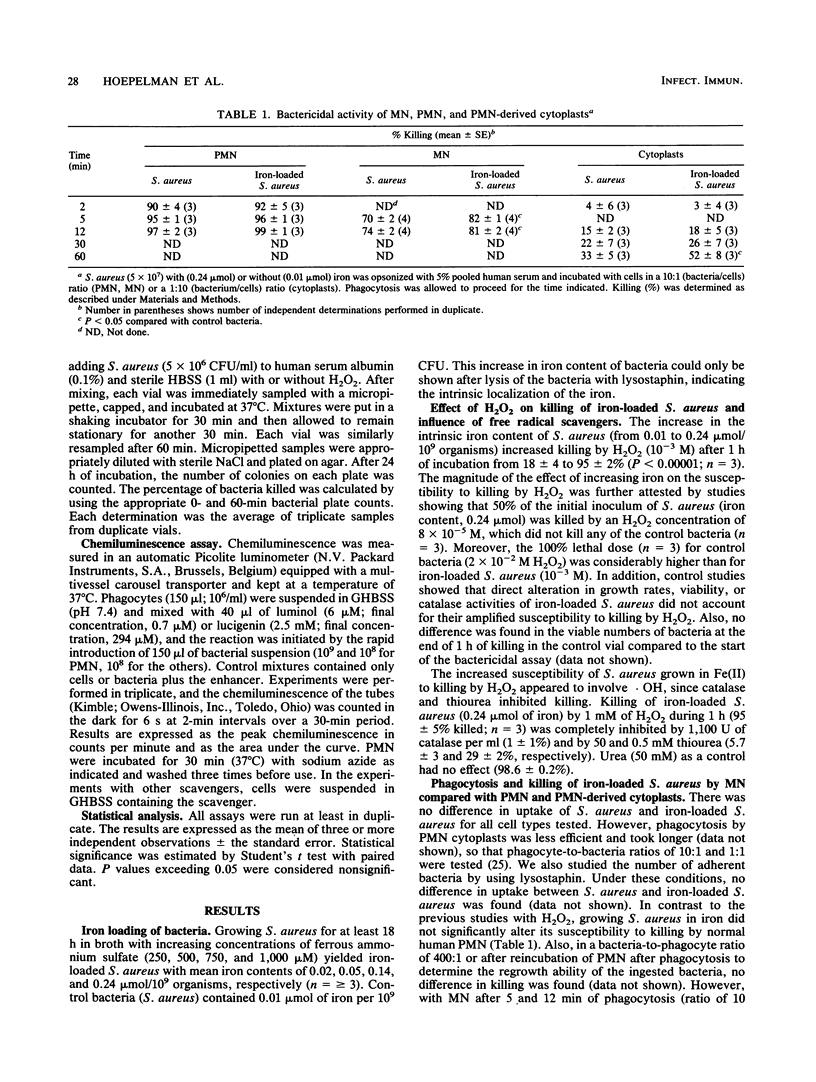
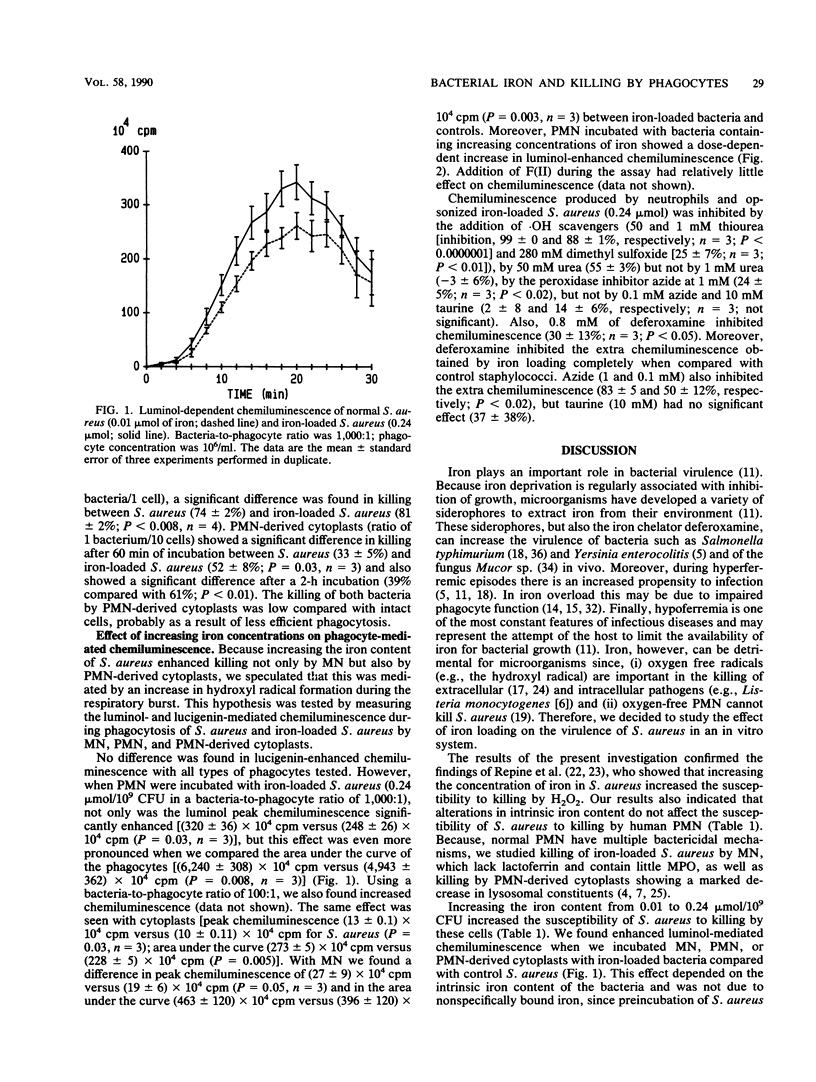
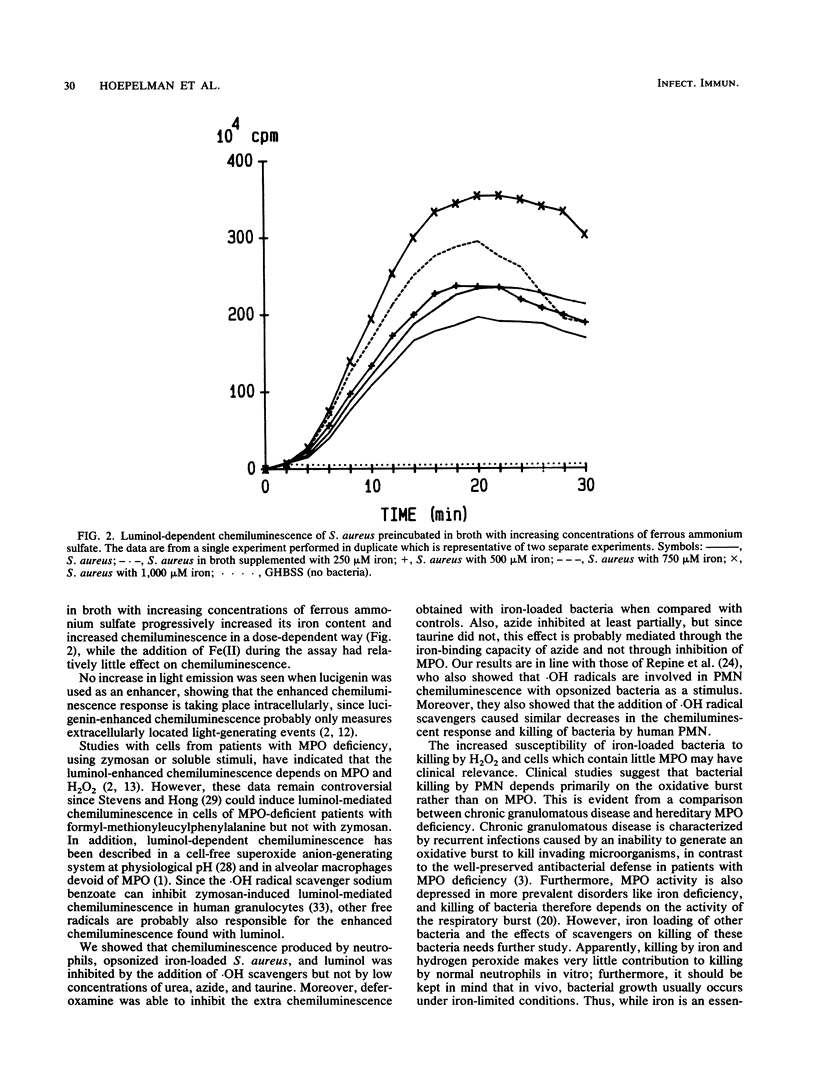
Abstract
It has been shown that increasing bacterial iron concentration enhances killing by hydrogen peroxide (H2O2) but not by polymorphonuclear granulocytes (PMN). It is possible that owing to the multiple bactericidal mechanisms of the PMN, differences in the killing rate of iron-loaded bacteria and control bacteria are obscured. We decided, therefore, to compare the killing of iron-loaded bacteria with that of control bacteria using human monocytes (MN), PMN, and PMN-derived cytoplasts. Incubation of Staphylococcus aureus with increasing concentrations of ferrous ammonium sulfate (0 to 1,000 microM) progressively increased the iron content in the bacteria (from 0.01 to 0.24 mumol of iron per 10(9) bacteria). Iron loading of the bacteria markedly increased their susceptibility to killing by H2O2. After 1 h of incubation with 1 mM H2O2, 95 +/- 2% of the iron-loaded bacteria were killed compared with 18 +/- 4% of the control bacteria (P less than 0.0001). Iron loading of bacteria did not alter their susceptibility to killing by human PMN. However, iron-loaded bacteria were more susceptible to killing by MN (after 12 min of incubation, 81 +/- 2 versus 74 +/- 2% killing; P less than 0.008) and to killing by PMN-derived cytoplasts (after 60 min of incubation, 52 +/- 8 versus 33 +/- 5%; P = 0.003) than the controls. Moreover, iron loading enhanced luminol-mediated chemiluminescence of MN, PMN, and PMN-derived cytoplasts. The hydroxyl radical scavenger thiourea inhibited H2O2-mediated killing of iron-loaded staphylococci as well as luminol-mediated chemiluminescence. These results suggest that alterations in intrinsic iron content increase killing of staphylococci by H2O2, MN, and PMN-derived cytoplasts by a free radical-mediated mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Aniansson H., Stendahl O., Dahlgren C. Comparison between luminol- and lucigenindependent chemiluminescence of polymorphonuclear leukocytes. Acta Pathol Microbiol Immunol Scand C. 1984 Dec;92(6):357–361. doi: 10.1111/j.1699-0463.1984.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Boelaert J. R., van Landuyt H. W., Valcke Y. J., Cantinieaux B., Lornoy W. F., Vanherweghem J. L., Moreillon P., Vandepitte J. M. The role of iron overload in Yersinia enterocolitica and Yersinia pseudotuberculosis bacteremia in hemodialysis patients. J Infect Dis. 1987 Aug;156(2):384–387. doi: 10.1093/infdis/156.2.384. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Vandenbroucke-Grauls C. M., van Asbeck B. S., Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987 Dec;55(12):3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos A., Wever R., Roos D. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta. 1978 Jul 7;525(1):37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Chai Y., Cohen M. S. Do human neutrophils make hydroxyl radical? Determination of free radicals generated by human neutrophils activated with a soluble or particulate stimulus using electron paramagnetic resonance spectrometry. J Biol Chem. 1986 Apr 5;261(10):4426–4431. [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Thompson B. Y., Chai Y., Cohen M. S. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986 Dec 25;261(36):17026–17032. [PubMed] [Google Scholar]
- Brock J. H. Iron and the outcome of infection. Br Med J (Clin Res Ed) 1986 Aug 30;293(6546):518–520. doi: 10.1136/bmj.293.6546.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Dahlgren C., Aniansson H., Magnusson K. E. Pattern of formylmethionyl-leucyl-phenylalanine-induced luminol- and lucigenin-dependent chemiluminescence in human neutrophils. Infect Immun. 1985 Jan;47(1):326–328. doi: 10.1128/iai.47.1.326-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Hoepelman I. M., Jaarsma E. Y., Verhoef J., Marx J. J. Effect of iron on polymorphonuclear granulocyte phagocytic capacity: role of oxidation state and effect of ascorbic acid. Br J Haematol. 1988 Dec;70(4):495–500. doi: 10.1111/j.1365-2141.1988.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Hoepelman I. M., Jaarsma E. Y., Verhoef J., Marx J. J. Polynuclear iron complexes impair the function of polymorphonuclear granulocytes. Br J Haematol. 1988 Mar;68(3):385–389. doi: 10.1111/j.1365-2141.1988.tb04219.x. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Peterson C. M., Grady R. W., Kumbaraci T., Cerami A., Graziano J. H. Effects of iron chelators and iron overload on Salmonella infection. Nature. 1977 May 5;267(5606):63–65. doi: 10.1038/267063a0. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa H., Bland C. E., Willis W. T., Dallman P. R. Iron deficiency and neutrophil function: different rates of correction of the depressions in oxidative burst and myeloperoxidase activity after iron treatment. Blood. 1987 May;69(5):1464–1468. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M., Harada R. N. Effect of staphylococcal iron content on the killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect Immun. 1981 Apr;32(1):407–410. doi: 10.1128/iai.32.1.407-410.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Repine J. E., Johansen K. S., Berger E. M. Hydroxyl radical scavengers produce similar decreases in the chemiluminescence responses and bactericidal activities of neutrophils. Infect Immun. 1984 Jan;43(1):435–437. doi: 10.1128/iai.43.1.435-437.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Voetman A. A., Meerhof L. J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983 Aug;97(2):368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., van Strijp J. A., Hoekstra W. P., Verhoef J. Effect of human polymorphonuclear and mononuclear leukocytes on chromosomal and plasmid DNA of Escherichia coli. Role of acid DNase. J Clin Invest. 1984 May;73(5):1254–1262. doi: 10.1172/JCI111327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Remington J. S. Effect of antimicrobial agents on chemiluminescence of human polymorphonuclear leukocytes in response to phagocytosis. J Antimicrob Chemother. 1982 Dec;10(6):505–515. doi: 10.1093/jac/10.6.505. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. J., Shirley P. S., Hedrick C. C., DeChatelet L. R. Role of free radical processes in stimulated human polymorphonuclear leukocytes. Biochemistry. 1986 Dec 2;25(24):8042–8048. doi: 10.1021/bi00372a037. [DOI] [PubMed] [Google Scholar]
- Veis J. H., Contiguglia R., Klein M., Mishell J., Alfrey A. C., Shapiro J. I. Mucormycosis in deferoxamine-treated patients on dialysis. Ann Intern Med. 1987 Aug;107(2):258–258. doi: 10.7326/0003-4819-107-2-258_1. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986 Aug;78(2):545–550. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck B. S., Marx J. J., Struyvenberg A., Verhoef J. Functional defects in phagocytic cells from patients with iron overload. J Infect. 1984 May;8(3):232–240. doi: 10.1016/s0163-4453(84)93955-0. [DOI] [PubMed] [Google Scholar]



