Abstract
Epidemiologic studies show a correlation between increased consumption of fruits and vegetables with reduced risk of ovarian cancer. One major bioactive compound found in cruciferous vegetables, particularly broccoli, is sulforaphane, derived from the breakdown of glucoraphanin. We observed potent antiproliferative effects of sulforaphane on human ovarian cancer cell line SKOV3 (IC50 40 μmol/L) and mouse ovarian cancer cell lines C3 and T3 (IC50 25 μmol/L each) by cell viability assays. The loss of viability is reflected by a down-regulation of cell cycle transition regulators cyclin D1, cyclin-dependent kinase 4 (cdk4), and cdk6. The upstream mediators of sulforaphane effects on the cell cycle in ovarian cancer are still unknown. However, because the Akt signal transduction pathway is overactivated in ovarian cancer, we investigated the effects of sulforaphane on this prosurvival pathway. Both total Akt protein and active phosphorylated levels of Akt (Ser473) and phosphoinositide 3-kinase were significantly decreased in sulforaphane-treated SKOV3, C3, and T3 cells with a concomitant inhibition of Akt kinase activity by sulforaphane in SKOV3 and C3 cells. This inhibitory effect of sulforaphane leads to a potent induction of apoptosis in all three cell lines, along with the cleavage of poly(ADP)-ribose polymerase. Our study is the first to report the antiproliferative effects of sulforaphane in ovarian cancer and identifying the Akt pathway as a target of sulforaphane, with implications for the inhibition of carcinogenesis by diet-based chemoprevention.
Introduction
Ovarian carcinoma is the most lethal gynecologic malignancy that remains almost silent before being diagnosed. The American Cancer Society estimates that in the United States, 20,180 women will be diagnosed with ovarian cancer and about 15,310 will die from it in 2006 (1). In about 75% of women, ovarian cancer is not diagnosed until after the spread of the disease beyond the ovary, which is typically stages III and IV, leading to 75% mortality rate within 5 years. However, >90% of the patients survive when the disease is diagnosed in stage I. Therefore, early detection and prevention are promising approaches to improve long-term survival of patients with ovarian cancer.
Epidemiologic studies show a correlation between increased consumption of fruits and vegetables with reduced risk of ovarian cancer (2, 3). Diet-derived bioactive agents such as indole-3-carbinol, diindolylmethane, retinoic acid, and sulforaphane (4-6) have been identified as having anticancer properties against different cancer types. However, there are only a few reports on the use of dietary components as chemopreventive agents for ovarian cancer (4, 7), although there are many reports with other cancers (5, 8). Cruciferous vegetables, particularly broccoli (9), have a high content of glucosinolates, and their hydrolysis results in the generation of metabolites responsible for the anticarcinogenic activity. One such bioactive compound is sulforaphane, which is derived from the breakdown of glucoraphanin (9). Sulforaphane inhibits the development of many cancers (6, 10) and is a potent inducer of phase II detoxification enzymes (9). There are, however, no studies on the anticancer and antiproliferative activity of sulforaphane in ovarian cancer.
The etiology of ovarian cancer is not well understood, although traditionally, the epithelium of the ovary is considered to be the origin of the neoplastic transformation. Proto-oncogenes and signaling pathways such as ras are mutated, and Akt, epidermal growth factor receptor, platelet-derived growth factor/vascular endothelial growth factor receptor, and vascular endothelial growth factor are overexpressed in ovarian cancer (11). Signal transduction pathways are major therapeutic targets in several cancers including ovarian cancer, and the serine-threonine kinase Akt is the focus of extensive research. Akt has wide-ranging receptors and is activated by phosphatidylinositol-3,4,5,-triphosphates, which are generated by phosphoinositide 3-kinase (PI3K; ref. 12) and mediates downstream events regulated by PI3K. Akt phosphorylates many different molecules that play key roles in cell cycle, glycogen synthesis, cell death, and cell survival. Deregulation of the Akt pathway has been observed in many cancer types, including breast, endometrial, thyroid cancers, and melanoma (12, 13). Components of the PI3K/Akt pathway are frequently overexpressed in ovarian cancer (14, 15) and are thought to play major roles in ovarian cancer carcinogenesis. The overexpression of the Akt pathway is frequently associated with poor prognosis and more aggressive phenotype (16). Therefore, it is critical to find agents that can directly target this pathway and the downstream molecules, thereby mitigating the oncogenic effects of Akt.
In this study, we examined the effects of sulforaphane on SKOV3, a human ovarian cancer cell line that is highly resistant to cytotoxic agents, such as the diphtheria toxin, cisplatin, adriamycin, and the tumor necrosis factor. We also studied the effects of sulforaphane on mouse ovarian cancer cell lines C3 and T3, which were engineered to constitutively overexpress activated Akt. Our results show that sulforaphane down-regulates PI3K, Akt, and phosphorylated Akt (pAkt). The consequent loss in the kinase activity leads to potent antiproliferative effects and induction of apoptosis. Our studies, for the first time, identify sulforaphane as a potent antiproliferative agent for ovarian cancer and implicate the Akt pathway as a target of sulforaphane in ovarian cancer.
Materials and Methods
Cell Culture
Human ovarian cancer cell SKOV3 was from the American Type Culture Collection (Manassas, VA) and routinely cultured in McCoy’s 5A medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Gemini, West Sacramento, CA), 50 IU/mL penicillin (Mediatech), 50 μg/mL streptomycin (Mediatech), and 2 mmol/L l-glutamine (Mediatech). C3 cell line was generated by overexpression of Akt and K-ras oncogenes in ovarian surface epithelial cells from K5-TVA-p53-/- mice using the avian RCAS retroviral delivery system (17). T3 cell line was derived from an i.p. tumor that developed upon injection of C3 cells into a nude mouse. C3 and T3 cells were routinely cultured in DMEM supplemented with 10% fetal bovine serum.
Antiproliferative Effects of Sulforaphane
The antiproliferative effects of sulforaphane (99.9% pure from LKT Labs, St. Paul, MN) on SKOV3, C3, and T3 cells were determined using 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt (XTT) essentially as described by Badithe et al. (18). Cells (2 × 103/well) were plated into 96-well plates and incubated overnight to allow cell adherence. The media were removed, and sulforaphane was added at concentrations of 10, 20, 40, 60, 80, and 100 μmol/L in a total volume of 200 μL and incubated for 24 and 48 h. The medium was replaced with fresh medium to which 50 μL of XTT solution (1 mg/mL in serum-free McCoy’s 5A medium or DMEM + 25 nmol/L phenazine methosulfate) was added. Plates were read after 3 to 4 h in a microplate reader at 450 nm and referenced at 630 nm. The mean absorbance values were calculated, and percentage survival in the treated cells was calculated compared with untreated control and plotted as a function of time and dose.
Clonogenic Assay
To determine the effect of sulforaphane on the clonogenicity, SKOV3, C3, and T3 cells were plated in six-well plates (400 per well). The cells were allowed to adhere overnight, after which sulforaphane was added at concentrations of 1, 5, 10, 20, and 40 μmol/L. After 14 days in culture, the cells were fixed and stained using 0.025% Coomassie brilliant blue R250 (in 50% methanol and 10% acetic acid) to visualize cell colonies. The colonies were counted, and percentage inhibition of clonogenicity and SD were calculated.
Western Blot Analysis of the Steady-State Level of Cellular Proteins
Cells were treated with sulforaphane (10, 20, and 40 μmol/L) for 12 and 24 h. At the end of the incubation period, the cells were harvested, washed with PBS, and lysed (1 × 106/100 μL of lysis buffer) using the radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 0.2% sodium deoxycholate, 0.1% SDS, 0.5% NP40, 1 μmol/L Pefabloc]. Lysates were subjected to Western blotting as described (19). For the detection of phosphorylated proteins, blocking was done with 5% bovine serum albumin in TBST. The following antibodies were used in this study: cyclin D1, cyclin-dependent kinase 4 (cdk4), cdk6 (Neomarkers, Fremont, CA), Akt, pAkt (Ser473), cleaved poly(ADP)ribose polymerase (Cell Signaling Technology, Danvers, MA), PI3K p85 subunit, and actin (Santa Cruz Biotechnology, Santa Cruz, CA).
Akt Kinase Assay
The kinase assay was done using the nonradioactive Akt kinase assay kit (Cell Signaling Technology) in SKOV3 and C3 cell extracts ± sulforaphane. Immobilized Akt (1G1) monoclonal antibody was used to immunoprecipitate Akt from cell extracts followed by an in vitro kinase assay using GSK-3 fusion protein as a substrate. Phosphorylation of GSK-3 was measured by Western blot analysis using phosphorylated GSK-3α/h (Ser21/9) antibody and chemiluminescent detection.
Cell Death Detection ELISA
The induction of apoptosis by sulforaphane in SKOV3, C3, and T3 cells was detected using the cell death ELISA kit (Roche Diagnostics Corporation, Indianapolis, IN) for quantitative determination of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes; ref. 5). Fifty thousand cells were plated in six-well plates overnight, and cells were either treated with sulforaphane (10, 20, 40, and 60 μmol/L) or left untreated to serve as the control. After 12 and 24 h, cells were harvested, and the ELISA was done as per manufacturer’s instructions. Absorbance was read at 405 nm. Data were plotted as the enrichment factor of the mononucleosomes and oligonucleosomes in the treated samples compared with the untreated control, using the control absorbance as 1.
Detection of Sulforaphane-Induced Changes in Cell Morphology
SKOV3 and C3 cells were plated in four-chamber culture slides at 50,000 cells per well and allowed to adhere followed by the addition of 40 μmol/L sulforaphane for 24 h. The cells were washed with PBS, fixed, and stained with 1 μg/mL 4′,6-diamidino-2-phenylindole and visualized under an inverted Zeiss Axiovert 200 microscope using Axiovision 4.3 software.
Results
Antiproliferative Effects of Sulforaphane
The antiproliferative effects of sulforaphane were investigated on SKOV3, C3, and T3 cell lines by the XTT assay. Cell survival decreased with increasing doses of sulforaphane, and almost total loss of cell viability was observed at 100 μmol/L sulforaphane. The IC50 was determined to be 40 μmol/L for SKOV3 cells and 25 μmol/L for C3 and T3 cells (Fig. 1). The results showsulforaphane to be a potent antiproliferative agent against ovarian cancer cells.
Figure 1.
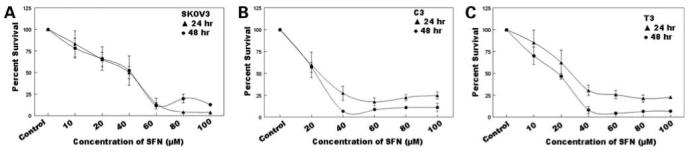
Dose- and time-dependent antiproliferative effect of sulforaphane (SFN) on SKOV3 (A), C3 (B), and T3 (C) cells by the XTT assay. Cells were seeded overnight, and varying concentrations (10, 20, 40, 60, 80, and 100 μmol/L) of sulforaphane were added to each well for 24 h (▲) and 48 h (○). Percentage cell survival was determined by XTT assay and plotted as a function of time and dose of sulforaphane. Representative of three independent experiments.
Effect of Sulforaphane on Clonogenicity of SKOV3, C3, and T3 Cells
The effects of sulforaphane were studied on the clonogenic ability of SKOV3, C3, and T3 cells. SKOV3, C3, and T3 cells showthe ability to form clones in the untreated control wells. However, with the addition of sulforaphane, a dose-dependent inhibition in clonogenicity was observed with a >50% inhibition seen with 1 μmol/L sulforaphane for SKOV3 cells and 5 μmol/L for C3 and T3 cells (Table 1).
Table 1.
Inhibition of clonogenicity of ovarian cancer cell lines by sulforaphane
| Sulforaphane concentration (μmol/L) | Percentage inhibition in clonogenicity | ||
|---|---|---|---|
| SKOV3 | C3 | T3 | |
| Control | 0 | 0 | 0 |
| 1 | 60.7 ± 18.5 | 6.15 ± 4.7 | 16.2 ± 7.6 |
| 5 | 75.4 ± 2.3 | 46.4 ± 11.1 | 85.2 ± 1.91 |
| 10 | 100 ± 0 | 74.9 ± 2.3 | 100 ± 0 |
| 20 | 100 ± 0 | 87.2 ± 5.6 | 100 ± 0 |
| 40 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
Effect of Sulforaphane on PI3K/Akt Pathway
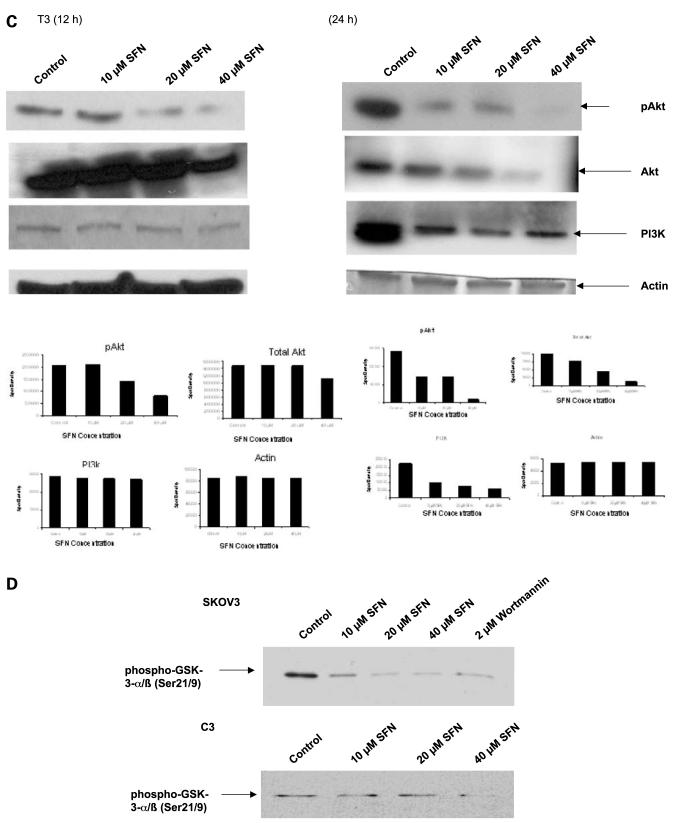
A key survival pathway operating in cells in response to growth factor stimuli is the PI3K/Akt pathway that is activated through membrane-bound receptors via PI3K leading to phosphorylation of numerous downstream molecules. We investigated whether sulforaphane altered the Akt pathway in SKOV3, C3, and T3 cells. Akt is constitutively expressed in all three of these cell lines. The treatment of SKOV3, C3, and T3 cells with sulforaphane resulted in down-regulation in the steady-state levels of total Akt protein as well as the active pAkt (Ser473) at 24 h (Fig. 2). A dose-dependent down-regulation of PI3K was also observed in all three cell lines upon treatment with sulforaphane at 24 h (Fig. 2). When C3 and T3 cells were treated with sulforaphane for 12 h, a decrease in pAkt (Ser473) was observed in T3 cells (Fig. 2C), but no significant change in C3 cells (Fig. 2B). A decrease in total Akt protein levels was observed at 40 μmol/L sulforaphane, with no significant changes observed in PI3K levels in C3 and T3 cell lines. In the case of SKOV3 cells, 12-h treatment of sulforaphane led to a decrease in pAkt levels, but no effects were observed on total Akt and PI3K levels. The manifestation of this activity occurs between 12 and 24 h of sulforaphane treatment, with significant changes in protein levels only at 24 h. The results show that sulforaphane inhibits the PI3K/Akt pathway by the down-regulation of the key enzymes in this pathway, indicating the Akt pathway as a possible target of sulforaphane.
Figure 2.
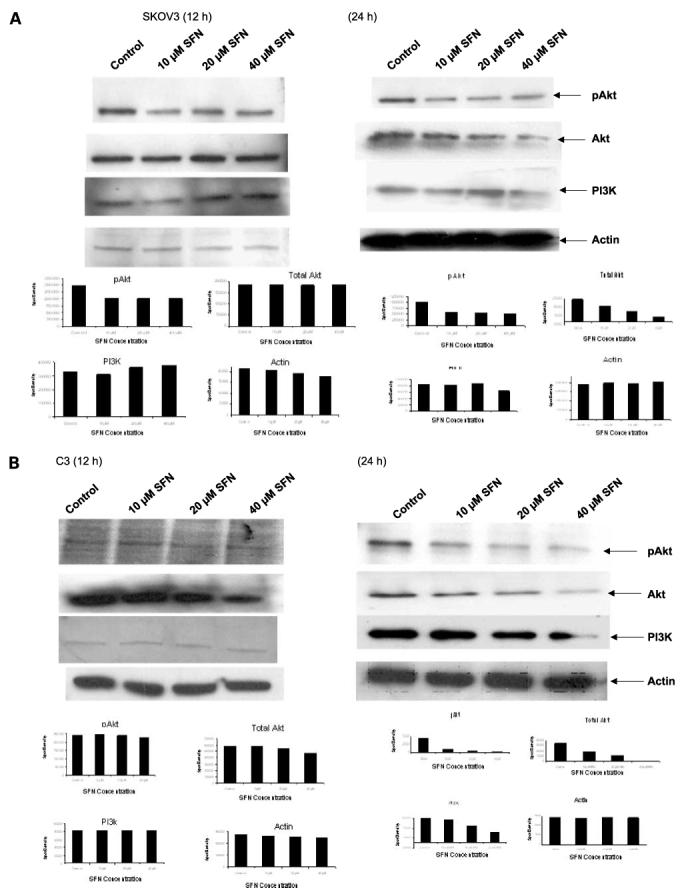
Effect of sulforaphane on the PI3K/Akt pathway. SKOV3 (A), C3 (B), and T3 (C) cells were either untreated or treated with 10, 20, and 40 μmol/L sulforaphane for 12 and 24 h, and the steady-state protein levels of Akt, pAkt (Ser473), and PI3K were determined by Western blot analysis. β-Actin was used as loading control. Representative of three independent experiments. D, effect of sulforaphane on Akt kinase activity. SKOV3 and C3 cells were plated and treated with 10, 20, and 40 μmol/L of sulforaphane for 24 h. Experimental details are described in Materials and Methods. Wortmannin was used as positive control to show the inhibition of Akt kinase activity.
Effect of Sulforaphane on Akt Kinase Activity
Because a down-regulation in Akt and pAkt levels was observed, we wanted to investigate if this corresponds to a decrease in the functional property of Akt. A significant reduction in the kinase activity of Akt was observed in SKOV3 and C3 cells (Fig. 2D) treated for 24 h with 10, 20, and 40 μmol/L sulforaphane. Our results show that sulforaphane inhibits the kinase activity of Akt that reflects the inhibition in pAkt levels and may account for the antiproliferative effects of sulforaphane in ovarian cancer.
Effect of Sulforaphane on Cell Cycle Markers
We investigated whether sulforaphane affects critical proteins involved in the G1-S transition of the cell cycle. SKOV3, C3, and T3 cells were treated with 10, 20, and 40 μmol/L sulforaphane for 12 and 24 h, and the whole-cell lysates were used to detect the steady-state levels of cyclin D1, cdk4, and cdk6. A down-regulation of cyclin D1, cdk4, and cdk6 levels was detected with increasing concentration of sulforaphane for 24 h in all three cell lines (Fig. 3), leading to a possible cell cycle arrest and decrease in proliferation. A 12-h sulforaphane treatment resulted in a slight down-regulation in cyclin D1 with 40 μmol/L in T3 cells, moderate down-regulation of cdk4 with 40 μmol/L in all cell lines, and no change in cdk6 levels in any cell line.
Figure 3.
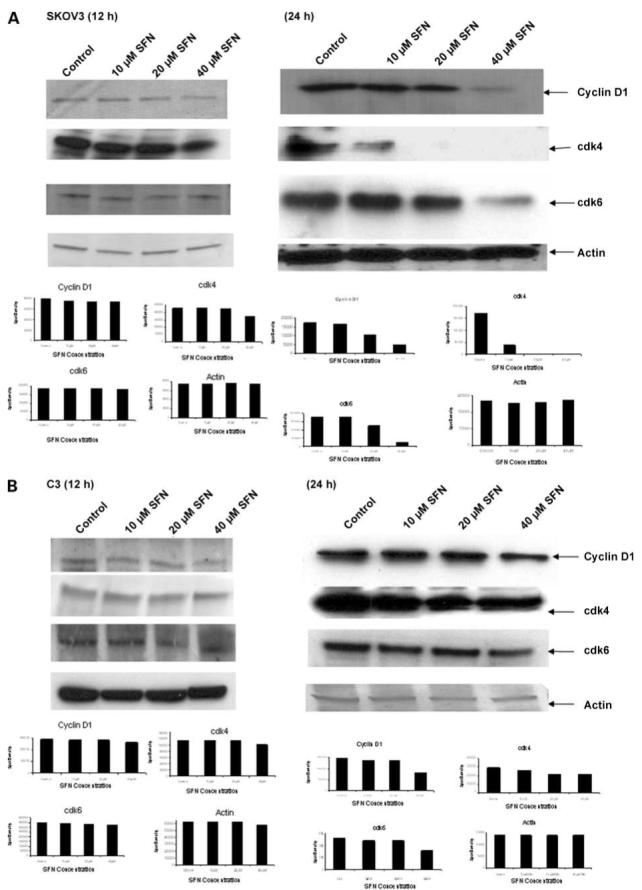
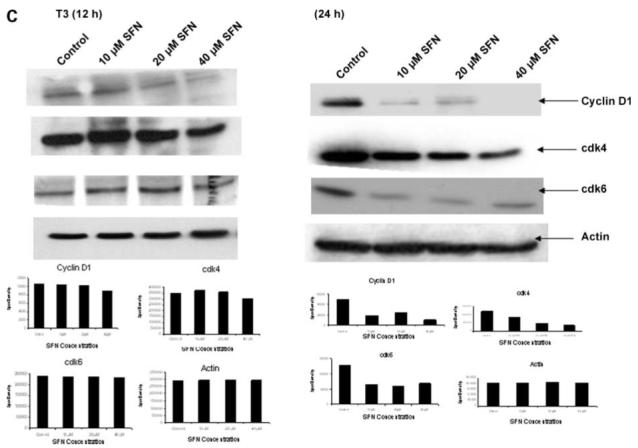
Effect of sulforaphane on steady-state levels of cell cycle proteins. SKOV3 (A), C3 (B), and T3 (C) cells were either untreated or treated with 10, 20, and 40 μmol/L sulforaphane for 12 and 24 h, and the whole-cell lysates were used for Western blot analysis of G1-S cell cycle transition markers, cyclin D1, cdk4, and cdk6. β-Actin was used as loading control. Representative of three independent experiments.
Effect of Sulforaphane on the Induction of Apoptosis
The induction of apoptosis by sulforaphane in SKOV3, C3, and T3 cells was studied by cell death detection ELISA. As shown in Fig. 4, sulforaphane was very effective in inducing apoptosis in all three cell lines at 24 h. This corroborates the antiproliferative effects of sulforaphane on three cell lines (Fig. 1), which is manifested by the induction of programmed cell death. In addition, Western blot analysis showed a dose-dependent increase in the levels of cleaved poly(ADP) ribose polymerase (Fig. 4, bottom) for all the three cell lines, indicating the induction of apoptosis. However, with 12 h of sulforaphane treatment, there was no induction of apoptosis in all the three cells and no changes were observed in the levels of cleaved poly (ADP)ribose polymerase.
Figure 4.
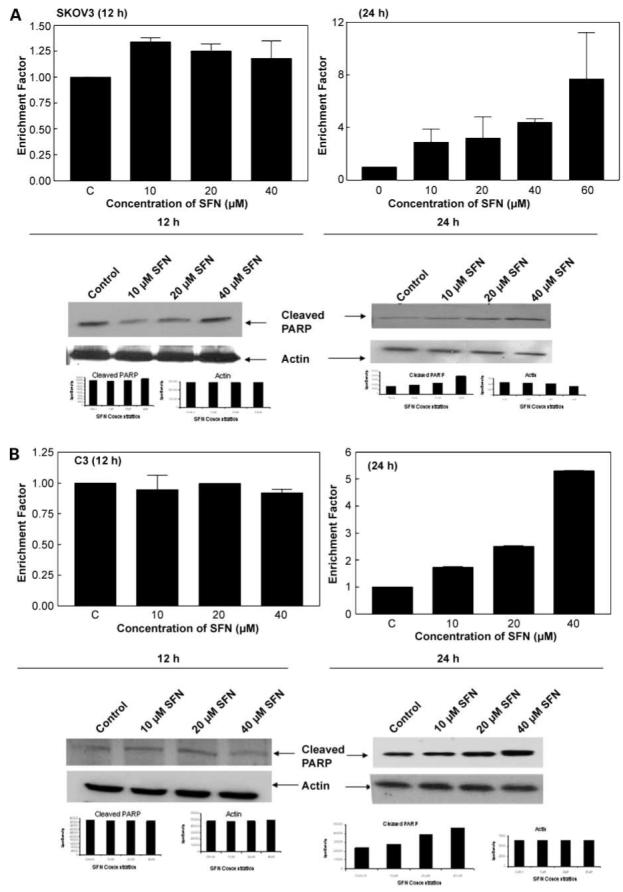
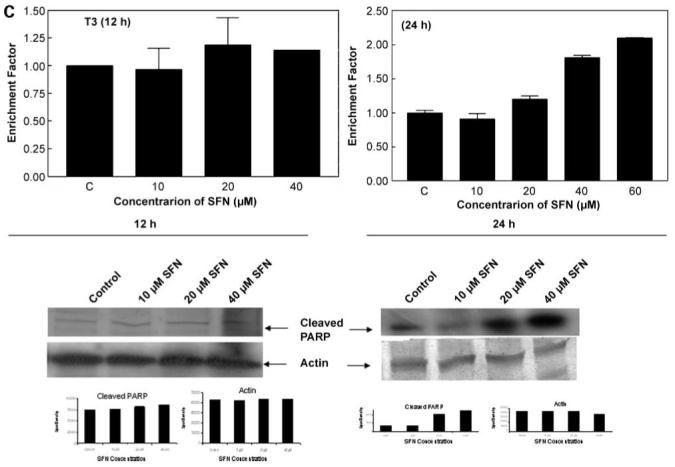
Induction of apoptosis by sulforaphane in SKOV3 (A), C3 (B), and T3 (C) cells. Cell death ELISA was done and expressed as enrichment factor compared with untreated control (top). Columns, mean in treated cells (n = 3) of three experiments; bars, SD. Induction of apoptosis was also measured by cleavage of poly(ADP)ribose polymerase (PARP) using a specific antibody (bottom).
Detection of Sulforaphane-Induced Changes in Cell Morphology
Besides the antiproliferative effects of sulforaphane and the induction of apoptosis, we also investigated the morphologic effects of sulforaphane on SKOV3 and C3 cells by staining with 4′,6-diamidino-2-phenylindole. The results show condensed chromatin, apoptotic bodies, and fragmented nuclei in sulforaphane-treated cells compared with controls (Fig. 5A and B). Increasing doses of sulforaphane added to SKOV3 and C3 cells drastically altered the cell morphology. SKOV3 is an epithelial cell line with a large cell size, and the control cells adhere well to the culture plate, are flattened, spread radially, and have a typical epithelial morphology. Distinct morphologic alterations were observed with 40 μmol/L sulforaphane in both cell lines. The changes induced by sulforaphane caused the cell membrane to shrink, and cells become ellipsoid or round with no visible radial spread. These alterations were accompanied by a decrease in cell number.
Figure 5.
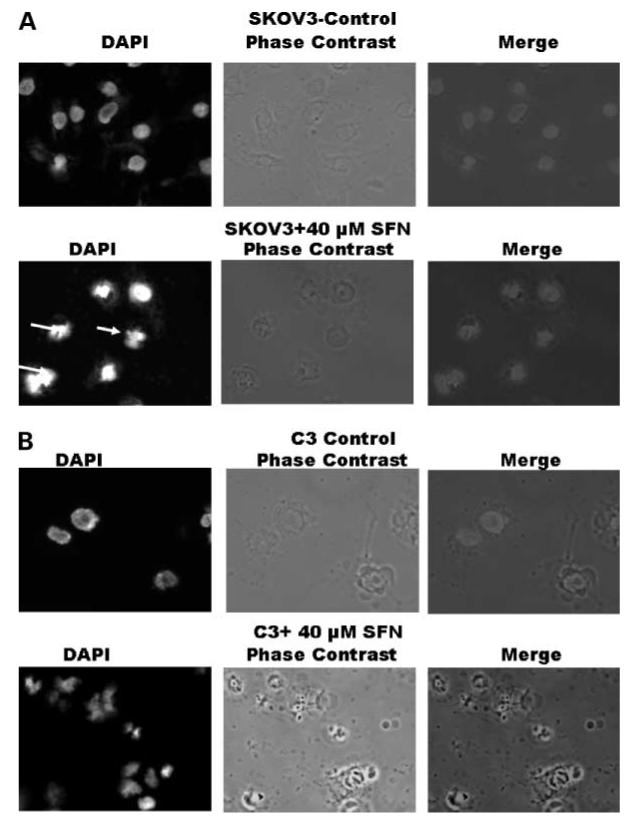
Effect of sulforaphane on cell morphology. SKOV3 (A) and C3 (B) cells were seeded overnight followed by the addition of 40 μmol/L sulforaphane for 24 h. The cells stained with 4′,6-diamidino-2-phenylindole (DAPI) were visualized under an inverted Zeiss Axiovert 200 microscope using Axiovision 4.3 software to observe gross morphologic and nuclear alterations induced by sulforaphane.
Discussion
Ovarian cancer is generally diagnosed in late stages, and, therefore, the management of the disease is extremely difficult owing to the fact that there is a frequent onset of i.p. metastases and ascites accumulation (11). This often is a major factor accounting for the mortality rates associated with ovarian carcinoma. In addition, there is a lack of effective therapeutic options for the late-stage disease. This is compounded by the development of resistance to available chemotherapeutic agents such as cisplatin, adriamycin, and tumor necrosis factor. Therefore, early detection and prevention that is geared toward improving the longterm survival of ovarian cancer patients is a promising approach. We are therefore developing dietary components present in cruciferous vegetables such as sulforaphane for ovarian cancer prevention.
In this study, we observed potent antiproliferative effects of sulforaphane in SKOV3, C3, and T3 cells. We also observed that sulforaphane inhibits the clonogenic ability of ovarian cancer cells, which is considered a marker of neoplastic propensity. This is significant considering that only a small percentage of cells in a tumor can survive and proliferate using autocrine factors without depending on paracrine factors and may be associated with up-regulated prosurvival pathways, and sulforaphane is able to target these cells. Our studies show that the inhibition of clonogenicity was observed with much lower doses than for the inhibition of Akt. It is therefore possible that Akt-independent prosurvival pathways, such as extracellular signal-regulated kinase/c-Jun-NH2-kinase/p38 may be responsible for inhibition in the clonogenic ability and long-term survival of ovarian cancer cells, which needs investigation (20, 21).
Our studies clearly showthat sulforaphane targets and inhibits the Akt pathway with a concomitant decreased functional kinase activity of Akt, thereby manifesting its anticancer and antiproliferative effects. It is important to note that only the pAkt levels are down-regulated at 12 h in all the cells studied with minimal changes in total Akt and no changes in PI3K with 12 h of sulforaphane treatment. A more pronounced down-regulation in pAkt, total Akt, and PI3K is observed only at 24 h. This indicates that there is an early effect of sulforaphane on the phosphorylated form of Akt with a greater inhibitory effect on the entire pathway at a later time point. These results are significant considering the fact that Akt is constitutively expressed and active in SKOV3 cells, and C3 and T3 overexpress Akt because they were generated by the overactivation of the Akt oncogene (17). It is therefore very significant that we have identified the Akt pathway as a target of sulforaphane for therapeutic and preventive interventions in ovarian cancer. The PI3K/Akt pathway is associated with the resistance to chemotherapy in ovarian cancer (22). Mutations in the regulatory subunit of PI3K (23) and the tumorsuppressor gene PTEN, a phosphoprotein/phospholipid phosphatase and negative regulator of Akt, have been observed in several cancer types, including ovarian cancer (24). Expression of PTEN in ovarian cancer cells suppresses tumor growth (25, 26) and its deletion in the mouse ovarian surface epithelium leads to preneoplastic ovarian lesions (27), indicating a strong role for this protein in ovarian cancer etiology. There is ample evidence for the role of the overactivated prosurvival PI3K/Akt pathway in ovarian cancer carcinogenesis (14, 15). There is only one study on the effect of sulforaphane on the Akt pathway, which suggests an induction of the pathway in CaCo-2 cells (28). However, in our studies, we clearly observed a down-regulation in the Akt levels along with a decrease in the functional kinase activity, making Akt an ideal target of sulforaphane.
Cell cycle progression is regulated by cyclins and cyclin-dependent kinases, such as cdk2, cdk4, and cdk6, which hyperphosphorylate the retinoblastoma gene product leading to the release of E2F transcription factor and cell cycle progression. Down-regulation of any of these molecules leads to cell cycle arrest and induction of apoptosis. At an early time point of 12 h, sulforaphane treatment led to a slight down-regulation in cdk4 and no changes in cdk6 in all the cells with no induction of apoptosis. However, a marked down-regulation of cyclin D1, cdk4, and cdk6 by sulforaphane at 24 h in our studies indicates the inability of cells to progress through the cell cycle, leading to a decrease in cell proliferation and possibly driving cells toward programmed cell death. Earlier reports have shown that sulforaphane is an inducer of programmed cell death in leukemia and prostate, bladder, and colon cancers (8, 9), and that PI3K inhibitors induce apoptosis by directly affecting pAkt levels (29). Based on our results, we postulate that the antiproliferative effect of sulforaphane manifests at 12 h on the Akt pathway, which becomes more pronounced at 24 h leading to significant down-regulation of pAkt, total Akt, and PI3K. The early effects on inhibition of pAkt, however, do not translate into the induction of apoptosis. Therefore, the effects of sulforaphane observed at 12 h is an initiating event that further leads to the down-regulation of cyclin D1, cdk4, and cdk6 and a potent induction of apoptosis at 24 h. A working model is shown in Fig. 6 that summarizes the potential target sites of sulforaphane in the Akt pathway in ovarian cancer.
Figure 6.
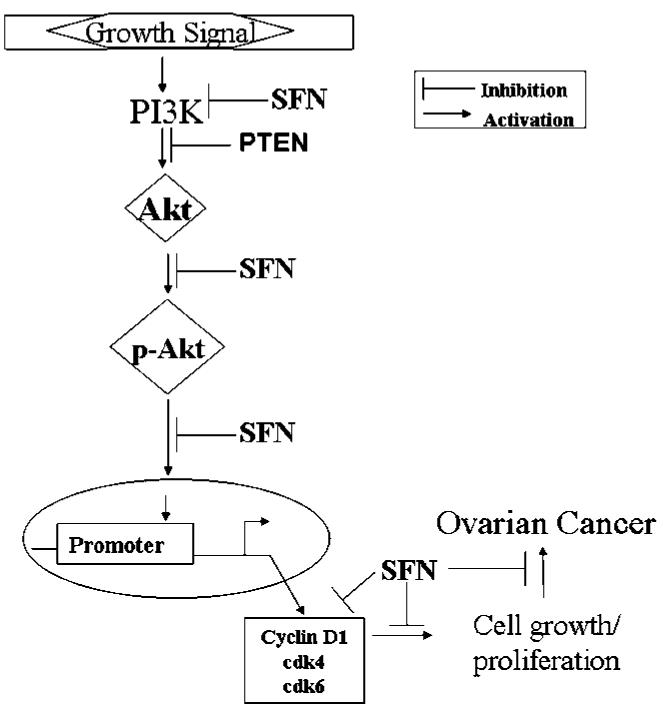
The antiproliferative targets of sulforaphane in ovarian cancer.
High levels of sulforaphane have been reported in mammalian cells and up to 20 μmol/L in rat plasma (30) and in urine of humans consuming broccoli (31, 32), suggesting that sulforaphane is bioavailable. The mean glucosinolates content is 100 mmol/L per 100 g of fresh weight corresponding to 26 mg glucoraphanin accounting for 55% of the glucosinolates (33). It has been estimated that 100 g broccoli yields ∼40 μmol/L sulforaphane (34, 35), which is the highest level that we have used in all our studies. It is thus presumable that this serum level of sulforaphane can be achieved by consumption of 100 g broccoli, and the doses that we have used are achievable physiologically.
Acknowledgments
We thank Dr. Raj Tiwari for valuable discussions and comments and Dr. Dana Mordue for help with microscopy.
Grant support: National Cancer Institute research grants CA29502 (B.T. Ashok) and RO1CA103924 (S. Orsulic).
References
- 1.American Cancer Society . Cancer facts and figures. American Cancer Society; Atlanta (GA): 2006. [Google Scholar]
- 2.IARC . Working Group on the Evaluation of Cancer-Preventive Strategies. In: Vaino H, Bianchini F, editors. IARC handbook of cancer prevention: fruit and vegetables. Vol. 8. IARC Press; Lyon: 2003. [Google Scholar]
- 3.Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–55. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- 4.Holmes WF, Soprano DR, Soprano KJ. Synthetic retinoids as inducers of apoptosis in ovarian carcinoma cell lines. J Cell Physiol. 2004;199:317–29. doi: 10.1002/jcp.10338. [DOI] [PubMed] [Google Scholar]
- 5.Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. Synthetic dimer of indole-3-carbinol: second generation diet derived anti-cancer agent in hormone sensitive prostate cancer. Prostate. 2006;66:453–62. doi: 10.1002/pros.20350. [DOI] [PubMed] [Google Scholar]
- 6.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opipari AW, Jr., Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 8.Ashok BT, Tiwari RK. Cruciferous vegetables and cancer chemoprevention. Recent Res Dev Nutr. 2004;6:83–94. [Google Scholar]
- 9.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anti-carcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–66. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 12.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JQ, Nicosia SV. Akt signal transduction pathway in oncogenesis. In: Schwab D, editor. Encyclopedic reference of cancer. Springer; Berlin: 2001. pp. 35–7. [Google Scholar]
- 14.Altomare DA, Wang HQ, Skele KL, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–7. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 15.Gao N, Flynn DC, Zhang Z, et al. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281–91. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- 16.Cheng JQ, Godwin AK, Bellacosa A, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89:9267–71. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse-model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashok BT, Kim E, Mittelman A, Tiwari RK. Proteasome inhibitors differentially affect heat shock protein response in cancer cells. Int J Mol Med. 2001;8:385–90. doi: 10.3892/ijmm.8.4.385. [DOI] [PubMed] [Google Scholar]
- 19.Ashok BT, Chen YG, Liu X, Bradlow HL, Mittelman A, Tiwari RK. Abrogation of estradiol mediated cellular and biochemical effects by indole-3-carbinol. Nutr Cancer. 2001;41:180–7. doi: 10.1080/01635581.2001.9680630. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Shen G, Yuan X, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–45. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 21.Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006;233:208–18. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicosia SV, Bai W, Cheng JQ, Coppola D, Kruk PA. Oncogenic pathways in ovarian epithelial cancer. Hematol Oncol Clin North Am. 2003;17:927–43. doi: 10.1016/s0889-8588(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 23.Philp AJ, Campbell IG, Leet C, et al. The phosphatidyl 3′-kinase p85α gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–9. [PubMed] [Google Scholar]
- 24.Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097–106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng JQ, Ruggeri B, Klein WM, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato N, Tsunoda H, Nishida M, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 27.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 28.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–52. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Stein RC. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr Relat Cancer. 2001;8:237–48. doi: 10.1677/erc.0.0080237. [DOI] [PubMed] [Google Scholar]
- 30.Hu R, Hebbar V, Kim BR, et al. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanates sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263–71. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Callaway EC. High cellular accumulation of sulforaphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364:301–7. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeulin M, van Rooijen HJ, Vaes WH. Analysis of isothiocyanates mercapturic acids in urine: a biomarker for cruciferous vegetable intake. J Agric Food Chem. 2003;51:3554–9. doi: 10.1021/jf0341316. [DOI] [PubMed] [Google Scholar]
- 33.Paolini M, Perocco P, Canistro D, et al. Induction of cytochrome P450, generation of oxidative stress and in vitro cell-transforming and DNA-damaging activities by glucoraphanin, the bioprecursor of the chemopreventive agent sulforaphane found in broccoli. Carcinogenesis. 2004;25:61–7. doi: 10.1093/carcin/bgg174. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemopreventive glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–100. [PubMed] [Google Scholar]
- 35.Conaway CC, Getahun SM, Liebes LL, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–78. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]