Abstract
Life on Earth developed in the presence and under the constant influence of gravity. Gravity has been present during the entire evolution, from the first organic molecule to mammals and humans. Modern research revealed clearly that gravity is important, probably indispensable for the function of living systems, from unicellular organisms to men. Thus, gravity research is no more or less a fundamental question about the conditions of life on Earth. Since the first space missions and supported thereafter by a multitude of space and ground-based experiments, it is well known that immune cell function is severely suppressed in microgravity, which renders the cells of the immune system an ideal model organism to investigate the influence of gravity on the cellular and molecular level. Here we review the current knowledge about the question, if and how cellular signal transduction depends on the existence of gravity, with special focus on cells of the immune system. Since immune cell function is fundamental to keep the organism under imnological surveillance during the defence against pathogens, to investigate the effects and possible molecular mechanisms of altered gravity is indispensable for long-term space flights to Earth Moon or Mars. Thus, understanding the impact of gravity on cellular functions on Earth will provide not only important informations about the development of life on Earth, but also for therapeutic and preventive strategies to cope successfully with medical problems during space exploration.
The "immune problem" in space
Early reports about disturbed immune cell function in space date back in the 70ties, where reduced reactivity of blood lymphoid cells has been discovered in crew members of Soyuz spaceships and of Skylab and Apollo [1,2]. Recently, a subclinical re-activation varicella zoster virus (VZV) has been reported in astronauts [3,4], a virus which becomes latent in the nervous system after primary infection, but is reactivated frequently in immune suppressed individuals, such as after organ transplantation, and in patients with cancer or AIDS. Whereas it is well known that gravity can be perceived by gravireceptors (statocyst-like organelles or gravisensitive ion channels in the cell membrane) in unicellular organisms such as Paramecium and Loxodes, where it strongly influences intracellular signal transduction and behaviour [5,6], the molecular mechanisms of gravisensitivity in mammalian cells are widely unknown. After the pioneering discovery of Cogoli et al. at the first Spacelab-Mission 20 years ago [7], it is known that proliferative response of lymphocytes after mitogenic stimulation is suppressed in microgravity [8]. In follow-up experiments in order to verify the result from Spacelab 1, it has been demonstrated clearly that factors other than microgravity can be excluded to be responsible for the depressed activation of lymphocytes. Whereas the phenomenon of reduced activation of T cells during microgravity is well described [9,10] and verified, the exact molecular mechanisms are not elucidated.
Signal transduction and cell-cell communication is disturbed in microgravity
Several investigations evidence alterations in signal transduction in lymphocytes. In lymphocytes, microgravity affected the protein kinase C [11,12] whereas delivery of first activation signal, patching and capping of conA-binding membrane proteins occurred normally in spaceflight [13]. These findings suggest the existence of gravisensitive cellular targets upstream from PKC and downstream from the TCR/CD3, where the lipid-raft-associated membrane-proximal signalosome complex is located. DNA array analysis of T cells subjected to simulated microgravity provided by the random-positioning machine (RPM) revealed an alteration of several signal moduls, in particular NF-kB and MAPK-signaling [14]. Also the expression of the early oncogenes c-fos, c-myc and c-jun is inhibited during spaceflight [summarized in [15]].
In other studies, gravisensitivity of pro- and antiapoptotic pathways has been reported in human mononuclear cells [16], human ML-1 thyroid-carcinoma cells [17] and astrocytes [18] in simulated microgravity. On the molecular level, simulated microgravity induced fas, p53 and bax and reduced bcl-2 [17,19]. Interestingly, the expression of fas was elevated in Jurkat-T-cells also during space flights of the shuttle missions STS-80 and STS-95 [20], suggesting an enhanced fas-fasL-mediated apoptosis of immune cells. During a 14-days space flight (SLS-2-mission) an accumulation of p53 has been found in keratinocytes and myocytes, indicating that central regulatory molecules of nuclear signal transduction and cell cycle are influenced by gravity [21]. The diminished proliferative response of T cells upon stimulation during microgravity could also be caused by a reduced expression of IL-2 receptor as demonstrated in simulated microgravity [22,23], resulting in an impairment of positive regulatory feedback loops. Overall, a decreased capacity of T-cells for the production of cytokines is a prominent effect of microgravity on leukocytes during spaceflight [24].
Microgravity also impaired monocyte function: During the spacelab-mission SLS-1 monocytes lost their capability of secreting IL-1 [25] and of expressing IL-2-receptor [26]. However, the molecular mechanisms are not identified. Examination of gene expression of monocytes under real microgravity demonstrated significant changes in gene induction associated with differentiation of monocytes into macrophages [27]. Kaur et al. [28] investigated monocytes isolated from astronauts before and after a mission and compared the results with control groups. They found a reduction of phagocytosis and a reduced oxidative burst- and degranulation-capacity. Meloni et al. [29] recently demonstrated that simulated weightlessness leads to massive alterations in the cytoskeleton of monocytes, which in turn influences motility and recently revealed during an ISS experiment a severe reduction in the locomotion ability of monocytic cells in microgravity [30]. Importantly, LFA-1 and ICAM-1 adhesion proteins expression seemed also to be sensitive to microgravity, whereas their interaction is not altered [30]. It seems that not all cell types of the immune system are sensitive to reduced gravity: Extensive studies with natural killer cells in simulated weightlessness and in real microgravity on board of the ISS revealed that neither cytotoxic effects nor interferon production is altered in microgravity [31]. Major gravi-sensitive signal transduction elements in mammalian cells are summarized in additional file 1 and figure 1.
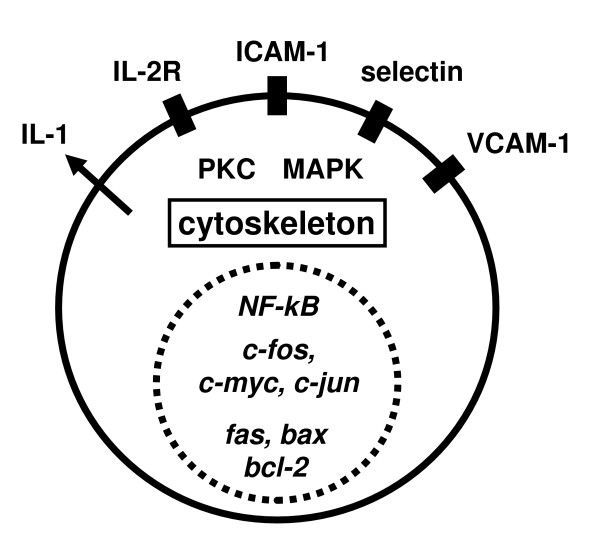
Figure 1.
Gravi-sensitive signal transduction elements in mammalian cells. Gravi-sensitive signal transduction elements has been detected at the cell surface, such as VCAM-1 (Vascular cell adhesion molecule 1), ICAM-1 (Intercellular adhesion and molecule 1) and IL-2R (interleukin-2 receptor), in the cytoplasma such as PKC (protein kinase C) and MAPK (mitogen-activated protein kinases) and in the nucleus such as expression of c-fos, c-jun and other genes. Microgravity severely affects also the cytoskeleton. However, the primary molecular mechanisms how microgravity influences cell signaling are unknown.
Cell migration in microgravity
Neutrophil granulocytes demonstrate the body's first line of host defense by recognizing and killing microorganisms. Neutrophil locomotion is integral for immune effector function, because the cells have to leave the blood vessels and navigate to places of infection and injury to fulfill their main task of phagocytosis. They are one of the most important cells regulating the immune response, because they can influence both induction and effector stage of immune reactions. Several studies provided evidence of a disturbed function of neutrophil granulocytes: Returning astronauts of spaceflight missions exhibited a strong increase of neutrophil granulocytes immediatedly after landing [32,33], and neutrophil chemotactic assays showed a 10-fold decrease in the optimal dose-response after landing [34]. In a parabolic flight experiment neutrophil granulocytes showed a dramatic increase of the superoxide-anion production [35]. Whereas some studies discuss an influence of space flights on the neutrophil phagocytotic activity and oxidative function [32], the influence of gravity on the migration of neutrophil granulocytes, which also determines the efficiency of an immunologial response, is still not known.
Cell migration is an essential characteristic of life. Multicellular organisms must be motile to obtain nourishment, evade being eaten in their own right, respond to environmental changes and reproduce. Likewise, unicellular organisms such as Paramecium or Loxodes must dynamically respond to fluctuations in ever-changing surroundings to assure survival [6]. However, cell migration is also an essential characteristic of many normal and abnormal biological processes within the human organism including embryonic development, defense against infections, wound healing and tumor metastasis [36,37]. In previous studies using simulated microgravity, changes in gravity demonstrated an inhibition of lymphocyte locomotion through type I collagen [38,39], and culture of human bone marrow CD34+ cells using NASA 's rotating wall vessels resulted in a decreased migration potential [40]. An altered movement in real microgravity was shown for leukocytes and Jurkat T cells, too [41,42], whereas the underlying signal transduction mechanisms are still illusive. On the other side, T cells become more motile after being cultured in 10 g hypergravity [43].
The cytoskeleton is responsible for giving a cell its shape and for generating the forces required for cell motility. It is an internal network of at least three types of cytosolic fibers: actin filaments, microtubules and intermediate filaments. Actin, one of the most highly conserved and abundant eukaryotic proteins, is constantly polymerized and depolymerized within cells to invoke cellular motility, tissue formation and repair [44,45]. Actin dynamics are considered to be the major component of the cytoskeleton responsible for cell motility. It has been shown to be essential for the migration of T lymphocytes as well as neutrophil granulocyte migration, a conclusion readily assumed as actin-depolymerizing drugs inhibit cellular motility [46,47]. In contrast, an intact microtubule network does not appear to be required for neutrophil migration, because microtubule-disrupting drugs such as colchicine even induce the migration of neutrophils [48], probably by inducing changes in the actin network.
Gravisensitivity of the cytoskeleton
Multiple investigators have reported that this complex network of fibers is sensitive to environmental factors such as microgravity and altered gravitational forces [49]. Several studies demonstrate modifications of the actin and microtubule cytoskeleton in microgravity. Already a few minutes of simulated weightlessness provided by 2D-clinorotation affected the cytoskeleton of lymphocytes, astrocytes, neurons and glial cells, disorganizing microtubules, intermediate filaments and microfilaments [50,51]. Morphological differences of both the microtubule and actin components of the cytoskeleton have been observed in cells grown in real and simulated microgravity [50,52]. Gruener and Hughes-Fulford reported that actin reorganization responded to the gravity level and showed abnormal assembly of actin stress fibers during spaceflight [53-55]. In human mesenchymal stem cells F-actin stress fibers were disrupted within three hours of initiation of modeled microgravity [56]. On the contrary, in Jurkat cells microgravity did not change the structure of actin but from vimentin [42]. Other studies have shown that microtubules are gravity sensitive, too [57]. Microtubule self-assembly is inhibited in the absence of gravity in space [58], and Lewis et al. observed that the microtubule filaments extended from a poorly defined centrosome in human Jurkat cells [52]. Moreover, cancer cells grown under microgravity exhibited an increased and highly disorganized vimentin as well as altered microtubules [59,60].
Many components of signal transduction pathways are known to regulate the cytoskeleton [11,52,54]. With regard to migration, neutrophils are the fastest moving cells at all with a speed maximum of 15 to 20 μm/min [61], and the starting signal for their migration to sites of inflammation is provided by early proinflammatory cytokines such as the bacterial peptide N-Formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) [62]. The bacterial peptide fMLP is the major chemotactic peptide produced by Escherichia coli and known to be a strong stimulator for the migration of neutrophil granulocytes. fMLP binds and activates a class of G-protein-coupled receptors. Ligand binding leads to the activation of two signalling pathways: (i) the activation of the PLC-gamma generates inositol-1,4,5-phosphate (IP3) and diacylglycerol (DAG), which results in IP3 mediated release of intracellularly stored calcium in the endoplasmatic reticulum and DAG-mediated activation of the protein kinase C (PKC). These are key events for the regulation of locomotory activity [62-64].(ii) the activation of the adenylyl cyclase leads to an increase of cytosolic cAMP, which results in an activation of the sarcoplasmatic/endoplasmatic reticulum calcium ATPase (SERCA) pump and calcium sequestration. Thus, stimulation of neutrophils with fMLP activates a signal transduction pathway ultimately leading to an elevation of cytosolic calcium which has been shown to be essential for the development of actin-based migration [65]. In addition, observations of migrating neutrophils within a three-dimensional collagen matrix revealed a frequent increase of calcium in those parts of the cells that underwent shape changes a few seconds later, and visualization of the calcium signal was shown to be a directionality marker for the orientation of neutrophils locomoting in a three-dimensional space [62]. With regard to cell migration, the inhibition of lymphocyte locomotion observed under microgravity culture conditions could be reversed by prior activation with phorbol myristate acetate (PMA), which directly activates the PKC [39].
Migration of immune cells is a crucial process during a multitude of physiological and pathophysiological conditions such as development, defense against infections and wound healing [36,37]. Leukocytes move through the body in order to keep the organism under immunological surveillance and to respond to pathogenic invading microorganisms. Migration within the body tissues and through endothelial barriers is strongly dependent and regulated both by cytoskeletal processes and by expression of surface adhesion molecules such as selectins and integrins [66], which interact with components of the extracellular matrices. Whereas the influence of microgravity on the cytoskeleton is well investigated [49], there is only little known about adhesion molecule expression in altered gravity. Importantly, the phenomenon of altered cytoskeletal organisation and migration in microgravity has been described well in non-adherent cells so far, but there is only little knowledge of cytoskeletal organisation in adherent cells, such as endothelial cells. Experiments on board of the Space Shuttle Mission STS-57 revealed a decrease of selectin-expression, but no change in ICAM-1 expression in splenocytes [10]. Moreover, long-term gravity vector changes modulate expression of ICAM-1, E-selectin and VCAM-1 on cultured endothelial cells, and increased adhesion of PMA-activated lymphocytes on endothelial monolayers in simulated and in real microgravity [67]. An experiment, which addressed the focal adhesion in connective tissue in microgravity, has been performed on board of STS-107 Spacelab [68], but got lost due to the fatal accident of the Space Shuttle Columbia in 2003. Thus, clear results about adhesion molecule expression after onset of altered gravity are still missing.
It is possible that the molecular and cellular structure of life on Earth may require gravity for survival, either in individual or in evolutionary terms, and it is therefore possible that exactly such gravity-dependent or gravity-sensing mechanisms will keep us dependent from the gravity field of Earth. No one can really neglect the importance of gravity on biological systems and only the facts that research platforms are rare and that access to altered gravity is limited, reduce the speed of progress in gravity research compared to other disciplines.
Technically, we are able to travel to Earth orbit or Moon for weeks up to months, and most probably, in the next decades we will be able to fly to Mars. But until now there is only limited knowledge about the biological and biomedical effects of weightlessness on organisms and humans, especially on the cellular and molecular level, where therapeutic or preventive countermeasures could be developed.
Competing interests
The authors declare that they have no competing interests.
Supplementary Material
Gravi-sensitive signal transduction elements in mammalian cells. The figure summarizes known gravi-sensitive signal transduction elements in mammalian cells. Please note, that the primary molecular mechanisms how microgravity influences cell signaling, are still unknown.
Contributor Information
Oliver Ullrich, Email: oliver.ullrich@anatom.uzh.ch.
Kathrin Huber, Email: kathrin.huber@anatom.uzh.ch.
Kerstin Lang, Email: lang@uni-wh.de.
References
- Konstantinova IV, Antropova YN, Legenkov VI, Zazhirey VD. Study of reactivity of blood lymphoid cells in crew members of the Soyuz-6, Soyuz-7 and Soyuz-8 spaceships before and after flight. Space Biol Med. 1973;7:48–55. [Google Scholar]
- Kimzey SL. Biomedical results from Skylab NASA-SP-377. National Aeronautics and Space Administration; 1977. Hematology and immunology studies; pp. 249–282. [Google Scholar]
- Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–1122. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- Häder DP, Hemmersbach R, Lebert M. Gravity and the behaviour of unicellular organisms. Cambridge, New York (United Kingdom, USA): Cambridge University Press; 2005. [Google Scholar]
- Hemmersbach R, Hader DP. Graviresponses of certain ciliates and flagellates. FASEB J. 1999;13:S69–75. doi: 10.1096/fasebj.13.9001.s69. [DOI] [PubMed] [Google Scholar]
- Cogoli A, Tschopp A, Fuchs-Bislin P. Cell sensitivity to gravity. Science. 1984;225:228–230. doi: 10.1126/science.6729481. [DOI] [PubMed] [Google Scholar]
- Cogoli A, Bechler A, Mueller O, Hunzinger E. Effects of Microgravity on Lymphocyte Activation. Exp 30011985, STS-61-A, Spacelab D1: Erasmus Experiment Archive. 1996.
- Cogoli A. Gravitational physiology of human immune cells: a review of in vivo, ex vivo and in vitro studies. J Gravit Physiol. 1996;3:1–9. [PubMed] [Google Scholar]
- Grove DS, Pishak SA, Mastro AM. The effect of a 10-day space flight on the function, phenotype, and adhesion molecule expression of splenocytes and lymph node lymphocytes. Exp Cell Res. 1995;219:102–109. doi: 10.1006/excr.1995.1210. [DOI] [PubMed] [Google Scholar]
- Hatton JP, Gaubert F, Cazenave JP, Schmitt D. Microgravity modifies protein kinase C isoform translocation in the human monocytic cell line U937 and human peripheral blood T-cells. J Cell Biochem. 2002;87:39–50. doi: 10.1002/jcb.10273. [DOI] [PubMed] [Google Scholar]
- Schmitt DA, Hatton JP, Emond C, Chaput D, Paris H, Levade T, Cazenave JP, Schaffar L. The distribution of protein kinase C in human leukocytes is altered in microgravity. FASEB J. 1996;10:1627–1634. doi: 10.1096/fasebj.10.14.9002555. [DOI] [PubMed] [Google Scholar]
- Cogoli A, Cogoli-Greuter M. Membrane binding of concanavalin A. Erasmus Experiment Archive; Exp 10041989, MASER 3.
- Boonyaratanakornkit JB, Cogoli A, Li CF, Schopper T, Pippia P, Galleri G, Meloni MA, Hughes-Fulford M. Key gravity-sensitive signaling pathways drive T cell activation. Faseb J. 2005;19:2020–2022. doi: 10.1096/fj.05-3778fje. [DOI] [PubMed] [Google Scholar]
- Bräucker R, Cogoli A, Hemmersbach R. Graviperception and Graviresponse at the Cellular Level. In: Horneck G, Baumstark-Khan C, editor. Astrobiology The Quest for the Conditions of Life. Berlin Heidelberg New York: Springer-Verlag; 2002. pp. 287–333. [Google Scholar]
- Bakos A, Varkonyi A, Minarovits J, Batkai L. Effect of simulated microgravity on human lymphocytes. J Gravit Physiol. 2001;8:P69–70. [PubMed] [Google Scholar]
- Kossmehl P, Shakibaei M, Cogoli A, Pickenhahn H, Paul M, Grimm D. Simulated microgravity induces programmed cell death in human thyroid carcinoma cells. J Gravit Physiol. 2002;9:P295–296. [PubMed] [Google Scholar]
- Uva BM, Masini MA, Sturla M, Tagliafierro G, Strollo F. Microgravity-induced programmed cell death in astrocytes. J Gravit Physiol. 2002;9:P275–276. [PubMed] [Google Scholar]
- Nakamura H, Kumei Y, Morita S, Shimokawa H, Ohya K, Shinomiya K. Antagonism between apoptotic (Bax/Bcl-2) and anti-apoptotic (IAP) signals in human osteoblastic cells under vector-averaged gravity condition. Ann N Y Acad Sci. 2003;1010:143–147. doi: 10.1196/annals.1299.023. [DOI] [PubMed] [Google Scholar]
- Cubano LA, Lewis ML. Fas/APO-1 protein is increased in spaceflown lymphocytes (Jurkat) Exp Gerontol. 2000;35:389–400. doi: 10.1016/s0531-5565(00)00090-5. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Takahashi A, Wang X, Ohnishi K, Ohira Y, Nagaoka S. Accumulation of a tumor suppressor p53 protein in rat muscle during a space flight. Mutat Res. 1999;430:271–274. doi: 10.1016/s0027-5107(99)00138-4. [DOI] [PubMed] [Google Scholar]
- Schwarzenberg M, Pippia P, Meloni MA, Cossu G, Cogoli-Greuter M, Cogoli A. Signal transduction in T lymphocytes – a comparison of the data from space, the free fall machine and the random positioning machine. Adv Space Res. 1999;24:793–800. doi: 10.1016/s0273-1177(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Walther I, Pippia P, Meloni MA, Turrini F, Mannu F, Cogoli A. Simulated microgravity inhibits the genetic expression of interleukin-2 and its receptor in mitogen-activated T lymphocytes. FEBS Lett. 1998;436:115–118. doi: 10.1016/s0014-5793(98)01107-7. [DOI] [PubMed] [Google Scholar]
- Cogoli A, Cogoli-Greuter M. Activation and proliferation of lymphocytes and other mammalian cells in microgravity. Adv Space Biol Med. 1997;6:33–79. doi: 10.1016/s1569-2574(08)60077-5. [DOI] [PubMed] [Google Scholar]
- Cogoli A. The effect of hypogravity and hypergravity on cells of the immune system. J Leukoc Biol. 1993;54:259–268. doi: 10.1002/jlb.54.3.259. [DOI] [PubMed] [Google Scholar]
- Hashemi BB, Penkala JE, Vens C, Huls H, Cubbage M, Sams CF. T cell activation responses are differentially regulated during clinorotation and in spaceflight. FASEB J. 1999;13:2071–2082. doi: 10.1096/fasebj.13.14.2071. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M, Chang T, Li CF. Effect of Gravity on Monocyte Differentiation. 10th ESA Life Sciences Symposium/29th Annual ISGP Meeting/24th Annual ASGSB Meeting/ELGRA Symposium "Life in Space for Life on Earth" Angers, France. 2008.
- Kaur I, Simons ER, Castro VA, Ott CM, Pierson DL. Changes in monocyte functions of astronauts. Brain Behav Immun. 2005;19:547–554. doi: 10.1016/j.bbi.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Meloni MA, Galleri G, Pippia P, Cogoli-Greuter M. Cytoskeleton changes and impaired motility of monocytes at modelled low gravity. Protoplasma. 2006;229:243–249. doi: 10.1007/s00709-006-0210-2. [DOI] [PubMed] [Google Scholar]
- Meloni MA, Galleri G, Pani G, Saba A, Pippia P, Cogoli-Greuter M. Effects of Real Microgravity Aboard International Space Station on Monocytes Motility and Interaction with T-Lymphocytes. 10th ESA Life Sciences Symposium/29th Annual ISGP Meeting/24th Annual ASGSB Meeting/ELGRA Symposium "Life in Space for Life on Earth" Angers, France. 2008.
- Buravkova LB, Rykova MP, Grigorieva V, Antropova EN. Cell interactions in microgravity: cytotoxic effects of natural killer cells in vitro. J Gravit Physiol. 2004;11:P177–180. [PubMed] [Google Scholar]
- Kaur I, Simons ER, Castro VA, Mark Ott C, Pierson DL. Changes in neutrophil functions in astronauts. Brain Behav Immun. 2004;18:443–450. doi: 10.1016/j.bbi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cogoli-Greuter M, et al. Mitogen binding, cytoskeleton patterns and motility of T-lymphocytes in microgravity. In: Cogoli A, Friedrich U, Mesland D, Demets R, editor. Life sciences experiments performed on sounding rockets (1985–1994) ESA SP-1206. pp. 59–70. [Google Scholar]
- Stowe RP, Sams CF, Mehta SK, Kaur I, Jones ML, Feeback DL, Pierson DL. Leukocyte subsets and neutrophil function after short-term spaceflight. J Leukoc Biol. 1999;65:179–186. doi: 10.1002/jlb.65.2.179. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Edelman LS, Chapes SK. Effects of corticosterone and microgravity on inflammatory cell production of superoxide. J Leukoc Biol. 1991;50:69–76. doi: 10.1002/jlb.50.1.69. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Horwitz AR, Parsons JT. Cell migration – movin' on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- Pellis NR, Goodwin TJ, Risin D, McIntyre BW, Pizzini RP, Cooper D, Baker TL, Spaulding GF. Changes in gravity inhibit lymphocyte locomotion through type I collagen. In Vitro Cell Dev Biol Anim. 1997;33:398–405. doi: 10.1007/s11626-997-0012-7. [DOI] [PubMed] [Google Scholar]
- Sundaresan A, Risin D, Pellis NR. Loss of signal transduction and inhibition of lymphocyte locomotion in a ground-based model of microgravity. In Vitro Cell Dev Biol Anim. 2002;38:118–122. doi: 10.1290/1071-2690(2002)038<0118:LOSTAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Plett PA, Abonour R, Frankovitz SM, Orschell CM. Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp Hematol. 2004;32:773–781. doi: 10.1016/j.exphem.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Cogoli-Greuter M, Meloni MA, Sciola L, Spano A, Pippia P, Monaco G, Cogoli A. Movements and interactions of leukocytes in microgravity. J Biotechnol. 1996;47:279–287. doi: 10.1016/0168-1656(96)01380-6. [DOI] [PubMed] [Google Scholar]
- Sciola L, Cogoli-Greuter M, Cogoli A, Spano A, Pippia P. Influence of microgravity on mitogen binding and cytoskeleton in Jurkat cells. Adv Space Res. 1999;24:801–805. doi: 10.1016/s0273-1177(99)00078-2. [DOI] [PubMed] [Google Scholar]
- Galimberti M, Tolic-Norrelykke IM, Favillini R, Mercatelli R, Annunziato F, Cosmi L, Liotta F, Santarlasci V, Maggi E, Pavone FS. Hypergravity speeds up the development of T-lymphocyte motility. Eur Biophys J. 2006;35:393–400. doi: 10.1007/s00249-006-0046-x. [DOI] [PubMed] [Google Scholar]
- Feldner JC, Brandt BH. Cancer cell motility – on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res. 2002;272:93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- Lee JS, Gotlieb AI. Microtubule-actin interactions may regulate endothelial integrity and repair. Cardiovasc Pathol. 2002;11:135–140. doi: 10.1016/s1054-8807(01)00080-1. [DOI] [PubMed] [Google Scholar]
- Hofman P, d'Andrea L, Guzman E, Selva E, Le Negrate G, Far DF, Lemichez E, Boquet P, Rossi B. Neutrophil F-actin and myosin but not microtubules functionally regulate transepithelial migration induced by interleukin 8 across a cultured intestinal epithelial monolayer. Eur Cytokine Netw. 1999;10:227–236. [PubMed] [Google Scholar]
- Verschueren H, Taelen I van der, Dewit J, De Braekeleer J, De Baetselier P, Aktories K, Just I. Effects of Clostridium botulinum C2 toxin and cytochalasin D on in vitro invasiveness, motility and F-actin content of a murine T-lymphoma cell line. Eur J Cell Biol. 1995;66:335–341. [PubMed] [Google Scholar]
- Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813–822. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- Schatten H, Lewis ML, Chakrabarti A. Spaceflight and clinorotation cause cytoskeleton and mitochondria changes and increases in apoptosis in cultured cells. Acta Astronaut. 2001;49:399–418. doi: 10.1016/s0094-5765(01)00116-3. [DOI] [PubMed] [Google Scholar]
- Uva BM, Masini MA, Sturla M, Prato P, Passalacqua M, Giuliani M, Tagliafierro G, Strollo F. Clinorotation-induced weightlessness influences the cytoskeleton of glial cells in culture. Brain Res. 2002;934:132–139. doi: 10.1016/s0006-8993(02)02415-0. [DOI] [PubMed] [Google Scholar]
- Uva BM, Strollo F, Ricci F, Pastorino M, Mason JI, Masini MA. Morpho-functional alterations in testicular and nervous cells submitted to modelled microgravity. J Endocrinol Invest. 2005;28:84–91. [PubMed] [Google Scholar]
- Lewis ML, Reynolds JL, Cubano LA, Hatton JP, Lawless BD, Piepmeier EH. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat) FASEB J. 1998;12:1007–1018. doi: 10.1096/fasebj.12.11.1007. [DOI] [PubMed] [Google Scholar]
- Gruener R, Roberts R, Reitstetter R. Reduced receptor aggregation and altered cytoskeleton in cultured myocytes after space-flight. Biol Sci Space. 1994;8:79–93. doi: 10.2187/bss.8.79. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M. Function of the cytoskeleton in gravisensing during spaceflight. Adv Space Res. 2003;32:1585–1593. doi: 10.1016/S0273-1177(03)90399-1. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M. Review of the biological effects of weightlessness on the human endocrine system. Receptor. 1993;3:145–154. [PubMed] [Google Scholar]
- Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford-Young SJ. Effects of microgravity on cell cytoskeleton and embryogenesis. Int J Dev Biol. 2006;50:183–191. doi: 10.1387/ijdb.052077sc. [DOI] [PubMed] [Google Scholar]
- Papaseit C, Pochon N, Tabony J. Microtubule self-organization is gravity-dependent. Proc Natl Acad Sci USA. 2000;97:8364–8368. doi: 10.1073/pnas.140029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infanger M, Kossmehl P, Shakibaei M, Bauer J, Kossmehl-Zorn S, Cogoli A, Curcio F, Oksche A, Wehland M, Kreutz R, et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell Tissue Res. 2006;324:267–277. doi: 10.1007/s00441-005-0142-8. [DOI] [PubMed] [Google Scholar]
- Vassy J, Portet S, Beil M, Millot G, Fauvel-Lafeve F, Gasset G, Schoevaert D. Weightlessness acts on human breast cancer cell line MCF-7. Adv Space Res. 2003;32:1595–1603. doi: 10.1016/S0273-1177(03)90400-5. [DOI] [PubMed] [Google Scholar]
- Entschladen F, Zanker KS. Locomotion of tumor cells: a molecular comparison to migrating pre- and postmitotic leukocytes. J Cancer Res Clin Oncol. 2000;126:671–681. doi: 10.1007/s004320000143. [DOI] [PubMed] [Google Scholar]
- Lang K, Hatt H, Niggemann B, Zaenker KS, Entschladen F. A novel function for chemokines: downregulation of neutrophil migration. Scand J Immunol. 2003;57:350–361. doi: 10.1046/j.1365-3083.2003.01247.x. [DOI] [PubMed] [Google Scholar]
- Entschladen F, Niggemann B, Zanker KS, Friedl P. Differential requirement of protein tyrosine kinases and protein kinase C in the regulation of T cell locomotion in three-dimensional collagen matrices. J Immunol. 1997;159:3203–3210. [PubMed] [Google Scholar]
- Schorr W, Swandulla D, Zeilhofer HU. Mechanisms of IL-8-induced Ca2+ signaling in human neutrophil granulocytes. Eur J Immunol. 1999;29:897–904. doi: 10.1002/(SICI)1521-4141(199903)29:03<897::AID-IMMU897>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stossel TP. The E. Donnall Thomas Lecture, 1993. The machinery of blood cell movements. Blood. 1994;84:367–379. [PubMed] [Google Scholar]
- Ullrich O, Diestel A, Eyupoglu IY, Nitsch R. Regulation of microglial expression of integrins by poly(ADP-ribose) polymerase-1. Nat Cell Biol. 2001;3:1035–1042. doi: 10.1038/ncb1201-1035. [DOI] [PubMed] [Google Scholar]
- Buravkova L, Romanov Y, Rykova M, Grigorieva O, Merzlikina N. Cell-to-cell interactions in changed gravity: ground-based and flight experiments. Acta Astronaut. 2005;57:67–74. doi: 10.1016/j.actaastro.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lapere C, Kholti A. Function of the Focal Adhesion of Plaque of Connective Tissue in microgravity (CONNECT) STS-107 Spacelab, Erasmus Experiment Archive. 2003.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gravi-sensitive signal transduction elements in mammalian cells. The figure summarizes known gravi-sensitive signal transduction elements in mammalian cells. Please note, that the primary molecular mechanisms how microgravity influences cell signaling, are still unknown.