Abstract
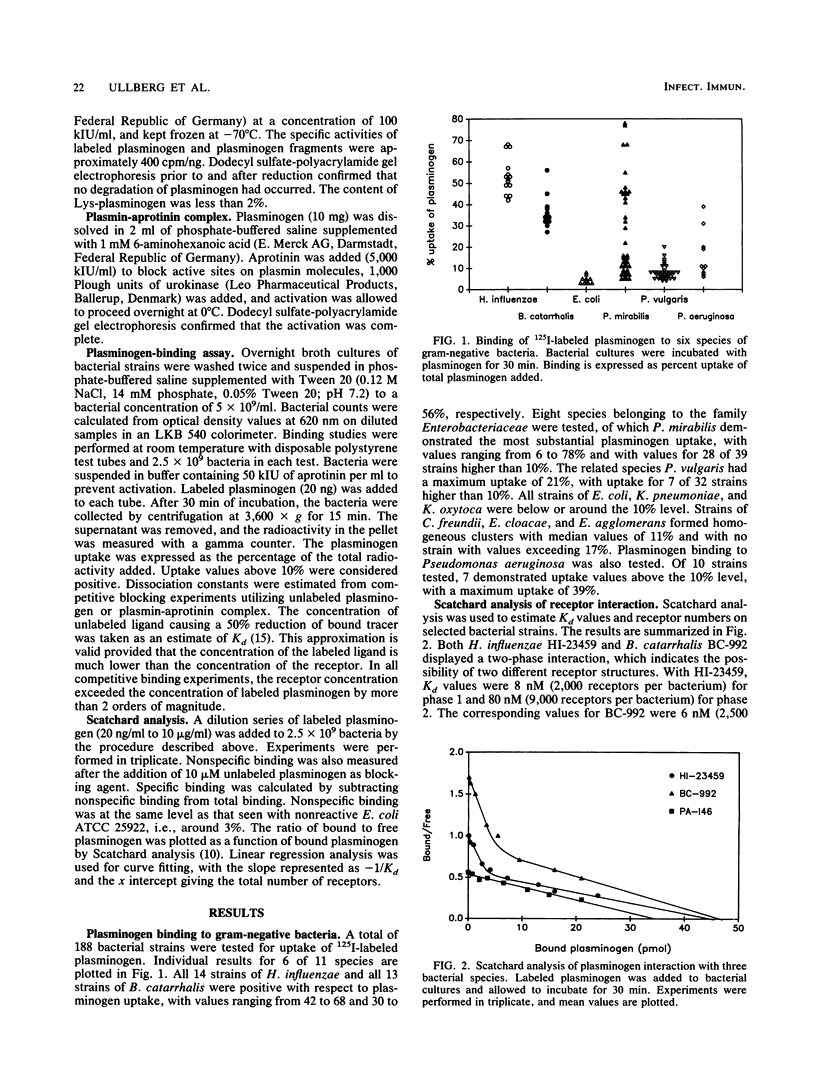
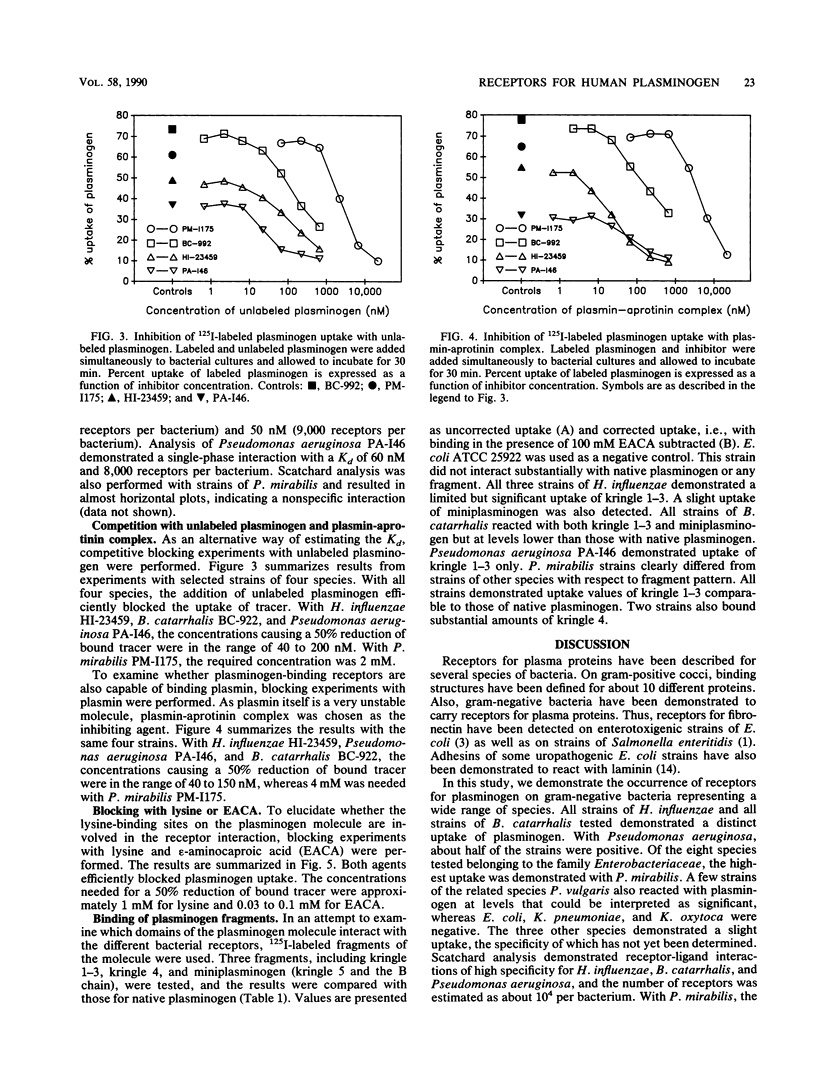
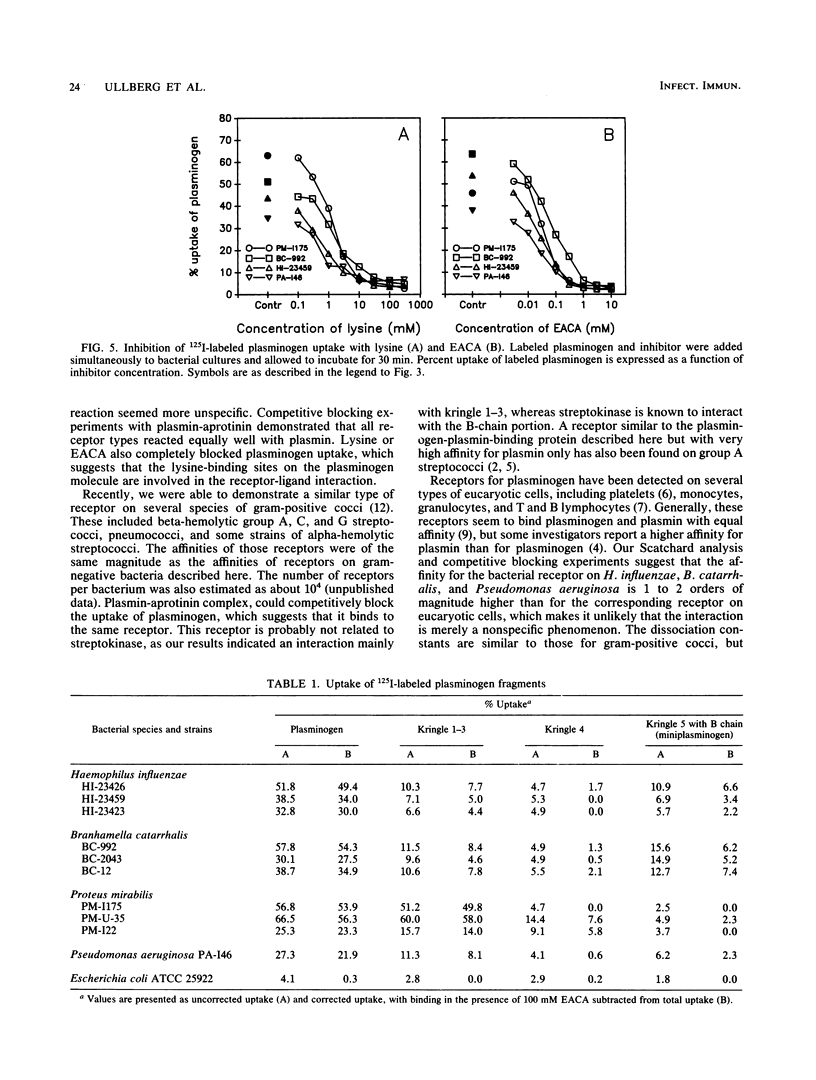
A total of 188 strains representing 11 species of gram-negative bacteria were examined for the ability to interact with human plasminogen. Highly purified human plasminogen was labeled with 125I, and its uptake by different bacterial strains was measured. All 14 strains of Haemophilus influenzae and all 13 strains of Branhamella catarrhalis tested were positive with respect to plasminogen uptake. Also, eight species belonging to the family Enterobacteriaceae were tested, and of those, Proteus mirabilis demonstrated the most substantial uptake, with 28 of 39 strains taking up more than 10% of the plasminogen. Ten strains of Pseudomonas aeruginosa were also tested, of which seven showed uptake values higher than 10%. With H. influenzae and B. catarrhalis strains, Scatchard analysis indicated a two-phase receptor interaction, one more-avid receptor with a Kd of 6 to 8 nM and 2,000 to 2,500 sites per bacterium and a second receptor with a Kd of 50 to 80 nM and 9,000 sites per bacterium. With Pseudomonas aeruginosa strains, a single receptor interaction was detected with a Kd of 60 nM and the number of sites was estimated as 8,000 per bacterium. Scatchard analysis with strains of P. mirabilis indicated binding of a less-specific nature. However, plasminogen uptake by this species could be reduced by 50% by the addition of 2 mM unlabeled plasminogen. This estimate of Kd, as well as uptake studies with plasminogen fragments, suggests different properties of this receptor. With all receptor types, the addition of plasmin-aprotinin complex inhibited plasminogen uptake, which demonstrates that both forms of the molecule react with the same receptors. Plasminogen uptake could be eliminated by the addition of lysine or epsilon-aminocaproic acid, which suggests that the lysine-binding sites of the plasminogen molecule are involved in the receptor-ligand interaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broeseker T. A., Boyle M. D., Lottenberg R. Characterization of the interaction of human plasmin with its specific receptor on a group A streptococcus. Microb Pathog. 1988 Jul;5(1):19–27. doi: 10.1016/0882-4010(88)90077-0. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Hajjar K. A., Harpel P. C., Jaffe E. A., Nachman R. L. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986 Sep 5;261(25):11656–11662. [PubMed] [Google Scholar]
- Lottenberg R., Broder C. C., Boyle M. D. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987 Aug;55(8):1914–1918. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985 Apr 10;260(7):4303–4311. [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Receptor mediated binding of the fibrinolytic components, plasminogen and urokinase, to peripheral blood cells. Thromb Haemost. 1987 Oct 28;58(3):936–942. [PubMed] [Google Scholar]
- Plow E. F., Freaney D. E., Plescia J., Miles L. A. The plasminogen system and cell surfaces: evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol. 1986 Dec;103(6 Pt 1):2411–2420. doi: 10.1083/jcb.103.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., Merenmies J., Rauvala H., Miettinen A., Järvinen A. K., Virkola R., Holthöfer H., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli: receptor-active domains in the canine urinary tract and in-vitro interaction with laminin. Microb Pathog. 1987 Aug;3(2):117–127. doi: 10.1016/0882-4010(87)90070-2. [DOI] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Wiman B., Rånby M. Determination of soluble fibrin in plasma by a rapid and quantitative spectrophotometric assay. Thromb Haemost. 1986 Apr 30;55(2):189–193. [PubMed] [Google Scholar]



