivo were performed at a TNF-α dose of 200 ng, where a significant accentuation in cryoinjury was observed, in both normal skin and tumor after FT (Fig. 3). Andrographolide (ANDRO), an NF-κB inhibitor, significantly reduced the TNF-α enhancement in cryoinjury for both normal skin and tumor (Fig. 5). In normal skin, the temperature threshold was reduced from −2.6 ± 2.7ºC to −18.3 ± 5.2ºC (p < 0.01), closer to the injury obtained with FT alone at −27.7 ± 5.0ºC (Fig. 5A). A similar reduction was seen in tumors where the temperature threshold changed considerably to −7.5 ± 2.2ºC from 2.1 ± 2.7ºC (p < 0.01) (Fig. 5B). Pre-treatment of tumor tissue with BAY, another NF-κB inhibitor, reduced the temperature threshold after TNF-α + FT to −8.0 ± 3.1ºC, which was statistically different (p < 0.01) from TNF-α + FT alone (Fig. 5B).
Fig. 5.
In vivo effect of using NF-κB inhibitors BAY 11-7085 (BAY), andrographolide (ANDRO) and caspase inhibitor Q-VD-OPH in normal skin (A) and tumor (B) grown in a dorsal skin fold chamber. The inhibition was performed at TNF-α pretreated dose of 200 ng for both normal skin and tumor. Values presented are mean ± standard deviation from 5–8 animals in each group. Groups were statistically different than groups pretreated with TNF- α(*, p < 0.01).
In contrast, inhibition with Q-VD-OPH, a caspase inhibitor, had a minimal effect on the accentuation of cryoinjury by 200 ng TNF-α for both normal skin and tumor. The temperature threshold with caspase inhibitor was measured to be −7.4 ± 1.9ºC and 1.0 ± 2.1ºC for normal skin and tumor, respectively (Fig. 5A and B). Although caspase inhibition was statistically significant (p < 0.05) in normal skin when compared to TNF-α + FT without inhibition, the reduction was significantly less than what was obtained after NF-κB inhibition with ANDRO (p < 0.05). None of the inhibitors (BAY, ANDRO, Q-VD-OPH) had a significant effect on temperature threshold obtained by FT alone (data not shown). Therefore, in vivo inhibition studies with NF-κB and caspase inhibitors show the presence of NF-κB-mediated inflammatory pathways in TNF-α-induced accentuation of cryoinjury.
Discussion
It is evident from both in vitro and in vivo results, that the administration of TNF-α before freezing increases the amount of injury significantly. This increase in injury is considerably higher in vivo, where complete cell destruction could be obtained up to the edge of the iceball (−0.5ºC). Inhibition of apoptotic or inflammatory pathways during cryosurgical enhancement with TNF-α presents contrasting results suggesting host-mediated inflammation responsible for augmentation in vivo and MVECs mediated apoptosis as the mechanism in vitro.
The in vitro data provides compelling evidence to suggest the role of endothelial cells in TNF-α induced accentuation in cryoinjury. MVECs have previously been shown to be sensitive to both FT injury and TNF-α induced NF-κB activation and is considered a better model than human umbilical vein cells (HUVECs) to study cryoinjury, because vaso-occlusion occurs primarily in the microvasculature (33, 34). In this study, MVECs were found to be more sensitive to freezing injury than LNCaP cells and therefore milder freezing conditions (−5ºC and 0 minute hold time) were used for this cell line as compared to LNCaP cells (−10ºC and 5 minutes hold time) (34). Increasing amount of cryoinjury to both MVECs and LNCaP cells was obtained in a dose-dependent manner with the addition of TNF-α. The sensitivity of MVECs to cell death in the presence of TNF-α was greater than LNCaP cells both with and without freezing. The resistance to TNF-α induced cell death in LNCaP cells as compared to MVECs could be due to the constitutive expression of anti-apoptotic proteins (ex. Inhibitor of Apoptosis Proteins), shown to be present in most tumor cell lines (35). The presence of PI-3/Akt survival pathway could be another mechanism for the resistance of LNCaP cells to TNF-α induced apoptotic injury (36).
Increased cryosensitivity of endothelial cells (with or without TNF-α) suggests that injury to the endothelium may help govern the extent and enhancement of injury in vivo, where many cell types are present. In fact several in vivo studies have shown endothelial injury and ensuing inflammation to be critical in governing the kill zone at the periphery of an iceball (12, 13, 25). The addition of TNF-α in vivo is suggested to cause even more endothelial cell death at higher subzero temperatures as observed in vitro, thereby increasing the extent of the vascular shutdown within the iceball.
The in vivo cryosurgical studies performed in the DSFC demonstrated a very significant dose dependency (Fig. 3). The temperature threshold of necrosis increased drastically with the addition of TNF-α, for both normal skin and tumor in the DSFC. At a dose of 200 ng, the edge of injury overlapped with the edge of the iceball (−0.5ºC) in tumor tissue, suggesting the ability to destroy all tumor within a cryosurgical iceball. This is clearly an augmentation that is not expected from the in vitro studies, where there was still significant viability remaining at −5ºC (in MVECs) and −10ºC (in LNCaP cells). It suggests the presence of other factors or mechanisms to account for the enhanced injury observed in vivo vs in vitro.
TNF-α is closely associated with in vivo vascular events and injury. Apart from endothelial cell apoptosis, the other reported actions of TNF-α in vivo comprise of proinflammatory reactions such as an increase in procoagulant activity, decrease in anticoagulant activity, recruitment and adherence of inflammatory cells such as neutrophils, and production of other cytokines (20, 21, 37, 38). TNF-α induced activation of NF-κB can promote the transcription of several genes that could increase the inflammatory/procoagulant response in the tissue by the expression of adhesion molecules such as ICAM, VCAM on the endothelium, facilitating leukocytes infiltration, and enhancing tissue factor expression while down regulating thrombomodulin (19, 21, 30). The increase in direct cell cryoinjury observed in vitro may partially be explained due to TNF-α induced apoptosis of endothelial and tumor cells. But the dramatic enhancement in cryoinjury observed in vivo also suggests the role of inflammatory prothrombotic events, which follow both after TNF-α and cryosurgical treatments. In order to investigate the significance of apoptosis and inflammation in TNF-α induced accentuation in cryoinjury, specific inhibitors to block caspases (to inhibit apoptosis) or NF-κB (to inhibit inflammation) were used both in vitro (cellular level) and in vivo (host level).
In vitro inhibition suggests active apoptosis pathway in MVECs in the presence of TNF-α and/or FT and NF-κB pathway in both the cell lines after FT. Caspase inhibition with Z-VAD-FMK showed significant rescue in MVECs, but no rescue in LNCaP cells (Fig. 4). This confirmed caspase mediated apoptosis as the injury mechanism in endothelial cells under TNF-α and FT insult. On the other hand, activation of NF-κB, results in the prevention of apoptosis through several anti-apoptotic proteins, including Bcl-XL (to inhibit cytochrome c leakage from the mitochondria), IAP (to inhibit caspase 3 and caspase 8 activation) and FLIP (to inhibit caspase 8 activation) (39–41). NF-κB inhibitor BAY 11-7085 reduced the viability of LNCaP cells and MVECs after both cryo and combined treatments (Fig. 4), confirming the activation of NF-κB as a protective mechanism after FT in vitro. The selection of inhibitors and dosage used in this work is based on previous literature reporting in vitro and in vivo models of NF-κB and caspase inhibition (26, 28, 29, 42–45).
In vivo blocking of apoptotic or inflammatory pathways suggests the role of TNF-α mediated inflammatory response to be the mechanism of observed enhancement in cryoinjury. In the DSFC both NF-κB inhibitors, BAY 11-7085 and andrographolide, produced a remarkable reduction in the TNF-α induced increase in temperature threshold of necrosis of cryoinjury for both normal skin and tumor (Fig. 5). On the other hand, treatment with Q-VD-OPH, a caspase inhibitor, didn’t produce a significant change in the temperature threshold of necrosis (Fig. 5). This is in contrast to in vitro results, where the application of pan-caspase inhibitor reduced injury (in MVECs), and NF-κB inhibitor to the contrary, enhanced cell injury (in LNCaP cells).
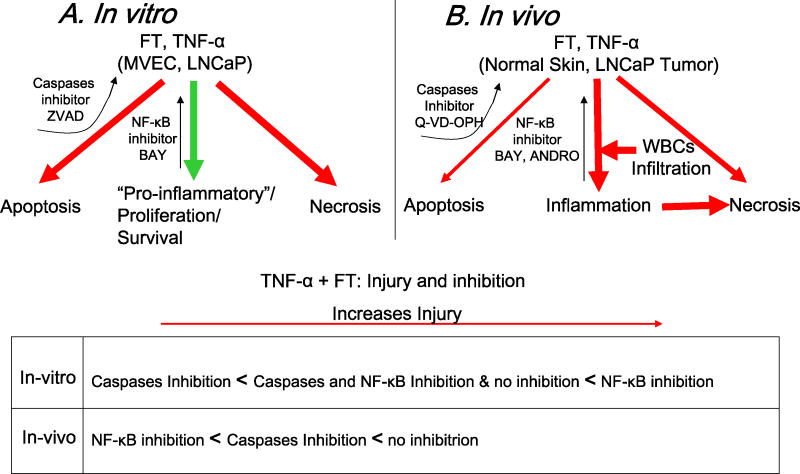
The results using apoptosis and inflammatory inhibitors observed both in vitro and in vivo supports our initial hypothesis of TNF-α induced apoptosis to be the mechanism for increased cell death observed in vitro but TNF-α induced host-mediated inflammatory response to be critical in vivo (Fig. 6). Although these in vitro and in vivo adjuvant augmentations are apparently divergent and affect two phenomena mediated by two distinct mechanisms, these contrasting results may represent two levels of the same in vivo phenomenon, ischemic necrosis, due to TNF-α effects at the cellular and tissue level.
Fig. 6.
Summary of in vitro and in vivo pathways of TNF-α enhanced cryoinjury. (A) In vitro response showing a prominent apoptotic vs. pro-inflammatory enhancement of injury which can be blocked by caspases (leading to rescue in MVECs) and NF-κB inhibition (leading to even greater death in LNCaP cells) respectively. (B) In vivo response showing a predominantly inflammatory vs. apoptotic enhancement of injury in vivo which can be blocked by NF-κB inhibition and to a less extent of caspases inhibition. An important part of the blockable inflammatory enhancement is suggested to be the inflammatory infiltrate (neutrophils and macrophages) recruited to the wound site.
Previous work with cryoadjuvants (particularly chemotherapeutics) has shown augmentation of cryoinjury, but fall short of obtaining an overlap of the kill zone with the edge of the iceball (4, 6, 7, 46). These studies have focused on increasing cryosurgical efficacy by using drugs to activate apoptotic based cell death pathways specifically near the edge of the iceball. The bulk of this work has been performed in vitro and none of the studies thus far have shown synergistic effects in vivo, as observed in this study. Though very few studies have confirmed the presence of apoptosis in vivo after a FT treatment, the overall significance of this mechanism of cell death in a cryosurgical procedure is not well defined and still under interrogation (16, 47).
Host-mediated vascular changes such as inflammation have been observed and implicated in a large number of studies related to freezing induced injury (9, 10). This work is the first effort to investigate the underlying mechanisms of vascular targeting drugs such as TNF-αin the augmentation of cryosurgical injury. The results show that though apoptotic based cellular level pathways may augment cryoinjury in vitro, the host-mediated vascular inflammatory pathways are more critical and produce a synergistic effect in cryoinjury augmentation in vivo. Future work is needed at the molecular level to determine the critical factors in the NF-κκB pathway that produce the observed synergistic augmentation in vivo. This study advocates the use of vascular targeting drugs such as TNF-α, or perhaps less toxic molecules that activate the NF-κB pathway in order to produce an augmentation in cryoinjury. Further work in a translational model is also needed to advance the clinical goal of complete cell death within a cryosurgical iceball.
Acknowledgments
Institute of Engineering and Medicine (IEM), University of Minnesota; NIH R01 NCI CA07528 for financial support.
Abbreviations
- ANDRO
Andrographolide
- DSFC
Dorsal Skin Fold Chamber
- FT
Freeze Thaw
References
- 1.Pease GR, Wong ST, Roos MS, Rubinsky B. MR image-guided control of cryosurgery. J Magn Reson Imaging. 1995;5:753–60. doi: 10.1002/jmri.1880050623. [DOI] [PubMed] [Google Scholar]
- 2.Rubinsky B. Cryosurgery. Annu Rev Biomed Eng. 2000;2:157–87. doi: 10.1146/annurev.bioeng.2.1.157. [DOI] [PubMed] [Google Scholar]
- 3.Long JP, Bahn D, Lee F, Shinohara K, Chinn DO, Macaluso JN., Jr Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology. 2001;57:518–23. doi: 10.1016/s0090-4295(00)01060-8. [DOI] [PubMed] [Google Scholar]
- 4.Ikekawa S, Ishihara K, Tanaka S, Ikeda S. Basic studies of cryochemotherapy in a murine tumor system. Cryobiology. 1985;22:477–83. doi: 10.1016/0011-2240(85)90159-2. [DOI] [PubMed] [Google Scholar]
- 5.Chao BH, He X, Bischof JC. Pre-treatment inflammation induced by TNF-alpha augments cryosurgical injury on human prostate cancer. Cryobiology. 2004;49:10–27. doi: 10.1016/j.cryobiol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Mir LM, Rubinsky B. Treatment of cancer with cryochemotherapy. Br J Cancer. 2002;86:1658–60. doi: 10.1038/sj.bjc.6600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42:274–85. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 8.Goel R, Swanlund D, Coad J, Paciotti GF, Bischof JC. TNF-alpha-based accentuation in cryoinjury--dose, delivery, and response. Molecular cancer therapeutics. 2007;6:2039–47. doi: 10.1158/1535-7163.MCT-06-0676. [DOI] [PubMed] [Google Scholar]
- 9.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60:40–9. doi: 10.1016/s0090-4295(02)01683-7. [DOI] [PubMed] [Google Scholar]
- 11.Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Annals of the New York Academy of Sciences. 1965;125:658–76. doi: 10.1111/j.1749-6632.1965.tb45420.x. [DOI] [PubMed] [Google Scholar]
- 12.Rabb JM, Renaud ML, Brandt PA, Witt CW. Effect of freezing and thawing on the microcirculation and capillary endothelium of the hamster cheek pouch. Cryobiology. 1974;11:508–18. doi: 10.1016/0011-2240(74)90120-5. [DOI] [PubMed] [Google Scholar]
- 13.Marzella L, Jesudass RR, Manson PN, Myers RA, Bulkley GB. Morphologic characterization of acute injury to vascular endothelium of skin after frostbite. Plast Reconstr Surg. 1989;83:67–76. doi: 10.1097/00006534-198901000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann NE, Bischof JB. In: Mechanisms of injury caused by in vivo freezing., in Life in Frozen State. Benson E, Fuller B, lane N, editors. Taylor & Francis; 2003. pp. 437–81. [Google Scholar]
- 15.Eberl T, Amberger A, Herold M, et al. Expression of stress proteins, adhesion molecules, and interleukin–8 in endothelial cells after preservation and reoxygenation. Cryobiology. 1999;38:106–18. doi: 10.1006/cryo.1999.2154. [DOI] [PubMed] [Google Scholar]
- 16.Forest V, Peoc’h M, Campos L, Guyotat D, Vergnon JM. Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology. 2005;50:29–37. doi: 10.1016/j.cryobiol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Tatsutani KN, Joye JD, Virmani R, Taylor MJ. In vitro evaluation of vascular endothelial and smooth muscle cell survival and apoptosis in response to hypothermia and freezing. Cryo letters. 2005;26:55–64. [PubMed] [Google Scholar]
- 18.Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565–73. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe N, Niitsu Y, Umeno H, et al. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988;48:2179–83. [PubMed] [Google Scholar]
- 21.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 22.Park H, Lyons JC, Griffin RJ, Lim BU, Song CW. Apoptosis and cell cycle progression in an acidic environment after irradiation. Radiat Res. 2000;153:295–304. doi: 10.1667/0033-7587(2000)153[0295:aaccpi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann NE, Bischof JC. Cryosurgery of normal and tumor tissue in the dorsal skin flap chamber: Part I--thermal response. J Biomech Eng. 2001;123:301–9. doi: 10.1115/1.1385838. [DOI] [PubMed] [Google Scholar]
- 24.Papenfuss HD, Gross JF, Intaglietta M, Treese FA. A transparent access chamber for the rat dorsal skin fold. Microvasc Res. 1979;18:311–8. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann NE, Bischof JC. Cryosurgery of normal and tumor tissue in the dorsal skin flap chamber: Part II--injury response. J Biomech Eng. 2001;123:310–6. doi: 10.1115/1.1385839. [DOI] [PubMed] [Google Scholar]
- 26.Pierce JW, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. The Journal of biological chemistry. 1997;272:21096–103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 27.Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of inhibitor of nuclear factor-kappaB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin Cancer Res. 2004;10:7645–54. doi: 10.1158/1078-0432.CCR-04-0958. [DOI] [PubMed] [Google Scholar]
- 28.Xia YF, Ye BQ, Li YD, et al. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol. 2004;173:4207–17. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- 29.Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell death and differentiation. 2007;14:387–91. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 30.Fukumura D, Salehi HA, Witwer B, Tuma RF, Melder RJ, Jain RK. Tumor necrosis factor alpha-induced leukocyte adhesion in normal and tumor vessels: effect of tumor type, transplantation site, and host strain. Cancer Res. 1995;55:4824–9. [PubMed] [Google Scholar]
- 31.Hirata H, Takahashi A, Kobayashi S, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. The Journal of experimental medicine. 1998;187:587–600. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas R, Garcia I, Donati YR, et al. Both TNF receptors are required for direct TNF-mediated cytotoxicity in microvascular endothelial cells. European journal of immunology. 1998;28:3577–86. doi: 10.1002/(SICI)1521-4141(199811)28:11<3577::AID-IMMU3577>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–9. [PubMed] [Google Scholar]
- 34.Berrada MS, Bischof JC. Evaluation of freezing effects on human microvascular-endothelial cells (HMEC) Cryo Letters. 2001;22:353–66. [PubMed] [Google Scholar]
- 35.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 36.Pfeil K, Eder IE, Putz T, et al. Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells. The Prostate. 2004;58:259–68. doi: 10.1002/pros.10332. [DOI] [PubMed] [Google Scholar]
- 37.Nawroth P, Handley D, Matsueda G, et al. Tumor necrosis factor/cachectin-induced intravascular fibrin formation in meth A fibrosarcomas. J Exp Med. 1988;168:637–47. doi: 10.1084/jem.168.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renard N, Lienard D, Lespagnard L, Eggermont A, Heimann R, Lejeune F. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha) Int J Cancer. 1994;57:656–63. doi: 10.1002/ijc.2910570508. [DOI] [PubMed] [Google Scholar]
- 39.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 40.Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–9. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–32. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279:23477–85. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- 43.Huerta-Yepez S, Vega M, Jazirehi A, et al. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene. 2004;23:4993–5003. doi: 10.1038/sj.onc.1207655. [DOI] [PubMed] [Google Scholar]
- 44.Rokhlin OW, Gudkov AV, Kwek S, Glover RA, Gewies AS, Cohen MB. p53 is involved in tumor necrosis factor-alpha-induced apoptosis in the human prostatic carcinoma cell line LNCaP. Oncogene. 2000;19:1959–68. doi: 10.1038/sj.onc.1203453. [DOI] [PubMed] [Google Scholar]
- 45.Granville DJ, Shaw JR, Leong S, et al. Release of cytochrome c, Bax migration, Bid cleavage, and activation of caspases 2, 3, 6, 7, 8, and 9 during endothelial cell apoptosis. Am J Pathol. 1999;155:1021–5. doi: 10.1016/S0002-9440(10)65202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forest V, Peoc’h M, Ardiet C, Campos L, Guyotat D, Vergnon JM. In vivo cryochemotherapy of a human lung cancer model. Cryobiology. 2005;51:92–101. doi: 10.1016/j.cryobiol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Steinbach JP, Weissenberger J, Aguzzi A. Distinct phases of cryogenic tissue damage in the cerebral cortex of wild-type and c-fos deficient mice. Neuropathology and applied neurobiology. 1999;25:468–80. doi: 10.1046/j.1365-2990.1999.00206.x. [DOI] [PubMed] [Google Scholar]