Abstract
Platelet-derived growth factor (PDGF) is a potent stimulator of wound healing. PDGF gene therapy may promote greater periodontal regeneration than local protein application, due to sustained growth factor delivery to the target tissue. This investigation tested the ability of recombinant adenoviruses (rAds) encoding PDGF-A or PDGF-1308 (a PDGF-A dominant-negative mutant that disrupts endogenous PDGF bioactivity) to affect cells derived from the periodontium. Osteoblasts, periodontal ligament fibroblasts, and gingival fibroblasts were transduced with rAds, and gene expression, DNA synthesis, and cell proliferation were evaluated. The results revealed strong message for the PDGF-A gene for 7 days following gene delivery. Ad2/PDGF-A enhanced the mitogenic and proliferative response in all cell types, while Ad2/PDGF-1308 potently inhibited mitogenesis and proliferation. In conclusion, Ad2/PDGF can effectively transduce cells derived from the periodontium and promote biological activity equivalent to PDGF-AA. These studies support the potential use of gene therapy for sustained PDGF release in periodontal tissues.
Keywords: gene transfer, platelet-derived growth factor, growth factors, adenovirus, tissue engineering
INTRODUCTION
Platelet-derived growth factor (PDGF) is an important molecule in the promotion of wound repair. PDGF is released following injury from platelets and is produced by numerous cell types during tissue regeneration. PDGF mediates its signal via two distinct high-affinity transmembrane receptors possessing intrinsic tyrosine kinase activity, termed PDGFR-α and –β (Rosenkranz and Kazlauskas, 1999). PDGF is a potent mitogen and chemoattractant for many cell types, including those derived from the periodontium, such as periodontal ligament (PDL) fibroblasts, gingival fibroblasts, and osteoblasts (Piché et al., 1989). Following acute cutaneous injury, genes for PDGF and corresponding PDGF receptors are induced at the site of the wounding (Antoniades et al., 1991; Green et al., 1997).
PDGF also has powerful effects in stimulating wound healing of a multitude of tissue types, such as skin, bone, and periodontium (Grotendorst et al., 1985; Park et al., 1995; Mitlak et al., 1996). Local administration of PDGF to periodontal osseous defects leads to significant regeneration of bone, cementum, and periodontal ligament (Giannobile et al., 1996). However, results from studies utilizing topical application of these factors have shown incomplete periodontal regeneration (Giannobile, 1999). One limitation of local protein delivery of PDGF is the short half-life of the molecule in the wound site (approximately 4 hrs) (Lynch et al., 1991). Thus, alternative methods to deliver PDGF may stimulate greater regeneration of periodontal structures.
Gene therapy is a recognized methodology for the delivery of therapeutic levels of proteins for extended periods of time to tissues (Baum and Mooney, 2000). Examples of various delivery systems include adenovirus, retrovirus, and DNA-lipid conjugates. Adenovirus has been a popular method for the incorporation of transgenes, due to its well-characterized genome and ability to infect both replicating and quiescent cells (Kozarsky and Wilson, 1993).
Therefore, the use of DNA delivery systems may serve as another method of targeting proteins to a wound site, since protein delivery systems provide such a short duration of action of the applied factor (Bonadio et al., 1998). Furthermore, assessing the longer-term (more than a few hours) exposure of cells to PDGF may aid in better understanding of the mechanisms of tissue regeneration.
The objectives of this study were to test the ability of recombinant adenoviruses encoding PDGF transgenes, PDGF-A and PDGF-1308, to transduce and modulate proliferative activity of cells associated with the periodontium.
MATERIALS & METHODS
Construction of Recombinant Adenoviruses
Methods for the construction of replication-deficient adenoviral vectors have been described previously (Schmid and Hearing, 1999). Briefly, first, the full-length murine PDGF-A or PDGF-1308 cDNA (gifts of Dr. Charles D. Stiles, Boston, MA, USA) was subcloned into a shuttle plasmid pAD2/CMV/SVIX (obtained from Genzyme Corporation, Cambridge, MA, USA), under the control of the cytomegalovirus promoter. The viral backbone DNA Ad2/EGFP was pre-cut with PshAI, and the shuttle plasmid containing either PDGF-A or PDGF-1308 cDNA was linearized with XbaI. The linearized shuttle plasmid and viral backbone DNA were co-transfected into 293 packaging cells (human embryonic kidney cells transformed with adenovirus) by means of calcium phosphate transfection. Recombination between the shuttle plasmid and the GFP viral backbone resulted in replacement of the GFP cDNA with PDGF-A or PDGF-1308 cDNA. At 8–10 days post-transfection, recombinant viral plaques were readily identified under inverted fluorescent microscope by lack of fluorescence. Recombinant plaques were picked, plaque-purified for three rounds, and expanded for large-scale purification of the viral stocks by ultracentrifugation with a cesium chloride gradient. The cesium-chloride-containing viral stocks were de-salted with Econo-Pac® 10 DG chromatography columns (Bio-Rad, Hercules, CA, USA). The viral stocks were stored in 10 mM Tris-HCl, pH 7.4, 1 mM MgCl2, 10% glycerol at −80°C. Titers of the virus stocks were determined on 293 cells by plaque formation assay and expressed as the number of plaque-forming units (pfu) per mL. Typical titers achieved were in the range of 109–1011 pfu/mL.
Gene Transfer to Periodontal Cells
Cells associated with the periodontium included human gingival fibroblasts (hGFs), murine periodontal ligament cells (SV-PDL) (kind gifts of Dr. Martha Somerman, University of Michigan; D’Errico et al., 1999), and MG-63 osteogenic cells which were plated at subconfluence in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% bovine calf serum (10% BCS) and antibiotics. Cells were then transduced with rAds (Ad2/GFP, Ad2/PDGF-A, or Ad2/PDGF-1308) by multiplicities of infection (MOIs), ranging from 0.1–100, depending on the experiment. After a period of 4–5 hrs of being shaken, the medium was replenished with fresh 10% BCS, and the cells were maintained and evaluated for subsequent experiments (below).
Flow Cytometry
To ascertain transduction efficiency of the Ad2/CMV/GFP (which was used to generate PDGF-A and PDGF-1308 recombinant adenoviruses) to MG-63, hGF, and SV-PDL cells, we performed flow cytometry. The cells were plated at a seeding density of 1 × 106 cells/dish in 60-mm culture dishes. Cells received either no treatment (NT) or treatment with an ascending dose range of Ad2/GFP (MOI = 0.1–100). The cell monolayers were harvested at days 1, 2, 4, and 7 after infection. Single cell suspensions were collected and assessed by cell sorting at the University of Michigan Flow Cytometry Facility. Fluorescence intensity was plotted against total cell number to calculate the proportion of cells demonstrating fluorescence (i.e., percentage of cells expressing GFP).
Northern Blotting
MG-63 osteogenic cells, SV-PDL cells, and hGFs were plated at a density of 4 × 105 cells/well in six-well plates. After 24 hrs, the cells were transduced with either Ad2/PDGF-A or control virus (Ad2/GFP) (at MOIs 1–100)) for 4–5 hrs. After 24 hrs, total RNA was isolated from the cells and quantified by spectrophotometry as previously described (Xie and Rothblum, 1991). A 3-µg quantity of total RNA was electrophoresed on 6% formaldehyde-1.2% agarose gels, transferred to nylon membranes (Strategene, Inc., LaJolla, CA, USA), and immobilized by UV crosslinking (Strategene). Membranes were hybridized with the murine PDGF-A cDNA probe (gift of Dr. Charles D. Stiles, Boston, MA, USA) and labeled by means of a horseradish peroxidase chemiluminescence technique (Amersham Life Science, Buckinghamshire, England). Blots were exposed to autoradiographic film (Amersham) for 20 min to 24 hrs. Relative loading of wells was evaluated by ethidium bromide staining of the original agarose gel and hybridization of blots with 18S rRNA (Renkawitz and Kunz, 1975). Transduction efficiency of Ad2/PDGF-A gene transfer was compared over the dose range of 1–100 MOI. PDGF-A gene expression was also analyzed at 7 days post-Ad2/GFP or Ad2/PDGF-A transduction, for the evaluation of prolonged gene expression.
Immunohistochemistry
For cell culture staining experiments, cells were plated at a density of 2.0 × 104 cells/well in 4-well RS-coated Lab-Tek® II chamber slides (Nalge Nunc Int., Naperville, IL, USA). After 24 hrs, the medium was changed to DMEM supplemented with 5% platelet-poor plasma. The cells were subsequently transduced with either Ad2/GFP (MOI = 100) or Ad2/PDGF-A (MOI = 100). After 24 hrs, the cells were fixed with 2% paraformaldehyde, permeabilized with 0.5% IGEPAL (Sigma Chemical Co., St. Louis, MO, USA), and blocked with 2% normal goat serum. Following treatment with primary antibody (1:50 dilution) specific for PDGF-A (Santa Cruz Biotechnology, Santa Cruz, CA, USA), the cells were processed for peroxidase staining by means of a commercial avidin-biotin complex method kit (Vector Laboratories, Burlingame, CA, USA) and True Blue (KPL Laboratories, Gaithersburg, MD, USA) substrate.
DNA Synthesis Assay
Mitogenic activity due to rAd delivery was evaluated by means of a standard BALB/c 3T3 bioassay as previously described for PDGF biological activity (Klenow and Flodgaard, 1983). These cells were utilized because of their well-documented response to platelet-derived growth factor isoforms. These cells were used as a tool for assessment of the effects of PDGF-A and -1308 transgenes on cell growth (compared with the variable growth characteristics of some of the transformed cells used) [i.e., MG-63 and SV-PDL] prior to cell proliferation studies. The cells were plated at a seeding density of 2500 cells/well in 96-well plates for 5 days to a stage of quiescence (with cells depleting the majority of growth factor activity by 5 days in trial experiments [data not shown]). Cells were then washed 3 times with PBS and stimulated in serum-free medium containing either negative control (1% bovine serum albumin [BSA]); positive control (10% BCS); rhPDGF-AA (Upstate Biotechnology, Inc., Lake Placid, NY, USA; at either 2 or 20 ng/mL); Ad2/PDGF-A (MOIs 1–100), or Ad2/PDGF-1308 (MOIs 1–100) or Ad2/GFP (MOI = 100) for a period of 24 hrs. To confirm activity of PDGF-A in relation to PDGF-1308, we conducted a second set of experiments which involved co-infecting the BALB/c 3T3 cells with both Ad2/PDGF-A (MOI = 100) and Ad2/PDGF-1308 (MOI 1–100) or Ad2/GFP (1–100). These experiments determined the reversibility of DNA synthesis stimulation of PDGF-A gene transfer by PDGF-1308. One µCi of [3H]thymidine (NEN/DuPont)/well (5 µCi/mL) was added to the cells during the final 6 hrs of incubation. Each well was fixed with ice-cold trichloroacetic acid (TCA) for 20 min. The wells were then washed three times with TCA. The plates were subjected to the addition of 0.25 N NaOH (37°C) for 15–30 min, and 0.75 N HCl was added to neutralize the solution. The contents from each well were transferred to scintillation vials for measurement of [3H]thymidine uptake. Tritium levels were assessed by measurement on a Wallac 1410 liqulid scintillation counter. Mitogenic activity was plotted as CPM vs. treatment concentration. The results were expressed as CPM per 10 wells of 3 separate experiments.
Cell Proliferation
MG-63, hGF, and SV-PDL cells were plated in 24-well plates at a density of 2.5 × l04 Cells/well in 10% BCS. After a period of 24 hrs to allow for cell attachment, the medium was changed to 5% platelet-poor plasma supplemented with either 20 ng/mL rhPDGF-AA, Ad2/GFP (MOI = 100)( Ad2/PDGF-A (MOI = 100), or Ad2/PDGF-1308 (MOI = 100). The medium was changed every other day, and cells were harvested at days 1, 2, 4, and 7 after treatment. Cell counts were made by means of a hemocytometer on triplicate cell culture wells.
Statistical Analysis
We performed an ANOVA with the Bonferroni-Dunn multiple-comparison procedure to evaluate DNA synthesis and proliferation comparing treatments with corresponding controls. An alpha level of 0.05 was used to determine statistical differences between groups.
RESULTS
Results generated from these studies showed the effective transduction of the recombinant adenoviruses to the cells derived from periodontal tissues with corresponding various effects on the modulation of DNA synthesis and cell proliferation.
Flow Cytometry
Transduction of cells by means of the control virus Ad2/GFP demonstrated a dose-dependent effect on GFP expression (From MOI 0.1–100) for up to 7 days following gene delivery. The percentage of cells exhibiting fluorescence as an indicator of GFP protein expression was maintained at 95%, 94.1%, and 89%, respectively, for hGFs, SV-PDL cells, and MG-63 cells at MOI = 100 by 7 days. MOI = 100 was chosen for subsequent gene transfer experiments, since the highest transduction efficiency was shown by this MOI without altering cell growth (data not shown).
Northern Blotting
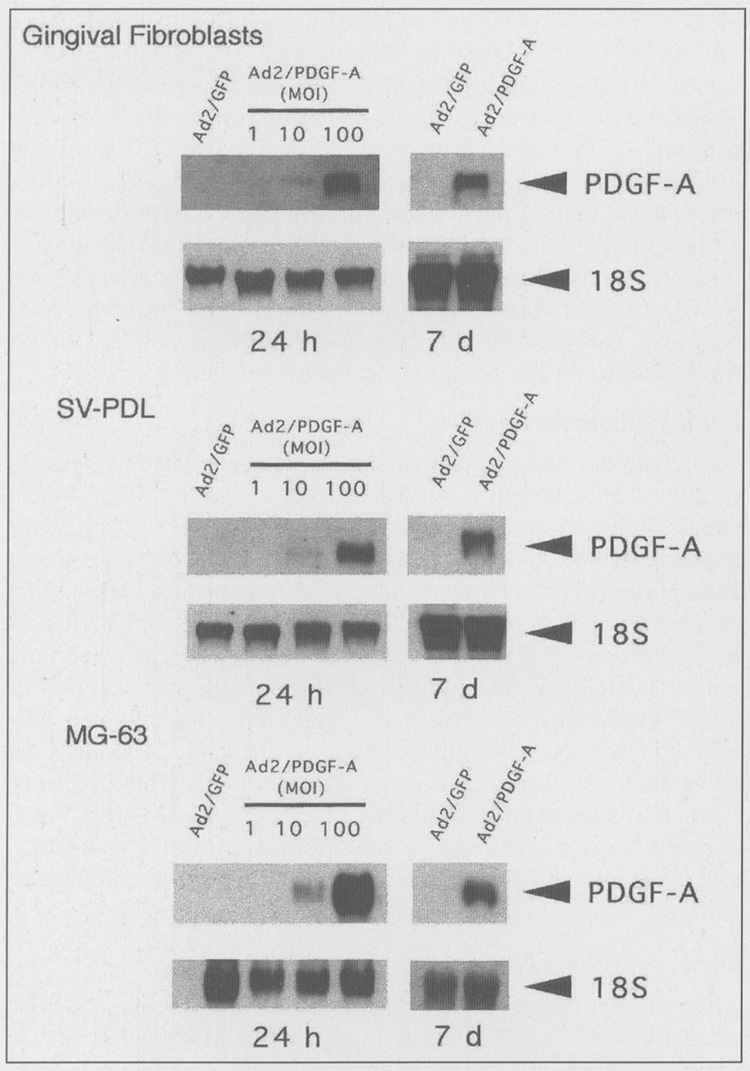
The dose-dependent PDGF-A transduction efficiency elicited by Ad2/PDGF-A on hGF, SV-PDL, and MG-63 cells is shown in Fig. 1. The ascending dose range of Ad2/PDGF-A (MOI = 1–100) to the cells revealed increasing gene expression that was detectable at 24 hrs at MOI = 10 and 100. High-level gene expression was noted for all cell types at MOI = 100. Prolonged gene expression of PDGF-A was also seen at 7 days following gene delivery. In contrast, no detectable PDGF-A gene expression was found in Ad2/GFP-transduced cells.
Figure 1.
A representative Northern analysis demonstrating dose-dependent gene expression of PDGF-A by infected periodontal cells 24 hrs and 7 days after transduction. Gingival fibroblasts, SV-PDL, and MG-63 osteogenic cells were transduced with Ad2/GFP at MOI = 100 or Ad2/PDGF-A at MOI = 1, 10, or 100, as described in MATERIALS & METHODS. A dose response of PDGF-A gene expression was noted following Ad2/PDGF-A delivery. Prolonged gene expression was found 7 days after PDGF-A gene transfer.
Immunohistochemistry
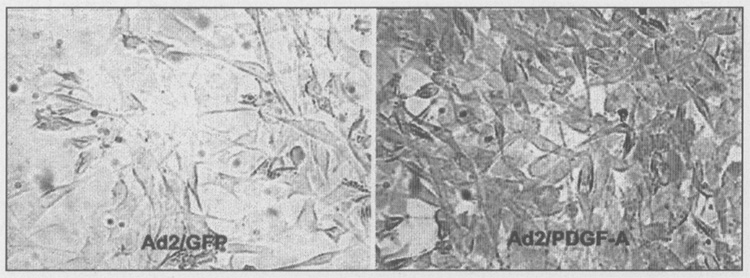
Immunohistochemical analysis of periodontal cells transduced with Ad2/PDGF-A revealed positive staining with the PDGF-AA antibody (Fig. 2) within 24 hrs of gene transfer. For comparison, cells transduced with Ad2/GFP failed to demonstrate measurable levels of PDGF-AA protein.
Figure 2.
Immunohistochemical detection of PDGF-AA protein by MG-63 osteogenic cells transduced with Ad2/PDGF-A. MG-63 cells were plated at subconfluence for 24 hrs in DMEM supplemented with 10% BCS. The medium was changed to DMEM supplemented with 5% platelet-poor plasma and cells transduced with either Ad2/GFP or Ad2/PDGF-A at MOI = 1 00. After 24 hrs, the cells were fixed with 2% paraformaldehyde, permeabilized with 0.5% IGEPAL, and blocked with 2% normal goat serum. Following treatment with primary antibody specific for PDGF-A, the cells were processed for peroxidase staining by means of a commercial avidin-biotin complex method kit and True Blue (KPL Laboratories, Gaithersburg, MD, USA) substrate. MG-63 cells transduced with Ad2/PDGF-A display positive staining consistent with PDGF-AA protein expression, while cells exposed to Ad2/GFP show a paucity of staining. Magnification, x20, phase contrast.
DNA Synthesis
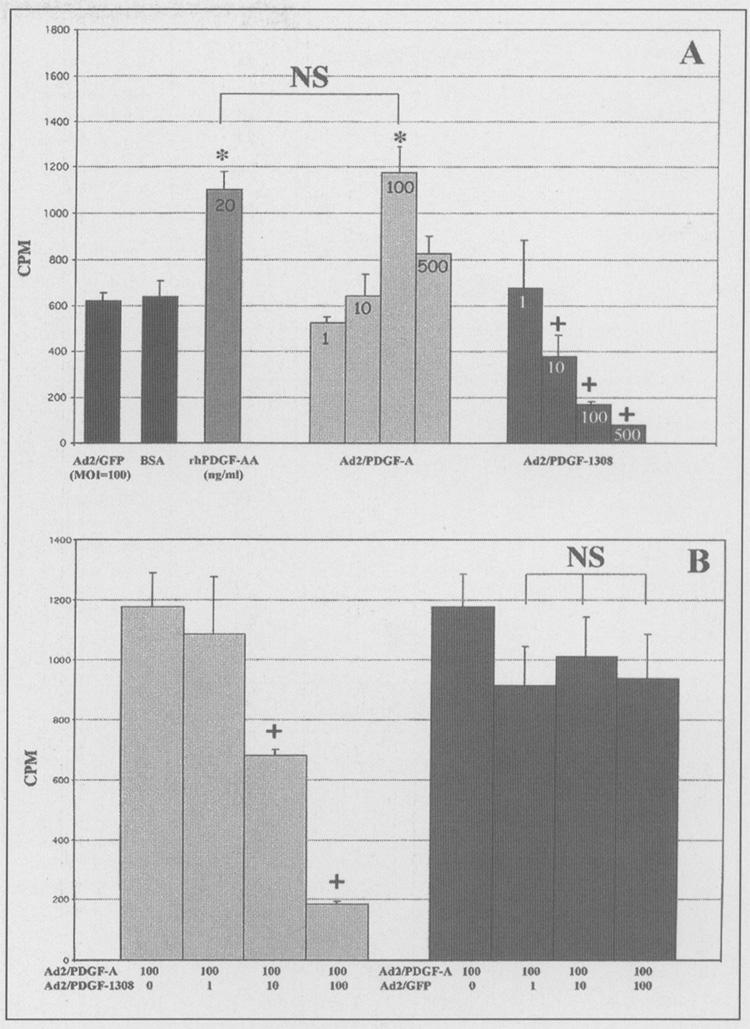
Assessment of biological activity as measured by [3H]thymidine uptake of DNA synthesis is shown in Fig. 3. Confirmation of transduction efficiency was evaluated qualitatively by Ad2/GFP and noting that efficiency approached 100% by visual assessment of fluorescence 24–48 hrs after infection (data not shown). Ad2/PDGF-A treatment of BALB/c 3T3 fibroblasts significantly stimulated DNA synthesis above that of control virus (Ad2/GFP) or 1% BSA (Fig. 3A). Furthermore, when Ad2/PDGF-A was compared with 20ng/mL of rhPDGF-AA, the stimulation was indistinguishable (p > 0.05) at MOI = 100. In contrast, there was a reduction in DNA synthesis at all tested MOIs with Ad2/PDGF-1308 (Fig. 3A). Fig. 3B illustrates the reversibility of Ad2/PDGF-A DNA synthesis stimulation when the cells were co-infected with Ad2/PDGF-1308 (at MOI = 10 and 100), while Ad2/GFP failed to influence Ad2/PDGF-A stimulation of [3H]thymidine uptake.
Figure 3.
Recombinant adenoviruses encoding platelet-derived growth factors modulate DNA synthesis in vitro. (A) BALB/c 3T3 fibroblasts were plated at a density of 2500 cells/well in 96-well plates. After 5 days quiescent cells were treated with either 1% BSA (control), Ad2/GFP (control reporter virus), rhPDGF-AA (20 ng/mL), Ad2/PDGF-A, or Ad2/PDGF- 1308. Data are plotted as counts per minute (CPM) vs. treatment group. Note statistically significant (p < 0.01) stimulation of DNA synthesis above controls with rhPDGF-AA and Ad2/PDGF-A at MOI = 10 and 100. No significant difference was found between rhPDGF-AA and Ad2/PDGF-A at MOI = 10 and 100. A significant reduction in [3H]thymidine uptake was found when Ad2/PDGF-A MOI = 500 was used. A dose response in inhibition of DNA synthesis was noted as MOI increased from 1 to 100 when the dominant-negative mutant (PDGF-1308) was used (p < 0.01). (B) PDGF-1308 reverses the effects of Ad2/PDGF-A on DNA synthesis when Ad2/PDGF-1308 and Ad2/PDGF-A are co-infected in BALB/c 3T3 cells (p < 0.01). No significant reversal of Ad2/PDGF-A mitogenic stimulation was noted with Ad2/GFP (MOI = 1–100). NS (not significant); *p < 0.01 (significant increase vs. Ad2/GFP); +p < 0.01 (significant decrease vs. Ad2/PDGF-A [MOI = 100] and Ad2/GFP). N = 10 for each of 3 separate experiments in panel A; N = 5 for 2 separate experiments in panel B. Bars represent ± the standard deviation.
Cell Proliferation
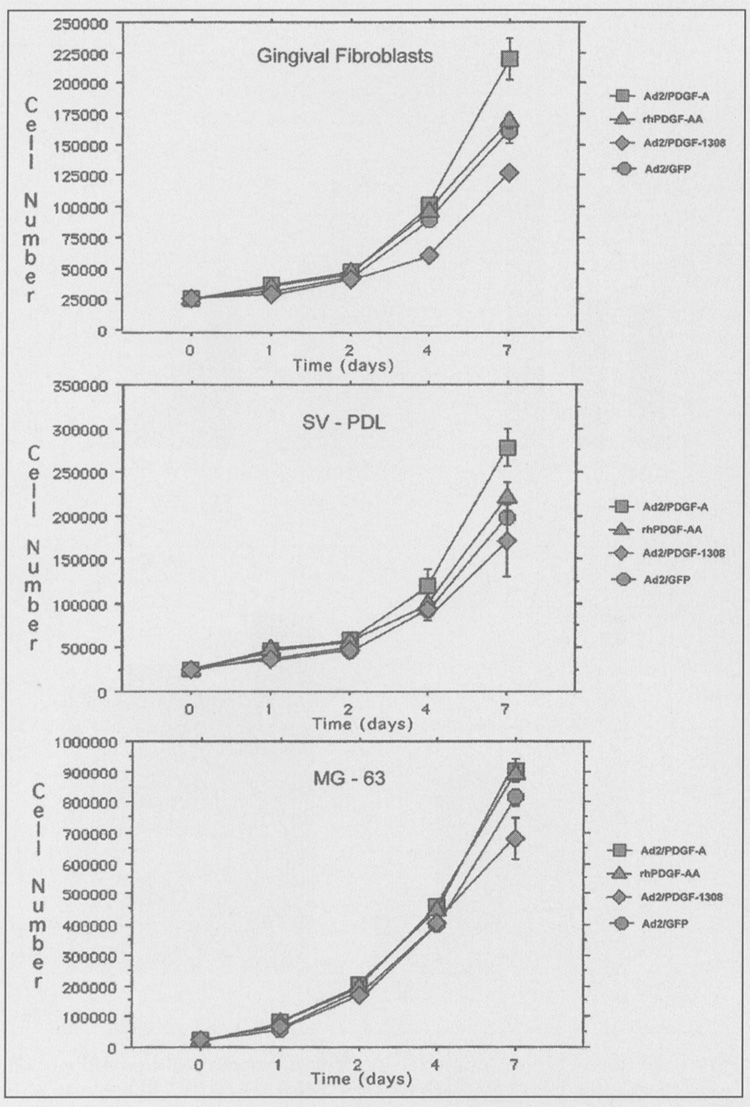
Since PDGF is a potent growth stimulator of periodontal cells, we compared continuous exposure of cells to PDGF vs. PDGF gene transfer using adenovirus (Fig. 4). A statistically significant increase (p < 0.05) in gingival fibroblast proliferation was found at day 7 between Ad2/PDGF-A and all other treatments. Ad2/PDGF-1308 significantly decreased gingival fibroblast proliferation compared with all other treatments at both days 4 and 7 (p < 0.05). Ad2/PDGF-A significantly enhanced SV-PDL cell proliferation above all other treatments at both days 4 and 7 (with the exception of rhPDGF-AA at day 4), while Ad2/PDGF-1308 significantly decreased SV-PDL growth at day 7 compared with all treatments (p < 0.05). Ad2/PDGF-A only minimally affected MG-63 cell proliferation, and increases were not statistically significant (p > 0.05). However, Ad2/PDGF-1308 significantly (p < 0.05) inhibited MG-63 cell growth by day 7 compared with the reporter virus, rhPDGF-AA and Ad2/PDGF-A (Fig. 4).
Figure 4.
Proliferation of periodontal cells following treatment with recombinant adenoviruses. Gingival fibroblasts, SV-PDL cells, and MG-63 cells were plated at subconfluence in DMEM supplemented with 10% BCS for 12 hrs. At day 0, the medium was changed to DMEM containing either 5% platelet-poor plasma treated by Ad2/GFP (circles), Ad2/PDGF-A (squares), rhPDGF-AA (triangles), or Ad2/PDGF-1308 (diamonds) The cells were counted at days 1, 2, 4, and 7 after treatment. The experiment was repeated thrice, with typical results shown above. A statistically significant increase (p < 0.05) in gingival fibroblast proliferation was found at day 7 between Ad2/PDGF-A and all other treatments. Ad2/PDGF-1308 significantly decreased gingival fibroblast proliferation compared with all other treatments at both days 4 and 7 (p < 0.05) Ad2/PDGF A significantly enhanced SV-PDL cell proliferation above all other treatments at both days 4 and 7 (with the exception of rhPDGF-AA at day 4), while Ad2/PDGF-1308 significantly decreased SV-PDL growth at day 7 compared with all treatments (p < 0.05). Ad2/PDGF-A affected MG-63 cell proliferation only minimally, and increases were not statistically significant (p > 0.05). However, Ad2/PDGF-1308 significantly (p < 0.05) inhibited MG-63 cell growth by day 7 compared with the reporter virus, rhPDGF-AA, and Ad2/PDGF-A. All viruses were delivered to the cells at MOI = 100. Error bars represent ± standard error for n = 3 when error bars are not visible, the error is encompassed within the corresponding data point.
DISCUSSION
We have shown the construction of recombinant adenoviruses encoding the genes PDGF-A and PDGF-1308. These studies demonstrate the effective, prolonged expression of the PDGF-A gene in a variety of cells associated with the periodontium. Furthermore, we report the modulation of DNA synthesis and cellular proliferation by PDGF transgenes.
These data showing prolonged expression of rAds give encouraging possibilities for a sustained growth factor delivery to periodontal tissues. However, given the presence of foreign adenoviral proteins that are produced, a shorter-term transduction would be expected in vivo. It has been reported that a local, dose-dependent, inflammatory response is found in target tissues from immunocompetent hosts (Herz and Gerard, 1993). However, investigations have shown that transgene expression can be extended in immunodeprived or immunodeficient hosts (Engelhardt et al., 1994). There is promise in reducing the effect of cytotoxic T-cell-mediated response by the use of “gutless viruses” which lack the expression of many viral proteins (Hardy et al., 1997). Hence, the development of second- and third-generation adenoviral vectors may enhance the potential for long-term expression of transgenes by adenovirus in vivo.
Ad2/PDG F-A potently stimulated DNA synthesis in the standard BALB/c 3T3 bioassay as well as cellular proliferation in all of the cell types tested. The level of stimulation was either superior to or equivalent to rhPDGF-AA application in both assay systems, suggesting that gene delivery can produce biological activity in vitro similar to rhPDGF-AA. Of added interest was the reduction of DNA synthesis and cellular proliferation with use of the dominant-negative mutant PDGF-1308. Furthermore, co-infection of Ad2/PDGF-1308 and Ad2/PDGF-A reversed Ad2/PDGF-A stimulation of DNA synthesis. Platelet-derived growth factor-1308 is a mutant of the murine PDGF-A gene that contains a substitution of serine for Cys129 which destabilizes PDGF-A and PDGF-B subunits within the cell (Mercola et al., 1990). Genes incorporating PDGF-1308 suppress wild-type PDGF-A gene expression in a trans-dominant fashion. Suppression occurs when the mutant PDGF subunits dimerize with wild-type subunits to form inactive or unstable heterodimers. Shamah et al. (1993) demonstrated that PDGF-1308 breaks autocrine loops in human astrocytoma cell lines and reverts the phenotype of BALB/c 3T3 cells transformed by the PDGF-A or PDGF-B (c-sis) gene. Analysis of our data suggests that gene delivery of Ad2/PDGF-1308 results in a diminution of cell proliferation due to inactivation of endogenous PDGF-A or PDGF-B chains assembled which may have an effect on autocrine activity. Future use of Ad2/PDGF-1308 will allow for local “knock-outs” of PDGF expression in vivo, which may aid in a better understanding of the role of PDGF in wound repair. The magnitude of stimulation of cell proliferation by Ad2/PDGF-A for MG-63 cells was not as profound as was shown for hGFs and was indistinguishable from PDGF-AA application. Indeed, MG-63 cells appear to possess altered growth characteristics and responses to growth factors, which may explain the minimal response to Ad2/PDGF (e.g., PDGF induces c-myc in MG-63 cells without corresponding significant enhancements in cell growth) (Womer et al., 1987). Thus, other cell lines should be assessed for the determination of responses to PDGF gene transfer.
Although PDGF application stimulates bone regeneration in vivo (Mitlak et al., 1996) and is produced in the healing fracture callus (Andrew et al., 1995), long-term PDGF exposure in orthotopic defects may yield differing results, depending on the mode of delivery. PDGF gene transfer to skin by particle bombardment (Eming et al., 1999) or released from biodegradable polymers (Shea et al., 1999) promotes granulation tissues formation and angiogenesis. However, recent studies have shown that prolonged exposure of osteoblastic cells to PDGF stimulates proliferation, while decreasing the expression of the osteoblast phenotype (with subsequent inhibition of mineralization in vitro [Yu et al., 1997; Hsieh and Graves, 1998]). Furthermore, “pulses” of PDGF for 3 days significantly enhances bone formation (Hsieh and Graves, 1998). These results may be due to the fact that chronic PDGF exposure to wounds results in fibrosis and granulation tissue formation (as seen in chronically inflamed tissues such as pulmonary fibrosis and failing dental implants) (Antoniades et al., 1990; Salcetti et al., 1997), while short-term bolus delivery results in osteogenesis. Therefore, regulation of the spatial and temporal levels of PDGF gene expression in vivo is likely to have a major influence on the resulting composition of newly developing or regenerating tissues exposed to Ad/PDGFs.
In conclusion, the results from this study demonstrate, for the first time, successful gene transfer of PDGFs to cells which comprise the periodontium. PDGF gene transfer results in prolonged gene expression that modulates DNA synthesis and cellular proliferation. This approach potentially offers a new mode of growth factor delivery to periodontal tissues. Current studies are in progress to improve our understanding of the role of PDGF gene transfer in periodontal wound repair in vivo.
ACKNOWLEDGMENTS
The authors acknowledge the helpful discussions with Dr. Charles D. Stiles. The technical assistance of Mr. Matthew Tomala and Ms. Marjon Jahromi is appreciated. This study was funded by NIH/NIDCR Grants DE 11960 and DE 13047 to WVG.
REFERENCES
- Andrew JG, Hoyland JA, Freemont AJ, Marsh DR. Platelet-derived growth factor expression in normally healing human fractures. Bone. 1995;16:455–460. doi: 10.1016/8756-3282(95)90191-4. [DOI] [PubMed] [Google Scholar]
- Antoniades HN, Bravo MA, Avila RE, Galanopolus T, Neville-Golden J, Maxwell M, et al. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PGDF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci USA. 1991;88:565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ, Mooney DJ. The impact of tissue engineering on dentistry. J Am Dent Assoc. 2000;131:309–318. doi: 10.14219/jada.archive.2000.0174. [DOI] [PubMed] [Google Scholar]
- Bonadio J, Goldstein SA, Levy RJ. Gene therapy for tissue repair and regeneration. Adv Drug Del Rev. 1998;33:53–69. doi: 10.1016/s0169-409x(98)00020-9. [DOI] [PubMed] [Google Scholar]
- D’Errico JA, Ouyang H, Berry JE, MacNeil RL, Strayhorn C, Imperiale MJ, et al. Immortalized cementoblasts and periodontal ligament cells in culture. Bone. 1999;25:39–47. doi: 10.1016/s8756-3282(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Eming SA, Whitsitt JS, He L, Krieg T, Morgan JR, Davidson JM. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J Invest Dermatol. 1999;112:297–302. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile WV. Periodontal tissue regeneration by growth factor polypeptides and gene transfer. In: Lynch SE, Genco RJ, Marx RE, editors. Tissue engineering: color applications in maxillofacial surgery and periodontics. Vol. 1. Chicago: Quintessence; 1999. pp. 231–243. [Google Scholar]
- Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D’Andrea M, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. J Periodontal Res. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Martin GR, Pencev D, Sodek J, Harvey AK. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985;76:2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci USA. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SC, Graves DT. Pulse application of platelet-derived growth factor enhances formation of a mineralizing matrix while continuous application is inhibitory. J Cell Biochem. 1998;96:169–180. doi: 10.1002/(sici)1097-4644(19980501)69:2<169::aid-jcb7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Klenow H, Flodgaard H. Both hypoxanthine and adenosine stimulate DNA synthesis independently in serum-starved L cells treated with platelet protein. Proc Natl Acad Sci USA. 1983;80:7420–7423. doi: 10.1073/pnas.80.24.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky KF, Wilson JM. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991;62:458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- Mercola M, Deininger PL, Shamah SM, Porter J, Wang CY, Stiles CD. Dominant-negative mutants of a platelet-derived growth factor gene. Genes Dev. 1990;4:2333–2341. doi: 10.1101/gad.4.12b.2333. [DOI] [PubMed] [Google Scholar]
- Mitlak BH, Finkelman RD, Hill EL, Li J, Martin B, Smith T, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238–247. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, et al. Periodontal regeneration in Class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 1995;66:462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- Piché JE, Carnes DL, Jr, Graves DT. Initial characterization of cells derived from human periodontia. J Dent Res. 1989;68:761–767. doi: 10.1177/00220345890680050201. [DOI] [PubMed] [Google Scholar]
- Renkawitz R, Kunz W. Independent replication of the ribosomal RNA genes in the polytrophic-meroistic ovaries of Calliphora erythrocephala, Drosophila hydei, and Sarcophaga barbata. Chromosoma. 1975;53:131–140. doi: 10.1007/BF00333041. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- Salcetti JM, Moriarty JD, Cooper LF, Smith FW, Collins JG, Socransky SS, et al. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants. 1997;12:32–42. [PubMed] [Google Scholar]
- Schmid SI, Hearing P. Adenovirus DNA packaging: construction and analysis. In: Wold WSM, editor. Adenovirus methods and protocols. Totowa, NJ: Humana Press; 1999. pp. 47–60. [Google Scholar]
- Shamah SM, Stiles CD, Guha A. Dominant-negative mutants of platelet-derived growth factor revert the transformed phenotype of human astrocytoma cells. Mol Cell Biol. 1993;13:7203–7212. doi: 10.1128/mcb.13.12.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering [see comments] Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- Womer RB, Frick K, Mitchell CD, Ross AH, Bishayee S, Scher CD. PDGF induces c-myc mRNA expression in MG-63 human osteosarcoma cells but does not stimulate cell replication. J Cell Physiol. 1987;132:65–72. doi: 10.1002/jcp.1041320109. [DOI] [PubMed] [Google Scholar]
- Xie WQ, Rothblum LI. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991;11:324–327. [PubMed] [Google Scholar]
- Yu X, Hsieh SC, Bao W, Graves DT. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol. 1997;272:C1709–C1716. doi: 10.1152/ajpcell.1997.272.5.C1709. [DOI] [PubMed] [Google Scholar]