Abstract
Emerging evidence suggests that the capacity of a tumor to grow and propagate is dependent on a small subset of cells within a tumor, termed cancer stem cells. In fact, cancer cells, like stem cells, can proliferate indefinitely through a dysregulated cellular self-renewal capacity. Cancer stem cells may originate due to the distribution into self-renewal and differentiation pathways occurring in multi-potential stem cells, tissue-specific stem cells, progenitor cells and cancer cells. Recent studies have shown that ovarian cancer also contains stem cells or tumor-initiating cells. Moreover, ovarian serous adenocarcinomas were disaggregated and subjected to growth conditions to select for self-renewing, non-adherent spheroids previously shown to be derived from tissue stem cells. A recent study showed that epithelial ovarian cancer was derived from a sub population of CD44+, CD117+ and CD133+ cells. The existence of cancer stem cells would explain why only a small minority of cancer cells is capable of extensive proliferation of the tumor. In this review, we have discussed the studies on ovarian cancer stem cells along with the molecular pathways that could be involved in these cancer stem cells.
Introduction
Ovarian cancer is the fifth leading cause of cancer deaths and has the highest mortality rate among gynecologic cancers. It is the most lethal malignancy of the female reproductive system, at the initial stage the five-year survival rate is nearly 45%, which declines to 30% for patients with an advanced disease [1,2]. Greater than 90% of ovarian cancers arise from the surface epithelium [3], and tumorigenesis has been associated with ovulation-associated wound repair and/or inflammation, possibly leading to abnormal stem cell expansion [3,4]. Over the last several years, it has been increasingly evident that a small population (less than 5%) of cancer cells, referred to as "cancer stem cells (CSCs)", is responsible for the aggressiveness of the disease, metastasis and resistance to therapy [5-7]. Cancer stem cells, like somatic stem cells, are thought to be capable of self-renewal or unlimited proliferation [7]. The recent discovery that CSCs express certain 'stem cell-specific' markers has renewed interest and provided a rise in the idea that CSCs may arise from somatic stem/progenitor cells. Considerable research efforts have been directed toward the identification of cancer stem cell markers in ovarian cancer.
Stem cells, as classically defined, are cells with a capacity for self-renewal and generation of daughter cells that can differentiate into all the way down different cell lineages found in the mature tissue [8]. Stem cells always undergo asymmetric cell divisions, with each cell generating two cells; one that is identical to itself in stemness and another which is committed to a certain lineage. The daughter cell with stem cell like properties maintains its own compartment over time, while its sister cell undergoes a series of cell divisions [9]. Self-renewal allows stem cells to persist during the entire the lifetime of the organism, while their differentiation potential allows them to perform functions like tissue genesis, tissue maintenance, and regeneration following stress or injury [9].
Of all the types of stem cell, hematopoietic stem cells (HSCs) are the best characterized adult stem cell [10]. HSCs can differentiate to form mature blood cells but can also reproduce themselves, which is known as self-renewal [10]. It is reside in distinct stem-cell niches that vary in location depending on the developmental stages of organism [11]. The human HSCs express high level of CD34 and low or absent level of CD33, CD38, thy-1, and CD71, appears to be enriched for primitive progenitor and HSC activity, while more mature progenitors express one or more of these markers [12]. Furthermore, in therapeutic target hematopoietic stem cells are the only stem cells developed up to therapy for the cancer and other disorders for the blood [11] and following HSC study for other stem cells will lead to improve therapy for other cancers.
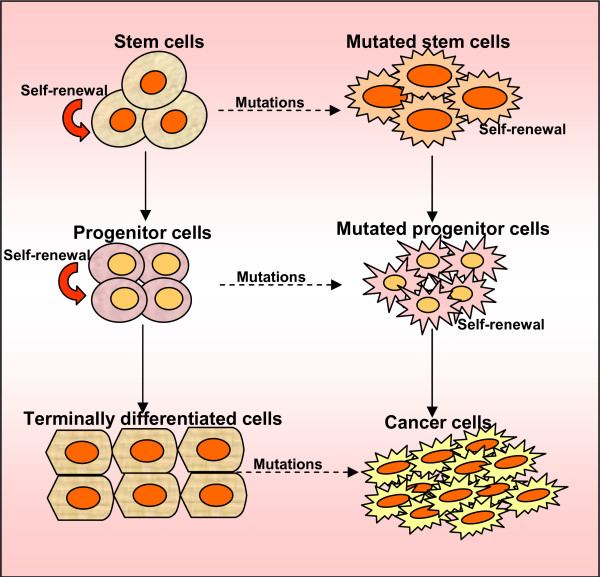
Cancer stem cells may arise following transforming mutations that occur in untransformed stem cells, progenitor cells, mature cells, and cancer cells. The genetic program controlling self-renewal and differentiation plays a key role in the genesis of cancer stem cells (Figure 1). Cancer stem cells (CSCs) have been demonstrated to have roles in several cancers, including cancers of the ovaries, breast, brain, prostate, pancreatic, hepatocellular, head and neck cancers and hematological malignancies [5-7,13-27]. According to the CSC model, only a specific subset of the cancer cell population (i.e., the long-lived CSC subset) should be able to sustain in vivo tumor growth, whereas all other subsets (i.e., the tumor counterparts of short-lived differentiated cells) should not. Indeed, this assumption has now been repeatedly confirmed in several tumor systems. Three key observations classically define the existence of a CSC population: (i) Only the minority of cancer cells within each tumor are usually endowed with tumorigenic potential when transplanted into immunodeficient mice; (ii) Tumorigenic cancer cells are characterized by a distinctive profile of surface markers and can be differentially and reproducibly isolated from non-tumorigenic ones by flow cytometry or other immunoselection procedures; and (iii) Tumors grown from tumorigenic cells contain mixed populations of tumorigenic and non-tumorigenic cancer cells, thus recreating the full phenotypic heterogeneity of the parent tumor [28]. Furthermore, recent studies have been shown the functions of normal and malignant stem/progenitor cells in tissue regeneration, cancer progression and targeting therapies [29,30]. In this review we aim to provide insight into the evaluation of the evidence that supports the existence of cancer stem cells and the characterization studies that have tried to identify ovarian cancer stem cells. We also discuss how taking this subpopulation of cells into account may affect the way we treat ovarian cancers in the future.
Figure 1.
Origin of cancer stem cells. Self-renewal and differentiation potentials are the features of stem cells. Progenitor cells, the product of stem cells that lose the activity of self-renewal, could differentiate into mature cells, which have the feature of differentiation. The hypothesis is that cancer stem cells are caused by transforming mutations occurring in multi-potential stem cells, tissue-specific stem cells, progenitor cells, mature cells, and cancer cells.
Cancer stem cells
The identification of a reservoir of stem cells within many adult tissues raises the interesting possibility that all adult tissues have stem cells. Stem cell populations within normal tissues are defined by certain common characteristics: self-renewal to maintain the stem cell pool over time; regulation of stem cell number through a strict balance between cell proliferation, cell differentiation and cell death; and the ability to give rise to a broad range of differentiated cells [31,32]. It is observed that like stem cells, cancer cells are widely thought to be able to proliferate indefinitely through a deregulated self-renewal capacity. In fact, cancer stem cells can thus only be defined experimentally by their ability to generate continuously growing tumors. CSCs have the capacity to self-renew, undergoing divisions that allow the generation of more CSCs and ultimately some of them differentiate into the various cell types that compose the tumor mass. To date, the practical translation of this definition, and the gold standard to define the 'stemness' of cancer cells, has been their ability to generate a phenocopy of the original malignancy in immuno-compromised mice [7].
Evidence for the existence of cancer stem cells
To assay the cancer stem cells, a xenograft model for breast cancer was developed that allowed specific cancer tumors isolated directly from a patient to be passaged reliably in vivo. In this model, only a subset of cancer cells had the ability to form new tumors [5]. The cancer stem cells isolated from tumors are mostly isolated by flow cytometry as the CD44+ CD24-/low lineage cell population [5]. Furthermore, dilution assays demonstrated that as few as 100 tumorigenic cancer cells were able to form tumors, while tens of thousands of the other (non-CSCs) populations of cancer cells failed to form tumors in nude mice. These tumorigenic cells have been serially generated in new tumors containing additional CD44+ CD24-/low lineage tumorigenic cells as well as the phenotypically mixed population of non-tumorigenic cancer cells [5,7]. In addition, when cultured cells were isolated based on the expression of CD133, a marker expressed by normal CNS stem cells [33], only the CD133+ fraction of cells was capable of forming spheres. These studies suggest that CNS tumors of neural origin contain a stem cell population. Li et al. reported that a highly tumorigenic subpopulation of pancreatic cancer cells expresses the cell surface markers CD44, CD24 and epithelial-specific antigen (ESA) [18]. Table 1 summarizes the studies which have described the direct isolation of populations containing cancer stem cells in various malignancies. Another phenotype used to distinguish these cells is their presence within the Side Population fraction as determined by their ability to exclude the Hoechst dye [34].
Table 1.
Cancer type and specific marker for cancer stem cell populations
| S. No | Cancer type | Markers for CSC population | References |
| 1. | Brain Tumors | CD133+ | [23] |
| 2. | Breast Cancer | CD24-/low/CD44+/ESA+ | [5] |
| 3. | Ovarian Cancer | CD133+/Side population (SP)/CD44+, CD117+ | [25,27,59] |
| 4. | Lung Cancer | CD133+ | [15] |
| 5. | Prostate Cancer | CD44+/α2β1high/CD133+ | [14] |
| 6. | Pancreatic Cancer | CD44+/CD24+/ESA/CD133+ | [16,18] |
| 7. | Hepatocellular Cancer | CD133+ | [24,26] |
| 8. | Hematological Malignancies | CD34+/CD38- | [17] |
| 9. | Colon Cancer | CD133+/CD44+/Lin-/ESA+ | [22,28,44] |
| 10. | Head and Neck Cancer | CD44+ | [21] |
Therapeutic targets for cancer stem cells
The field of stem cell research has given new hope for the treatment and even a cure for incurable diseases in human. Particularly, the identification of a rare population of adult stem cells in most tissues/organs in humans has emerged as an attractive source of multiple stem/progenitor cells for cell replacement-based therapies and tissue engineering in regenerative medicine. Our recent review discussed that cancer stem/progenitor cell research also offers the possibility of targeting these undifferentiated and malignant cells that provide critical function in cancer initiation and relapse for treating patients diagnosed with advanced and metastatic cancer [30,35,36]. Various strategies consisting of molecular targeting of distinct oncogenic signaling elements activated in the cancer progenitor cells and their local microenvironment during cancer progression can be explored [37]. Furthermore, overcoming the intrinsic and acquired resistance of cancer stem/progenitor cells to current clinical treatments represents a major challenge in treating and curing the most aggressive and metastatic cancers [38]. In addition, hematopoitic stem cells are the most characterized stem cells and it has been used for the therapy to cure cancer [11]. In this review we also described that the molecular mechanisms involved in the intrinsic and acquired resistance of cancer cells to current cancer therapies [38].
Pathways of self-renewal and carcinogenesis
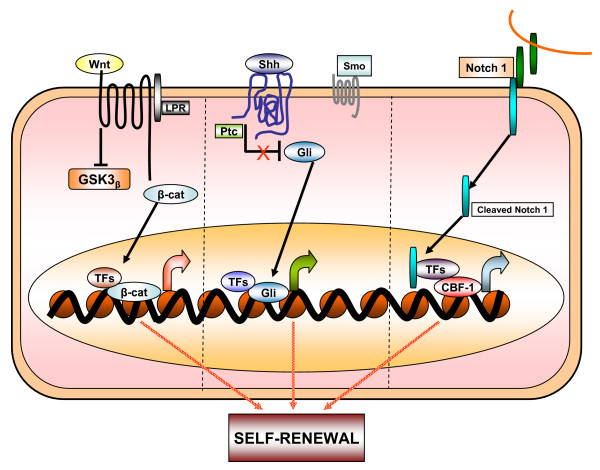
Since the cancer stem cells share common properties with normal stem cells, it is reasonable to think that they have overlapping regulatory mechanisms. Indeed, one of the most outstanding questions concerning the biology of stem cells is: how do multi-potent stem cells select a particular differentiation pathway and start to differentiate? Another question is how do stem cells decide to maintain self-renewal properties and continue to proliferate? Recent studies demonstrate that the presence of various genes and signaling pathways are involved in the regulation of the aforementioned processes. Among these, the Sonic Hedgehog (Shh), Notch and Wnt signaling transduction pathways play a major role in the self-renewal of stem cells [39-41]. Recent advances in the understanding of the role of Wnt, Hedgehog, Shh, and Notch signaling pathways in regulating stem cell self-renewal have shed new light on carcinogenesis (Figure 2) [7,42,43]. The next obvious question is the possible connection between tumors and the (Hedgehog) Hh and Wnt pathways and how the activation of these pathways leads, in some cases, to such highly efficient tumorigenesis. Recent genetic evidence suggests that somatic stem cells are the producers of CSCs; that the Wnt and Hh pathways function in the normal regulation of stem-cell number in at least some tissues; and that expansion of the somatic stem-cell population may be the first step in the formation of at least some types of cancers [44-46]. Numerous arguments support a stem-cell origin for human cancer. Foremost is the observation that stem cells possess many of the features that characterize the malignant phenotype, including self-renewal and unlimited replicative potential [47]. Also, the mutations that initiate tumor formation seem to accumulate in cells that persist throughout life, as suggested by the exponential increase of cancer incidence with age. This is thought to reflect a requirement for four to seven mutations in a single cell to effect malignant transformation [47]. Although similar signaling pathways may regulate self-renewal in normal stem cells and cancer stem cells, there are mechanistic differences in some cancers. Interestingly, the mechanistic differences in self-renewal between normal stem cells and cancer stem cells can thus be targeted to deplete cancer stem cells without damaging normal stem cells.
Figure 2.
Schematic diagram of signaling pathways that are involved in normal and cancer stem cell biology. Wnt, Shh and Notch1 pathways have been shown to contribute to the self-renewal of stem cells and/or progenitors in a variety of organs, including the ovarian system. When deregulated, these pathways can contribute to oncogenesis. Mutations of these pathways have been associated with a number of carcinomas.
Ovarian tumors
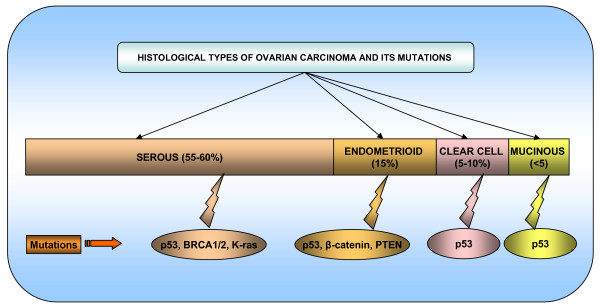
The ovaries contain three main types of cells germ cells, stromal cells and epithelial cells which give rise to germ cell, stromal and epithelial ovarian tumors, respectively. Epithelial ovarian cancers (EOC) were the most common type of ovarian cancers. Comprising nearly 90% of all ovarian cancers EOCs are derived from relatively pluripotent cells of the celomic epithelium or "modified mesothelium". These cells originate from the primitive mesoderm and can undergo metaplasia. Approximately 10% to 20% of epithelial ovarian neoplasms are borderline or low malignant potential tumors and are characterized by a high degree of cellular proliferation in the absence of stromal invasion. Of the invasive epithelial ovarian cancers, about 55–60% are serous, 15% endometrioid, 5–10% clear cell and <5% mucinous [48] (Figure 3). The various histological subtypes of ovarian carcinoma have identifiable precursor lesions and multiple early genetic alterations. Figure 3 explains the various histological subtypes of ovarian cancer and their associated specific mutations. Mutations may be one of the major factors contributing to the origin of ovarian cancer stem cells. Many of the histological subtypes resemble the epithelial component of the lower genital tract, including papillary serous tumors that have an appearance resembling the glandular epithelium lining the fallopian tube. Mucinous tumors, on the other hand, contain cells resembling endocervical glands, and endometrioid tumors contain cells resembling the endometrium. Non-epithelial types of ovarian cancer include sex cord-stromal tumors (6% of ovarian cancers) and germ cell tumors (3% of all malignant ovarian neoplasms) [49-51]. The histological subtypes of ovarian carcinoma have identifiable precursor lesions and early genetic alterations. Figure 3 explains the histological subtypes and its specific mutations in ovarian carcinoma. Mutations are one of the major alteration factors for the origin of cancer ovarian stem cells.
Figure 3.
Schematic diagram representing the histological types and its specific mutations in ovarian carcinoma.
Markers and their roles in ovarian tumors
In general, tumor markers can be used for one of four purposes: (i) screening a healthy population or a high risk population for the presence of cancer; (ii) making a diagnosis of cancer or of a specific type of cancer; (iii) determining the prognosis of a patient; and (iv) monitoring the course in a patient in remission or while receiving surgery, radiation, or chemotherapy.
Furthermore, recent studies have identified different prognostic and diagnostic surface markers for ovarian cancer [52] and these markers need to be analyzed for their role in ovarian cancer. One of the well-known tumor antigens is the epithelial cell mucin MUC1, a transmembrane glycoprotein that is differentially expressed on tumor cells compared with normal epithelial cells [53,54]. MUC1 is expressed either not at all or in small amounts on various normal epithelia but aberrantly or neoexpressed at high levels on the majority of adenocarcinomas. Tumor-associated alterations of MUC1 are characterized by hypoglycosylation, increased sialylation, and altered carbohydrate core-type expression [53]. Engelmann et al reported that MUC1 molecule is not only expressed on mature cancer cells, but also on tumor cells that have multiple characteristics of stem and progenitor cells [55]. This study demonstrates MUC1 expressed breast cancer cell line MCF7 as a source of a minor population of cells with characteristics of tumor stem/progenitor cells to show for the first time that these cells also express the hypoglycosylated (tumor) form of MUC1, previously described only on mature MCF7 cells and other tumors and tumor cell lines. Moreover, these cells give rise to MUC1+ tumors in vivo and that these tumors maintain a small population of MUC1+ cells with the stem/progenitor characteristics [55]. Our recent finding demonstrated the tumor-specific expression of Tumor Associated Glycoprotein-72 (TAG-72) in ovarian cancer and its association with disease stage may serve as a potential marker for effective disease management [56]. In addition, surface marker mucins are overexpressed in many epithelial malignancies including ovarian cancer, suggesting a possible role in the pathogenesis of these cancers. Other studies from our laboratory have provided experimental evidence that the MUC4 mucin interacts with HER2 potentiates its downstream signaling and enhances the motility of ovarian cancer cells. Our findings provide experimental support for the hypothesis that MUC4 mucin expression is associated with a higher metastatic potential and thereby a poor prognosis in ovarian cancer [57]. The future direction of these studies will be to explore the roles of MUC4 and TAG-72 in ovarian cancer stem/progenitor cells.
Ovarian cancer stem cells
A recent study describes that ovarian cancer cell lines were shown to possess "side population" (SP) cells that have been described as cancer stem cells due to their stem-like characteristics including the ability to differentiate into tumors with different histologies. These putative cancer stem cells reflect the various histological subtypes observed in ovarian carcinoma. They also provide a model of cancer metastasis in which these cells are able to colonize, expand, and differentiate into heterogeneous tumor phenotypes similar to primary tumors. In such a model, both the primary tumors and metastasis would display similar genetic and expression profiles because both populations are supposedly derived from the same lineage of cancer stem cells [58]. Ovarian cancer stem cells, like somatic stem cells, are shown to be capable of unlimited self-renewal and proliferation. In general, multi-potent cancer stem cells may account for the histological heterogeneity often found in tumors [25,27,59]. Moreover, ovarian somatic stem cells would be expected to divide asymmetrically, yielding both a daughter cell that proceeds to terminal differentiation, and an undifferentiated copy capable of self-renewal. Repeated asymmetric self-renewal sets of somatic stem cells or their immediate progenitor's stem cells lead to the accrual of mutations over time, which might ultimately lead to their transformation into cancer stem cells and malignant progression.
Furthermore, another study describes that two mouse ovarian cancer cell lines such as MOVCAR7 and 4306 contain candidate cancer stem cells [25]. These two murine ovarian cancer cells have large SP, making them suitable to study ovarian cancer stem cell biology. A similar, albeit very small, SP was also identified in the human ovarian cancer stem cell lines IGROV-1, SKOV-3 and OVCAR-3 and also in cells claimed from patient ascetic fluid [25]. Further, a study proved that isolated and characterized ovarian cancer-initiating cells (OCICs) are fully capable of reestablishing their original tumor hierarchy in vivo. These cells are very organized self-renewing, anchorage-independent spheres and were reproducibly dividable using antibodies against both CD44 and CD117 [27]. These OCICs were capable of intraperitoneal tumorigenesis and could serially propagate tumors in animals. Consequently, this study fulfills all currently accepted criteria for the existence of a subpopulation of tumor-initiating cells [27], and their specific detection and targeting could be highly valuable for therapy of recurrent, chemo-resistant disease. Whereas advanced ovarian cancer is generally initially responsive to standard chemotherapies (ciaplatin and paclitaxel), that responsive almost inevitably followed by drug resistant phenotype [2,60]. One accepted hypothesis about chemoresistance is standard therapies failed to target tumor progenitors, which are have like normal stem cells because of expression of membrane efflux transporters. Zhang et al showed that OCICs, under stem cell-selective conditions, over express ABCG2 and are more resistant to cisplatin and paclitaxel, suggesting a possible role for these cells in ovarian cancer chemoresistance [27].
Conclusion and perspective
The aforementioned studies showed that a so-called ovarian cancer stem cell, with high-proliferative capacity, self-renewal properties and multi-lineage potential, could be responsible for tumor development and the differentiation of more mature epithelial ovarian cells contributing to tumorigenesis. There are important consequences for cancer treatment if the growth of tumors is at least in part, dependent on a cancer stem cell population. The cancer stem cell hypothesis posits that cancer stem cells are a minor population of self-renewing cancer cells that fuel tumor growth and remain in patients after conventional therapy has been completed. The hypothesis predicts that effective tumor eradication will require obtaining agents that can target cancer stem cells while sparing normal stem cells. Experimental evidence suggests that ovarian cancer stem cells are relatively resistant to conventional chemotherapeutic agents. Current cancer therapies often engender severe toxicity because of their general effects on all rapidly dividing cells. Identification of candidate targets for more specific mechanism-based cancer therapy using techniques such as gene chips could reveal signature patterns of transcriptional output which are characteristic of activated self-renewal pathways.
Emerging evidence suggests that these pathways also control patterning and growth in self-renewing adult tissues by regulating the stem-cell compartment. Thus, pharmacological inhibition of these pathways in the worst case might result in severe toxicity due to a loss of normal stem-cell compartments. Further research will be needed to determine whether continuous pathway activity is required in normal and tumor tissues, and whether these requirements differ sufficiently as to allow therapeutic intervention. Even if pathway inhibition is prohibited by normal physiological requirements, other mechanism-based approaches that exploit aberrant pathway activation might be feasible. It has been proposed that malignancy is determined in all tissues by mis-regulation of a common set of genes that control growth by affecting cell proliferation, apoptosis, invasion and angiogenesis. This hypothesis is supported by the demonstration that multiple types of normal human cells can be made tumorigenic by the expression of a defined set of viral and cellular proteins. Therapeutic agents for the treatment of such tumors might target not only self-renewal pathway components, but also other critical transcriptional targets of the self-renewal pathways, or proteins that co-operate with them to deregulate growth.
It is important that agents directed against cancer stem cells discriminate between cancer stem cells and normal stem cells. This will require the identification of realistic drug targets unique to cancer stem cells. The identification of such targets and the development of anti-cancer agents will require a deeper understanding of normal stem cell biology as well as cancer biology. More importantly, identification of the ovarian cancer stem cell would provide a critical step in advancing the development of novel therapeutic strategies in the management of ovarian cancer. Furthermore, characterizations of such progenitor or cancer stem cells in drug resistant (Ciaplatin, Paclitaxel and etc) manner for ovarian cancer will likely lead to a greater understanding of early events leading to the genesis of this elusive disease, in addition to providing new therapeutics targets aimed at the cells directly responsible for its propagation.
Abbreviations
CSC: Cancer Stem Cell; CNS: Central Nervous System; ESA: Epithelial-Specific Antigen; Shh: Sonic Hedgehog; Hh: Hedgehog; EOC: Epithelial ovarian Cancer; OCIC: Ovarian Cancer Initiating Cells; SP: Side Population
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PPM participated in drafting the full manuscript and creating figures. SKB participated in substantial contribution to conception and revising it critically for important intellectual content.
Acknowledgments
Acknowledgements
The authors thank Kristi L.W. Berger (Eppley Institute) for editorial assistance. The authors on this article were supported by grants from the U.S. Department of Defense (OC04110) and National Institutes of Health (RO1 CA78590, CA 131944 and CA133774).
Contributor Information
Moorthy P Ponnusamy, Email: mpalanim@unmc.edu.
Surinder K Batra, Email: sbatra@unmc.edu.
References
- Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–770. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- Pecorelli S, Favalli G, Zigliani L, Odicino F. Cancer in women. Int J Gynaecol Obstet. 2003;82:369–379. doi: 10.1016/s0020-7292(03)00225-x. [DOI] [PubMed] [Google Scholar]
- Godwin AK, Testa JR, Hamilton TC. The biology of ovarian cancer development. Cancer. 1993;71:530–536. doi: 10.1002/cncr.2820710207. [DOI] [PubMed] [Google Scholar]
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der KD. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- McNiece I. The CD34+Thy1+ cell population: are they all stem cells? Exp Hematol. 2000;28:1312–1314. doi: 10.1016/s0301-472x(00)00609-3. [DOI] [PubMed] [Google Scholar]
- Brown MD, Gilmore PE, Hart CA, Samuel JD, Ramani VA, George NJ, Clarke NW. Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. Prostate. 2007;67:1384–1396. doi: 10.1002/pros.20620. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di VA, Conticello C, Ruco L, Peschle C, De MR. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De MR. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 2007;26:203–214. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;%19:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther. 2008;83:673–691. doi: 10.1038/sj.clpt.6100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Reguart N, He B, Taron M, You L, Jablons DM, Rosell R. The role of Wnt signaling in cancer and stem cells. Future Oncol. 2005;1:787–797. doi: 10.2217/14796694.1.6.787. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18:S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- Breedlove G, Busenhart C. Screening and detection of ovarian cancer. J Midwifery Womens Health. 2005;50:51–54. doi: 10.1016/j.jmwh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Hightower RD, Nguyen HN, Averette HE, Hoskins W, Harrison T, Steren A. National survey of ovarian carcinoma. IV: Patterns of care and related survival for older patients. Cancer. 1994;73:377–383. doi: 10.1002/1097-0142(19940115)73:2<377::aid-cncr2820730223>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- Ponnusamy MP, Venkatraman G, Singh AP, Chauhan SC, Johansson SL, Jain M, Smith L, Davis JS, Remmenga SW, Batra SK. Expression of TAG-72 in ovarian cancer and its correlation with tumor stage and patient prognosis. Cancer Lett. 2007;251:247–257. doi: 10.1016/j.canlet.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99:520–526. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien JR, Aletti G, Bell DA, Keeney GL, Shridhar V, Hartmann LC. Molecular pathogenesis and therapeutic targets in epithelial ovarian cancer. J Cell Biochem. 2007;102:1117–1129. doi: 10.1002/jcb.21552. [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S, Scambia G. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506–514. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]