Abstract
There are four opportunities for HIV prevention: before exposure, at the moment of exposure, immediately after exposure, and as secondary prevention focused on infected subjects. Until recently, most resources have been directed toward behavioral strategies aimed at preventing exposure entirely. Recognizing that these strategies are not enough to contain the epidemic, investigators are turning their attention to post-exposure prevention opportunities. There is increasing focus on the use of ART–either systemic or topical (microbicides)–to prevent infection at the moment of exposure. Likewise, there is growing evidence that ART treatment of infected people could serve as prevention as well. A number of ongoing clinical trials will shed some light on the potential of these approaches. Above all, prevention of HIV requires decision-makers to focus resources on strategies that are most effective. Finally, treatment of HIV and prevention of HIV must be considered and deployed together.
Introduction
The 2007 UNAIDS report estimated that for every one person who receives antiretroviral treatment, 4–6 other people acquire HIV [1]. Yet, as has been recently noted [2], HIV prevention programs and initiatives have made only modest progress, and only in some communities. Furthermore, where there have been gains, they have not always been sustainable. HIV prevention can only succeed under the following conditions: i) all the available strategies are used in combination as "highly active prevention" [3]; ii) the menu of options are driven by scientific results and not ideology; iii) affected communities work together with organizations committed to prevention; iv) we continue use our growing knowledge of prevention–biological, structural and behavioral–to move past the social, economic, and other constraints we face today. Given the often limited prevention resources, we must focus on strategies that work [2]. In this article we review the documented successes and focus on recent data that is likely to shape near-term HIV prevention strategies.
Prevention opportunities
There are four separate and discrete opportunities for HIV prevention: before exposure to HIV, at the moment of exposure, immediately after exposure, and among people who are HIV infected [4] (Figure 1).
Figure 1.
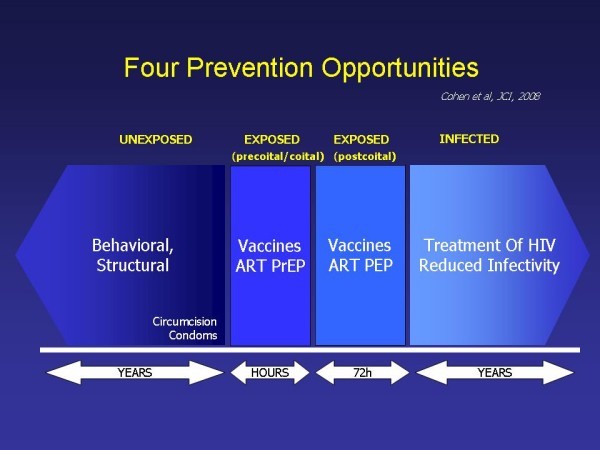
HIV Prevention opportunities, adapted from[4].
HIV prevention before exposure
Behavioral interventions directed at those who are not infected with HIV must educate people about prevention, encourage access to services such as treatment for sexually transmitted infections or drug abuse, delay onset of first intercourse, increase condom use, and reduce the number of sexual partners and/or sharing of syringes and needles. Interventions can be deployed at the level of the individual, couple, family, peer group or network, institution, or community [3] (Table 1). Voluntary counseling and testing, a cornerstone of any HIV prevention strategy, can be applied at each of these levels: to individuals, to couples (as in Rwanda and Zambia), with entire families (as in Uganda), with peer groups (Thailand), and with entire communities (as in Project Accept).
Table 1.
A multilevel approach to behavioural strategies for HIV prevention with HIV counselling and testing as an example.
| Examples | Applied to HIV counselling and testing | |
|---|---|---|
| Individual | Education; drug-related or sexual risk reduction counselling; skills building; prevention case management | HIV testing and counselling for individuals35 |
| Couple | Couples counselling | HIV counselling and testing for couples35–38 |
| Family | Family-based counselling programmes | Home-based family HIV counselling and testing39 |
| Peer group/network | Peer education; diffusion of innovation; network-based strategies | Voluntary counselling and testing for all network members |
| Institution (eg, school, workplace, prisons) | Institution-based programmes | Services for voluntary counselling and testing available within workplaces and other institutional settings40 |
| Community | Mass media; social marketing; community mobilisation | Community-based voluntary counselling and testing (eg, Project Accept);41,42 Mobilisation and media to promote HIV counselling and testing |
Adapted from [3].
Abstinence, be faithful, and condoms (ABC) has been the key message of the global HIV prevention effort, but political and religious influences have resulted in greater focus on abstinence only, despite the clear evidence that comprehensive approaches are far more effective [5]. As Collins and colleagues summarized,
It is time to scrap the ABCs and elevate the debate on HIV prevention beyond the incessant controversies over individual interventions. Small scale, isolated programs, however effective, will not bring the AIDS epidemic under control. To lower HIV incidence, especially in high transmission areas, policy makers, donors, and advocates need to demand national prevention efforts that re tailored to their epidemics, bring quality interventions to scale, and address environmental factors in vulnerability. That is why today's most commonly cited acronym for HIV prevention–"ABC"–falls severely short of what is needed to reduce HIV transmission. ABC infantilizes prevention, oversimplifying what should be an ongoing, strategic approach to reducing incidence [6].
Barriers before exposure
Among the behavior change strategies for prevention of HIV is the use of mechanical barriers during sexual intercourse. The benefits of male condoms have been thoroughly documented [7], but the drawback is that these devices need to be used properly and nearly 100% of the time. According to a Cochrane review, when used properly, condom effectiveness is around 85% [8]. As a result of prevention education efforts, a recent survey shows that young woman in sub-Saharan Africa report increased condom by their male partners [9]. Likewise, female condoms have been shown to be an effective barrier against transmission of STIs–including HIV–but they have gained little popularity since their introduction [10].
In 2007, a study was conducted on the use of diaphragms to prevent HIV acquisition [11]. Because the endocervix is so rich in cells receptive to HIV [12], researchers had reason to believe that a diaphragm would prevent HIV infection; however, this trial failed to demonstrate protection[11]. The study results could be due to HIV infection outside the cervix, because adherence was poor, or because concomitant condom usage in both arms of the study limited the ability to detect a benefit from the diaphragm [11].
More recently, male circumcision has been studied as a possible means for preventing HIV transmission. Circumcision essentially erects a permanent barrier against HIV through removal of the foreskin. The mucosal foreskin glans of the penis is rich in cells receptive to HIV infection [12]. Powerful observational data suggested that circumcised men were much less likely to acquire HIV, implying the glans is the main site of HIV acquisition in men. Three randomized controlled trials demonstrated a minimum of 60% reduction in HIV acquisition [13-15]. Consequently, circumcision has been sought for high-risk subjects, but the logistical challenges of providing enough procedures to make an immediate impact are daunting. Many infants born in resource-constrained countries lack access to safe circumcisions, and there has been a distinct lack of political will or patience to institute safe neonatal circumcision worldwide.
Other sexually transmitted diseases: a reappraisal
Classical STDs amplify the transmission of HIV by increasing the genital tract viral burden (infectiousness) and increasing susceptibility to HIV [16]. Overwhelming epidemiologic evidence links classical STDs and HIV [17], especially STDs that are more ubiquitous (e.g. HSV-2, trichomonas), produce lifelong infection (HSV-2), and/or produce ulcers (HSV-2, syphilis). Recognizing that STDs play a critical role in transmission, commitment to their treatment for the prevention of HIV is essential. Unfortunately, the results of clinical trials using treatment of STDs for prevention have been disappointing [18].
To some extent, this is not surprising. STD treatment can only prove effective if just the right person is treated for just the right STD with effective antibiotics for the right period of time. Most recently, daily suppressive treatment of HSV-2 in people with established HSV-2 infection failed to reduce HIV acquisition [19,20]. However, a substantial number of subjects developed genital ulcer, and it seems unlikely that acyclovir, the antibiotic administered, reduced the subclinical inflammation that is likely key to HIV transmission. A trial to determine whether suppression of HSV-2 in HIV-infected subjects can decrease sexual transmission of HIV-1 within a discordant couples is in progress [21].
Still, the disappointing results of these trials should not deliver the wrong message. First, the treatment of STDs is critically important on its own merits [22]. Second, people with STDs are much more likely to have unrecognized HIV [23], including incident infection [24]. Third, people with STDs who remain HIV negative have demonstrated HIV risk behaviors that demand emergent prevention efforts. HIV and classical STDs represent one, not two problems, and the merging of these (currently) separate disciplines is critical to reducing the incidence of both.
HIV prevention at the time of exposure: biology beyond barriers
If an HIV negative person has unprotected sexual exposure to an HIV positive person, transmission is possible. The transmission event is determined by the infectiousness of the "index case" and the susceptibility of "the host" [25](Figure 2). This topic has been extensively reviewed in the scientific literature [4]. The viral inoculum [26] and phenotype [27] play a critical role in transmission probability. The higher the concentration of the virus in the blood, the greater the probability of the transmission event [26,28]. In addition, the transmitted virus has unique properties: a single HIV variant launches sexually transmitted HIV infection 80% of the time [27], and the transmitted virus is generally capable of using the CCR5 receptor [27]. Conversely, polymorphisms and deletions in the CCR5 receptor reduce the probability of HIV acquisition [29]. In addition, the transmitted virus appears to be less well defended against antibody attack (i.e. a reduced glycan shield) [30].
Figure 2.
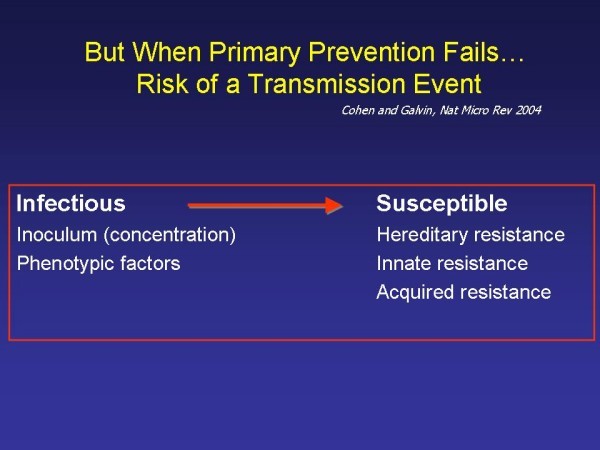
Infectiousness and susceptibility, adapted from[16].
Studies with HIV transmission in macaques show that both cell free (Miller C et al. JV 2005) and cell associated [31] HIV can be transmitted through vaginal exposure. In the presence of an ulcer, infection of mucosal cells and spread to lymph nodes is inordinately fast [31]. In humans, the probability of HIV transmission is likely amplified by sexually transmitted diseases that cause ulcer [16,32].
Beyond mechanical barriers, there are only two ways to prevent HIV infection at the moment of exposure: a credible host defense and/or antiviral therapy.
Development of a protective HIV vaccine
The limits of vaccine development have been extensively discussed [25,33]. Three immune options have received the greatest attention: innate immunity, humoral immunity (antibodies) and/or cell-mediated immunity (cytotoxic lymphocytes) [34].
Antibodies have the capacity to block the attachment of HIV to receptive cells, or to neutralize HIV [35]. Indeed, a group of monoclonal antibodies which neutralize HIV-1 have been identified and described [36]. These antibodies have been used successfully in passive immunity experiments to protect infant rhesus macaques from peroral infection [37]. However, this type of neutralizing antibodies is not generally formed in vivo, perhaps because they are similar to autoantibodies that could harm the host [38]. HIV infection gradually leads to the formation of other antibodies [39] which form weeks or months after infection and after the creation of HIV mutants that uniformly escape the action of these antibodies [40]. Most of these antibodies will not neutralize heterologous viruses. However, a small number of hosts will develop broad neutralizing antibodies long after initial infection. The precise molecular mechanisms by which neutralizing broad antibodies limit HIV replication are not understood. Parenthetically, antibodies directed against HIV form too late in primary infection to explain reduction in viral burden [39].
The time between exposure to HIV, infection, and viral replication is very short (Figure 3) [39]. Even if a vaccine that evokes protective antibodies is developed, it will be a challenge for an anamnestic (memory) antibody response to evolve sufficiently enough to prevent transmission. Protection from HIV infection might require antibody generation at the mucosal surface (IgG or IgA) [41], and no mucosal vaccines have been developed. In addition, acute HIV infection compromises the B cell immune response required for antibody formation. About 30% of patients with acute HIV infection have Rhematoid factor detected, indicating disturbed function of B cells [39]. To date, one vaccine designed to stimulate antibodies has been tested, and no protection from infection was observed, although this vaccine did not generate systemic or mucosal neutralizing antibodies [42].
Figure 3.
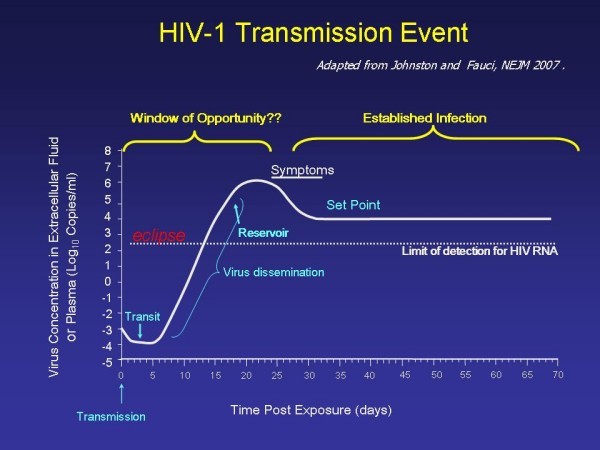
HIV-1 Transmission Event, adapted from[25].
Given the difficulty in developing an antibody-based vaccine, investigators gravitated toward development of vaccines that stimulate cell-mediated immunity [34]. It has been demonstrated that i) animals with lymphocyte depletion cannot control viral replication; ii) some vaccines that stimulate a cell-mediated immune response limit the peak of viremia and transiently decrease the viral burden at "set-point" in macaques; and iii) improve animal survival [43]. While there is virtually no evidence that a T-cell based vaccine can prevent infection, it has long been argued that a reduced peak and set point HIV burden could prevent secondary transmission of HIV, and benefit the health and survival of the host.
A recent trial of a vaccine (Merck V520) that stimulated HIV responsive T cells failed to prevent HIV acquisition [44]. However, the vaccine also failed to reduce viral load at set point. Furthermore, more infections were observed in the group that received the vaccine than in the control group, but the reason for this phenomenon is not known [45]. Another ongoing trial that tests HIV proteins delivered in a canary pox virus vector and boosted with gp120 will be completed in 2009.
ART for prevention
The use of antimicrobial agents to prevent the spread of infections has a long, broad and very successful history. It is surprising that to date, ART has not been more widely adapted as a prevention tool. ART is safe, available, becoming more affordable, and subject to structural modification(s) that might improve drug usage for public health purposes. While it is true that cost, toxicity, lack of adherence and viral drug resistance challenge the utility of the approach, it seems inevitable that ART will play a larger role in the HIV epidemic in the near future, but until recently this area of research has lacked a sense of urgency and adequate funding.
There are three ways ART could be employed: as pre-exposure prophylaxis (PreP), as post-exposure prophylaxis (PEP), and as prevention of secondary transmission from an infected person through suppression of viral concentration in the genital tract [46].
Pre-exposure Prophylaxis (PreP)
Studies with animals have strongly suggested that antiretroviral drugs delivered topically (i.e. "microbicides") or systemically can prevent the transmission of HIV-1 [47-49]. A series of studies from the US Centers for Disease Control and Prevention used multiple rectal mucosal viral challenge on macaques who were given daily antiretroviral agents [50,51]. Tenofovir delayed SHIVSF162P3 infection, but after repeated exposure infection was prevented in only 1 of 4 animals studied [51]. However, high-dose tenofovir and emtricitabine given subcutaneously protected 6 of 6 macaques from infection [50].
Based on this animal data, trials of oral pre-exposure prophylaxis for uninfected high-risk individuals are now under way in Peru, Ecuador, Thailand, Botswana, and the United States. These trials use either tenofovir or a combination of tenofovir and emtricitabine [52] (Table 2). Results will emerge as early as 2010. It should be noted, however, that all of these trials offer prolonged (i.e. one year or more) daily dosing, interventions which are expensive and potentially toxic. It seems likely that prevention benefits will ultimately be realized with a briefer combination of pre- and post-exposure prophylaxis (see below), particularly since the most recent macaque studies suggest that optimally timed doses of PreP and a single dose of PEP are sufficient for protection.
Table 2.
Current and Proposed Pre-Exposure Prophylaxis Trials, October 2007 Study (Sponsor) Study and Agent(s) (Dose) Population (Target N) Sites [52].
| Study (sponsor) | Study and Agent(s) (Dose) | Population (Target N) | Sites |
|---|---|---|---|
| US CDC-NCHSTP-4323 | Phase II daily TDF or daily oral placebo | MSM ages 18 to 60 (400) | US (anticipated completion 2009) |
| US CDC-NCHSTP-4370 | Phase II/III daily TDF or daily oral placebo | IDU ages 20 to 60 (2,000) | Thailand (anticipated completion 2008) |
| CDC-NCHSTP-4940; BOTUSA MB06 | Phase III daily Truvada or daily oral placebo | Men and women ages 18 to 29 (1,200) | Botswana (anticipated completion 2010) |
| iPrEX (NIAID/BMGF) | Phase III daily Truvada or daily oral placebo | MSM ages 18 and up (3,000) | Peru, Ecuador, Brazil, Thailand, South Africa, US (anticipated completion 2011) |
| FHI (USAID) | ) Phase III daily Truvada or daily oral placebo | High-risk women ages 18 to 35 (3,900) | Kenya, Malawi, South Africa, Tanzania, Zimbabwe (study planned, no anticipated completion date yet) |
| Partners Study (BMGF) | Phase III daily TDF, daily Truvada, or daily oral Placebo | Discordant heterosexual couples ages 18 to 60 (4,000) | Uganda, Kenya (study planned, no anticipated completion date yet) |
| VOICE/MTN 003 (NIAID) | Phase IIB safety and effectiveness of daily tenofovir gel (1%) or placebo gel, or daily TDF (300 mg), Truvada, or oral placebo | Nonpregnant premenopausal women ages 18 to 35 (2,400 oral, 1,600 gel) | South Africa, Zambia, Malawi, Uganda, Zimbabwe (study planned, no anticipated completion date yet) |
Antiretroviral therapy can also be delivered topically, via agents known as microbicides, a subject which has been extensively reviewed [53]. While the early days of microbicide research focused on drugs other than ART (e.g. detergents, surface active agents), a variety of more targeted biological products (antibodies and ART) are now being studied [53]. There are studies being conducted on antiviral agents including NNRTIs (s-DABO, TMC-120, U-781, and MIV-150), and the NRTI tenofovir is about to enter a phase 3 clinical efficacy trial.
Particularly exciting is the emergence of new, slow-release vaginal devices that might permit infrequent dosing of effective compounds [53]. One potential problem with topical ART is low-level, systemic absorption, which could promote antiviral resistance; however, in a completed PReP safety trial no women who acquired HIV developed mutations associated with tenofovir resistance [54].
Postexposure prophylaxis (PEP)
The only prevention treatment option after unprotected HIV exposure is emergent use of antiretroviral agents [55]. Prophylaxis following occupational exposure to HIV is considered standard of care in the United States [56] and in most other countries [52]. This protocol was developed primarily from studies in macaques [57] and a single case control study of health care workers with needle stick exposures [58]. In the latter study, thirty-three healthcare workers who sero-converted following percutaneous exposure were compared with control subjects selected from six hundred and seventy-nine individuals who did not seroconvert after postexposure prophylaxis. Zidovudine (in a few cases, other antiretrovirals) given to individuals after percutaneous exposure to HIV led to an 81% risk reduction (CI, 48% to 94%) in HIV seroconversion.
Conducting randomized, controlled, clinical trials of postexposure prophylaxis to prevent the occupational or sexual transmission of HIV in humans are not feasible because of the inefficient transmission of HIV per sexual exposure, and the prohibitive cost of enrolling the very large number of subjects that would be needed to establish benefit. Current CDC guidelines recommend the use of 3 antiretroviral agents for 28 days following high-risk sexual exposure to a known or suspected HIV-infected partner [56]. Based on animal experiments it seems clear that prophylaxis should be administered urgently. In failures reported in a PEP registry delayed administration was a critical risk factor [59].
Prevention for positives
Prevention for positives is only possible if a person knows his or her HIV status. Voluntary counseling and testing strategies (VCT), a cornerstone of HIV prevention, has generally been seen as a first defense against the spread of HIV disease, with the idea that a negative serological test, combined with prevention information, would inspire harm reduction [60-62]. Recognizing the critical role of knowledge of status, the US CDC and many other governments and organizations have recently moved to "opt-out testing" [63]. Others have championed the implementation of universal routinized testing [64].
One key and unresolved issue in preventing the sexual transmission of HIV is identifying the population most critical to the spread of the virus. Given the close relationship between viral load and transmission probability, one could assume that people with acute HIV and late infection (untreated) might be of greatest importance [65]. Indeed, in the only empiric study to address this issue a, substantial number of transmission events could be linked to index cases in precisely these stages of disease [26]. But a recent and compelling mathematical modeling exercise argued that subjects with unrecognized established infection with moderate viral loads in their blood (100,000 copies) are most critical [66], a finding which underscores the importance of knowledge of status.
A substantial number of couples are "discordant" (one partner is HIV-infected and the other is not). Recent massive household screening studies [21,67] have demonstrated that 49% of couples screened can be expected to be discordant, with some regional differences. Ongoing transmission within discordant couples occurs at a rate of about 8–11% per year, even in the face of counseling [68]. Thus the considerable danger of HIV transmission within untested discordant couples should not be underestimated [67,69].
These observations emphasize the importance of timely HIV detection and the need to develop effective counseling strategies to reduce the likelihood of secondary HIV transmission from people who know they are HIV infected. Studies on secondary transmission have raised the concern that ART treatment could actually increase the spread of HIV as a result of improved general health and increased libido after starting ART treatment [60]. Even more worrisome, this increase in secondary transmission would include resistant strains of the virus through patients who discontinue or fail therapy [60].
Recent studies have highlighted these concerns. Williamson et al. [70] found that HIV-infected men who have sex with men in the UK (and who knew their serostatus) had higher risk behaviors (including unprotected anal intercourse and intercourse) with partners of unknown or discordant serostatus than men who were negative or did not know their serostatus. Eisele et al. [71] found ongoing risk behaviors among men and women awaiting ART in Cape Town, South Africa; correlates of risk included failure to disclose serostatus and misconceptions about the relationship between ART and HIV transmission.
ART for suppression of HIV
When used properly, ART can be expected to suppress HIV in the blood and the male genital tract [72]; suppression of HIV in the female genital tract appears to be less rapid and reliable [73]. However, STDs can increase shedding of HIV even in men [74] and women [75] receiving ART. While episodic viral shedding is easily documented, the risk of a transmission event during shedding is unknown.
There are three lines of evidence to suggest that ART reduces infectiousness of treated patients: retrospective analysis, prospective observational studies and ecological data. In two retrospective studies, HIV transmission was greatly reduced when the index cases in couples were offered therapy [76,77]. Two prospective observational studies had similar findings. In one study, of 1034 discordant couples in Zambia and Rwanda, the index partners in 248 couples were receiving ART [78]. Among the 42 partners in this cohort who acquired HIV since 2003, only 2 had partners receiving ART. A similar prospective observational study of Ugandan patients initiating ART reported a 98% reduction in the estimated risk of HIV transmission following the start of ART [79].
Retrospective and observational studies are susceptible to the effects of unexpected modifiers, including unmeasured sexual behavior(s) and condom use. In addition, the periods of observation generally cannot determine long term benefit or detect transmission of resistance viruses (see below). Perhaps most importantly, the studies only include index subjects who require ART for low CD4 counts or advanced HIV disease, whereas ART for prevention might wisely be employed at a much higher CD4 count, especially in people at greatest risk for transmitting HIV.
Several ecologic studies of the preventative benefit of ART have been completed. In a large closed cohort of homosexual men in San Francisco, California, a 60% reduction in anticipated cases of HIV was attributed to availability of ART for infected sexual partners [80]. A study from Taiwan showed a 53% reduction in the expected cases of HIV following the free provision of ART in 1997 [81]. More recently, a study in British Columbia, Canada suggested that up to 50% of expected incident HIV cases were averted by ART [82].
However, ecological prevention benefits of ART have not been universal. No reduction in incident HIV infections among men who have sex with men in San Francisco was observed despite widespread availability of ART [83], and increases in HIV incidence were found among homosexual men attending sexually transmitted disease clinics in Amsterdam, the Netherlands from 1991 to 2001 [84] regardless of treatment roll-out. Ecologic studies are greatly limited by an "ecologic fallacy": the inability to relate the patients who receive therapy to the actual incidence or prevalence of HIV in the community. In addition, the accuracy of HIV prevalence and incidence data in these many of these settings is unknown.
A randomized, controlled trial is underway to try to define the impact of ART on HIV transmission. HPTN 052 is designed to compare the effectiveness of two different treatment strategies to prevent the sexual transmission of HIV among 1750 serodiscordant couples [85]. HIV-infected partners with a CD4 count between 350–550 cells/mm3 are randomly assigned to initiate ART at enrollment or to delay ART until their CD4 T-cell count falls below 250 cells/mm3 or they develop an AIDS-defining illness. The results should detect a 35% reduction in HIV transmission to sexual partners due to ART treatment of HIV-infected subjects. In addition, this study compares the benefits of early versus delayed ART (ACTG 5245), a critical question if ART is going to be used more broadly as a public health tool.
Rational selection of antiviral agents for prophylaxis or prevention
The number and choice of antiviral agents (whether systemic or topical) is vital to the success of any of the interventions discussed. The choice of ART regimen must also take into account the risk of HIV-resistant variants and the pharmacology of antiviral agents. The prevalence of de novo resistance in individuals with incident HIV infection differs greatly by country and region [86] but should be taken into account in selecting ART prophylactic regimens. Furthermore, resistance in the genital tract can be unique and sustained [87].
Recent findings on the pharmacology of antiretrovirals in the genital tract suggest that certain antiretroviral agents may be preferable for the prevention of HIV following sexual exposure (Figure 4). Lamivudine, emtricitabine, zidovudine, tenofovir and maraviroc concentrations in the female genital tract were higher than blood plasma, and lopinavir and atazanavir achieved low to moderate genital tract concentrations [88]. Efavirenz achieved female genital secretion concentrations <1% blood plasma. In addition, many antiretrovirals are detected in genital secretions within 1–2 hours after the first dose of ART. These results should be used to guide selection of agents for HIV PreP and PEP, and perhaps secondary prevention as well.
Figure 4.
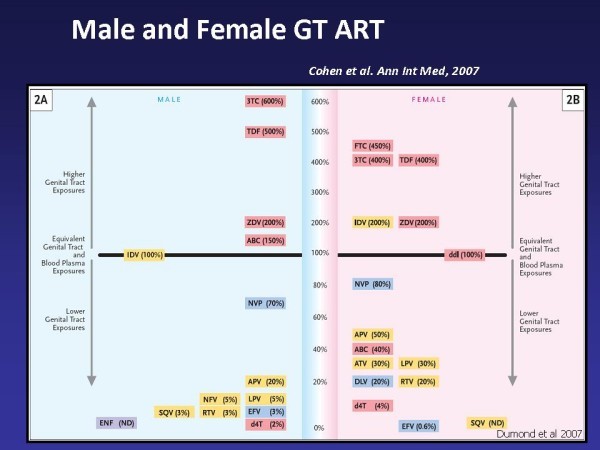
Male and Female GT ART, reproduced with permission from[46].
Ease of therapy is also an important consideration in choosing an ART regimen for non-occupational postexposure prophylaxis. Two case-controlled studies of non-occupational postexposure prophylaxis following high risk sexual exposures were conducted using tenofovir DF and lamivudine in 44 subjects and the combination of tenofovir DF and emtricitabine in an additional 68 subjects. Subjects in both studies with tenofovir-based dual regimens had higher completion rates of a 28-day postexposure regimen than historical controls taking 2- or 3-drug regimens containing zidovudine (P < 0.0001) [89]. Dropout rates during non-occupational postexposure prophylaxis treatment are high [90,91], particularly in cases of sexual assault [92-95]. Although the reasons for discontinuation of therapy may include reassessment of risk exposure and/or intolerable side effects, the evidence of increased adherence with simpler regimens should not be ignored. Finally, it seems clear that health care workers need more education about PEP [96].
ART and public health reality
There are many mathematical models of the effects of ART on the epidemic, both ART used as PreP [97] or provided to people with established infection [46,98,99]. These models are greatly limited by their assumptions, and none has been subjected to experimental investigation. The biggest questions include adherence, degree of benefit, and population volume served. In other words, are enough people at risk of or infected with the disease receiving the right ART at the right times and for long enough to make a difference? In addition, the public health benefits of ART for people with HIV are up for debate, since ART cannot be readily offered to people with acute HIV infection and people with very advanced disease, since neither group is aware of their status during maximal contagion.
What if?
We are at a critical juncture for HIV prevention research [2]. It is clear that we cannot simply treat all individuals who become infected. We do not have the tools to make an HIV vaccine [34], and there is no "magic bullet" solution on the horizon. Currently there is intense interest in multi-faceted approaches, but it seems unlikely that behavioral interventions alone will prove sufficient to change the course of the epidemic [3]. The tool currently most readily available is ART, and ART–as PreP, PEP or treatment–will likely play an increasing role in HIV prevention. Indeed, it is possible that the indications for ART treatment will evolve to consider the public health benefit(s) with the same intensity and urgency as the individual therapeutic benefit(s).
Perhaps the most immediate issue facing us is what to do with the things that work. For example, barrier methods (condoms and circumcision) are clearly effective, but they have by no means reached their full prevention potential. The difficulty in rolling out circumcision, especially in countries most greatly affected by HIV, has been a source of great frustration. Similarly, how do we prepare properly for the broad application of ART, should the trials underway demonstrate the anticipated success? And how do we deal with methods that do not work? How do we develop a strategy that recognizes the importance of STDs in HIV transmission without the expectation that treatment of STDs per se will alter the course of the epidemic? How do we demonstrate a commitment to vaccine development, short of conducting large-scale clinical trials unlikely to succeed?
These issues can only be properly addressed if the research community works well and creatively with public health leaders and agencies, and this has not always been the case [100]. Given the potential trajectory of HIV prevention, now is the time to address these questions. We have mastered some fundamental tools of HIV prevention, and many more are on the way. In the meantime, we must implement all the tools at our disposal, monitor their successes, and prevent the transmission of HIV.
Contributor Information
Myron S Cohen, Email: mscohen@med.unc.edu.
Pontiano Kaleebu, Email: Pontiano.Kaleebu@mrcuganda.org.
Thomas Coates, Email: tcoates@mednet.ucla.edu.
References
- UNAIDS. AIDS Epidemic Update. Vol. 11. Geneva, Switzerland: UNAIDS; 2007. [Google Scholar]
- Potts M, Halperin DT, Kirby D, Swidler A, Marseille E, Klausner JD, Hearst N, Wamai RG, Kahn JG, Walsh J. Public health. Reassessing HIV prevention. Science. 2008;11(5877):749–750. doi: 10.1126/science.1153843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: what will make them work better? Lancet. 2008. in press . [DOI] [PMC free article] [PubMed]
- Cohen MS, Hellmann N, Levy JA, DeCock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J Clin Invest. 2008;11(4):1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda J, Carpenter C, Curran J, Holzemer W, Smits H, Scott K, Orza M. PEPFAR Implementation: Progress and Promise. Washington, D.C.: National Academies Press; 2007. [Google Scholar]
- Collins C, Coates TJ, Curran J. Moving beyond the alphabet soup of HIV prevention. AIDS. 2008;11(Suppl 2):S5–8. doi: 10.1097/01.aids.0000327431.82795.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002. p. CD003255. [DOI] [PubMed]
- Pinkerton S, Abramson P. Effectiveness of condoms in preventing HIV transmission. Social Science & Medicine. 1997;11(9):1303–1312. doi: 10.1016/S0277-9536(96)00258-4. [DOI] [PubMed] [Google Scholar]
- Cleland J, Ali M. Sexual abstinence, contraception, and condom use by young African women: a secondary analysis of survey data. Lancet. 2006;11:1788–1793. doi: 10.1016/S0140-6736(06)69738-9. [DOI] [PubMed] [Google Scholar]
- Padian NS, Buvé A, Balkus J, Serwadda D, Cates W. , JrBiomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008. in press . [DOI] [PubMed]
- Padian NS, Straten A van der, Ramjee G, Chipato T, de Bruyn G, Blanchard K, Shiboski S, Montgomery ET, Fancher H, Cheng H, Rosenblum M, Laan M van der, Jewell N, McIntyre J. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;11(9583):251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A, Bailey RC. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;11(3):867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;11(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;11(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;11(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- Galvin S, Cohen M. The role of sexually transmitted diseases in HIV transmission. Nature Reviews Microbiology. 2004;11:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;11(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Wawer MJ. Reassessing the hypothesis on STI control for HIV prevention. Lancet. 2008;11(9630):2064–2065. doi: 10.1016/S0140-6736(08)60896-X. [DOI] [PubMed] [Google Scholar]
- Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, Cowan F, Casapia M, Ortiz A, Fuchs J, Buchbinder S, Koblin B, Zwerski S, Rose S, Wang J, Corey L. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;11(9630):2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, Tanton C, Ross D, Everett D, Clayton T, Balira R, Knight L, Hambleton I, Le Goff J, Belec L, Hayes R. Effect of Herpes Simplex Suppression on Incidence of HIV among Women in Tanzania. N Engl J Med. 2008;11(15):1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa BL Jr, Bukusi EA, Ngure K, Kavuma L, Inambao M, Kanweka W, Allen S, Kiarie JN, Makhema J, Were E, Manongi R, Coetzee D, De Bruyn G, Delany-Moretlwe S, Margaret A, Mugo N, Mujugira A, Ndase P, Celum C, Group PiPH-HTS. Regional Differences in Prevalence of HIV-1 Discordance in Africa and Enrollment of HIV-1 Discordant Couples into an HIV-1 Prevention Trial. PLoS ONE. 2008;11(1):e1411. doi: 10.1371/journal.pone.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, Hawkes S. Global control of sexually transmitted infections. Lancet. 2006;11(9551):2001–2016. doi: 10.1016/S0140-6736(06)69482-8. [DOI] [PubMed] [Google Scholar]
- Chen XS, Yin YP, Tucker JD, Gao X, Cheng F, Wang TF, Wang HC, Huang PY, Cohen MS. Detection of Acute and Established HIV Infections in Sexually Transmitted Disease Clinics in Guangxi, China: Implications for Screening and Prevention of HIV Infection. J Infect Dis. 2007;11(11):1654–1661. doi: 10.1086/522008. [DOI] [PubMed] [Google Scholar]
- Powers KA, Miller WC, Pilcher CD, Mapanje C, Martinson FE, Fiscus SA, Chilongozi DA, Namakhwa D, Price MA, Galvin SR, Hoffman IF, Cohen MS. Team MUPAHS. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS. 2007;11(16):2237–2242. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Fauci A. An HIV Vaccine–evolving concepts. New England Journal of Medicine. 2007;11:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;11(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY. et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;11(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, Patterson BK, Coombs RW, Krieger JN, Cohen MS. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. Aids. 2001;11(5):621–627. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, Begum K, Galvis MC, Kostecki V, Valente AJ, Murthy KK, Haro L, Dolan MJ, Allan JS, Ahuja SK. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA. Potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus. J Biol Chem. 2000;11(25):18946–18961. doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;11(5666):2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- Weiler AM, Li Q, Duan L, Kaizu M, Weisgrau KL, Friedrich TC, Reynolds MR, Haase AT, Rakasz EG. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J Virol. 2008;11(8):4154–4158. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KA, Poole C, Pettifor AE, Cohen MS. Systematic review and meta-analysis of the heterosexual infectivity of HIV-1: Have the methods confused the message? Lancet Infectious Diseases. in press . [DOI] [PMC free article] [PubMed]
- Kaufmann DE, Lichterfeld M, Altfeld M, Addo MM, Johnston MN, Lee PK, Wagner BS, Kalife ET, Strick D, Rosenberg ES, Walker BD. Limited durability of viral control following treated acute HIV infection. PLoS Med. 2004;11(2):e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B, Burton D. Towards an AIDS Vaccine. Science. 2008;11:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- Montefiori D, Sattentau Q, Flores J, Esparza J, Mascola J. Antibody-based HIV-1 vaccines: recent developments and future directions. PLoS Med. 2007;11(12):e348. doi: 10.1371/journal.pmed.0040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D, Desrosiers R, Doms R, Koff W, PD K. HIV vaccine design and the neutralizing antibody protection. Nat Immunol. 2004;11:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- Ruprecht RM, Ferrantelli F, Kitabwalla M, Xu W, McClure HM. Antibody protection: passive immunization of neonates against oral AIDS virus challenge. Vaccine. 2003;11(24):3370–3373. doi: 10.1016/S0264-410X(03)00335-9. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;11(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, deCamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen MS, Eron J, Hicks CB, Liao HX, Self SG, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Shaw GM, Greenberg ML, Morris L, Karim SSA, Blattner WA, Montefiori DC, Perelson AS, Haynes BF. The Initial Antibody Response To HIV-1: Induction of Ineffective Early Cell B Responses against gp41 by the Transmitted/Founder Virus. PLoS Pathogens. 2008. submitted Jul 14. [DOI] [PMC free article] [PubMed]
- Richmond JF, Lu S, Santoro JC, Weng J, Hu SL, Montefiori DC, Robinson HL. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;11(11):9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbod T, Kauel R, Reichard C, Kimani J, Ngugi E, Bwayo JJ, Nagelkerke N, Hasselrot K, Li B, Moses S, Group KHS, MacDonald KS, Broliden K. HIV-neutralizing immunoglobulin A and HIV-specific proliferation are independently associated with reduced HIV acquisition in Kenyan sex workers. AIDS. 2008;11(6):727–735. doi: 10.1097/QAD.0b013e3282f56b64. [DOI] [PubMed] [Google Scholar]
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;11(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;11(5779):1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafick-Pierre S. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? Journal of Experimental Medicine. 2008;11(1):7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J, Klasse P, Dolan M, Ahuja SK. AIDS/HIV. A STEP into darkness or light? Science. 2008;11(5877):753–755. doi: 10.1126/science.1154258. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;11(8):591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- Van Rompay KK, McChesney MB, Aguirre NL, Schmidt KA, Bischofberger N, Marthas ML. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J Infect Dis. 2001;11(4):429–438. doi: 10.1086/322781. [DOI] [PubMed] [Google Scholar]
- Van Rompay KK, Schmidt KA, Lawson JR, Singh R, Bischofberger N, Marthas ML. Topical administration of low-dose tenofovir disoproxil fumarate to protect infant macaques against multiple oral exposures of low doses of simian immunodeficiency virus. J Infect Dis. 2002;11(10):1508–1513. doi: 10.1086/344360. [DOI] [PubMed] [Google Scholar]
- Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;11:937–938. doi: 10.1097/00002030-200304110-00027. [DOI] [PubMed] [Google Scholar]
- Garcia-Lerma J, Otten R, Qari S, Jackson E, Luo W, Monsour M, Schinazi R, Janssen R, Folks T, Heneine W. Prevention of rectal SHIV transmission in macaques by tenofovir/FTC combination. PLoS Med. 2008;11(2):e30. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, Adams DR, Bashirian S, Johnson J, Soriano V, Rendon A, Hudgens MG, Butera S, Janssen R, Paxton L, Greenberg AE, Folks TM. Chemoprophylaxis with Tenofovir Disoproxil Fumarate Provided Partial Protection against Infection with Simian Human Immunodeficiency Virus in Macaques Given Multiple Virus Challenges. J Infect Dis. 2006;11(7):904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Kashuba AD. Antiretroviral therapy for prevention of HIV infection: new clues from an animal model. PLoS Med. 2008;11(2):e28. doi: 10.1371/journal.pmed.0050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;11:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Maslankowski L, Gai F, El-Sadr W, Justman J, Kwiecien A, Masse B, Eshleman S, Hendrix C, Morrow K, Rooney J, Soto-Torres L, Thiebot H, Team tHP. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;11(4):543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- Roland ME. Postexposure prophylaxis after sexual exposure to HIV. Curr Opin Infect Dis. 2007;11(1):39–46. doi: 10.1097/QCO.0b013e328012c5e0. [DOI] [PubMed] [Google Scholar]
- MMWR Recomm Rep. RR-11. Vol. 11. US Centers for Disease Control and Prevention; 2001. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis; pp. 1–52. [PubMed] [Google Scholar]
- Hosseinipour M, Cohen MS, Vernazza PL, Kashuba AD. Can antiretroviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1? Clin Infect Dis. 2002;11(10):1391–1395. doi: 10.1086/340403. [DOI] [PubMed] [Google Scholar]
- Cardo DM, Culver DH, Ciesielski CA, Srivastava PU, Marcus R, Abiteboul D, Heptonstall J, Ippolito G, Lot F, McKibben PS, Bell DM. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;11(21):1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- Roland ME, Neilands TB, Krone MR, Katz MH, Franses K, Grant RM, Busch MP, Hecht FM, Shacklett BL, Kahn JO, Bamberger JD, Coates TJ, Chesney MA, Martin JN. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis. 2005;11(10):1507–1513. doi: 10.1086/497268. [DOI] [PubMed] [Google Scholar]
- Crepaz N, Hart TA, Marks G. Highly Active Antiretroviral Therapy and Sexual Risk Behavior: A Meta-analytic Review. JAMA. 2004;11(2):224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- Denison JA, O'Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990–2005. AIDS Behav. 2008;11(3):363–373. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;11(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- Revised Recommendations for HIV Testing in Adults, Adolescents, and Pregnant Women in Health-Care Settings. MMWR. 2006;11(RR-14) [PubMed] [Google Scholar]
- Janssen RS. Implementing HIV screening. Clin Infect Dis. 2007;11(Suppl 4):S226–231. doi: 10.1086/522542. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Eron JJ, Galvin S, Gay C, Cohen MS. Acute HIV revisted: new opportunities for treatment and prevention. J Clin Invest. 2004;11(7):937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA. 2007;11(44):17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell R, Cherutich P. Universal HIV testing and counselling in Africa. Lancet. 2008;11(9631):2148–2150. doi: 10.1016/S0140-6736(08)60929-0. [DOI] [PubMed] [Google Scholar]
- Allen S, Meinzen-Derr J, Kautzman M, Zulu I, Trask S, Fideli U, Musonda R, Kasolo F, Gao F, Haworth A. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;11(5):733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, Greenberg L, Allen S. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;11(9631):2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- Williamson LM, Dodds JP, Mercey DE, Hart GI, Johnson AM. Sexual risk behaviour and knowledge of HIV status among community samples of gay men in the UK. AIDS. 2008;11:1071–1077. doi: 10.1097/QAD.0b013e3282f8af9b. [DOI] [PubMed] [Google Scholar]
- Eisele TP, Mathews C, Chopra M, Brown L, Silvestre E, Daries V, Kendall C. High Levels of Risk Behavior Among People Living with HIV Initiating and Waiting to Start Antiretroviral Therapy in Cape Town South Africa. AIDS Behav. 2008;11(4):570–577. doi: 10.1007/s10461-007-9279-7. [DOI] [PubMed] [Google Scholar]
- Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, Boggian K, Cohen MS, Fiscus SA, Eron JJ. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;11:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;11(4):455–480. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- Sadiq ST, Taylor S, Kaye S, Bennett J, Johnstone R, Byrne P, Copas AJ, Drake SM, Pillay D, Weller I. The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. AIDS. 2002;11(2):219–225. doi: 10.1097/00002030-200201250-00011. [DOI] [PubMed] [Google Scholar]
- Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Perre P Van de. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007;11(5):365–368. doi: 10.1136/sti.2007.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicco M, Lazzarin A, Nicolosi A, Gasparini M, Costigliola P, Arici C, Saracco A. Antiretroviral treatment of men infected with human immunodeficiency virus type 1 reduces the incidence of heterosexual transmission. Italian Study Group on HIV Heterosexual Transmission. Arch Intern Med. 1994;11(17):1971–1976. doi: 10.1001/archinte.154.17.1971. [DOI] [PubMed] [Google Scholar]
- Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;11(1):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- Kayitenkore K, Bekan B, Rufagari J, Marion-Landais S, Karita E, Allen S. The impact of ART on HIV transmission among HIV serodiscordant couples. XVI International AIDS Conference: 2006; Toronto, Canada. 2006. p. 32.
- Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako-Kajura W, Were W, Coutinho A, Liechty C, Madraa E, Rutherford G, Mermin J. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;11(1):85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- Porco TC, Martin JN, Page-Shafer KA, Cheng A, Charlebois E, Grant RM, Osmond DH. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;11(1):81–88. doi: 10.1097/00002030-200401020-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CT, Hsu HM, Twu SJ, Chen MY, Chang YY, Hwang JS, Wang JD, Chuang CY. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;11(5):879–885. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, Harrigan PR. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;11(9534):531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- Katz MH, Schwarcz SK, Kellogg TA, Klausner JD, Dilley JW, Gibson S, McFarland W. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;11(3):388–394. doi: 10.2105/ajph.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukers NH, Spaargaren J, Geskus RB, Beijnen J, Coutinho RA, Fennema HS. HIV incidence on the increase among homosexual men attending an Amsterdam sexually transmitted disease clinic: using a novel approach for detecting recent infections. AIDS. 2002;11(10):F19–24. doi: 10.1097/00002030-200207050-00001. [DOI] [PubMed] [Google Scholar]
- Godbole S, Kumarasamy N, Chen Y, Chariyalertsak S, Hakim J, Santos B, Grinsztejn B, Pilotto JH, Hosseinipour M, Kumwenda J, Filho E, Mayer K, Cohen MS, Team HP. Antiretroviral therapy to prevent the sexual transmission of HIV-1: initial results from HPTN 052. International AIDS Society Annual Meeting, Mexico City. 2008. TUPE0046.
- Vella S, Palmisano L. The global status of resistance to antiretroviral drugs. Clin Infect Dis. 2005;11(Suppl 4):S239–246. doi: 10.1086/430784. [DOI] [PubMed] [Google Scholar]
- Smith DM, Wong JK, Shao H, Hightower GK, Mai SHT, Moreno JM, Ignacio CC, Frost SDW, Richman DD, Little SJ. Long-Term Persistence of Transmitted HIV Drug Resistance in Male Genital Tract Secretions: Implications for Secondary Transmission. J Infect Dis. 2007;11:356–360. doi: 10.1086/519164. [DOI] [PubMed] [Google Scholar]
- Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, Bridges AS, Stewart PW, Cohen MS, Kashuba AD. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. Aids. 2007;11(14):1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KH, Mimiaga MJ, Cohen D, Grasso C, Bill R, Vanderwarker R, Fisher A. Tenofovir DF Plus Lamivudine or Emtricitabine for Nonoccupational Postexposure Prophylaxis (NPEP) in a Boston Community Health Center. J Acquir Immune Defic Syndr. 2008;11(4):494–499. doi: 10.1097/QAI.0b013e318162afcb. [DOI] [PubMed] [Google Scholar]
- Kahn JO, Martin JN, Roland ME, Bamberger JD, Chesney M, Chambers D, Franses K, Coates TJ, Katz MH. Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: the San Francisco PEP Study. J Infect Dis. 2001;11(5):707–714. doi: 10.1086/318829. [DOI] [PubMed] [Google Scholar]
- Winston A, McAllister J, Amin J, Cooper DA, Carr A. The use of a triple nucleoside-nucleotide regimen for nonoccupational HIV post-exposure prophylaxis. HIV Med. 2005;11(3):191–197. doi: 10.1111/j.1468-1293.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Wiebe ER, Comay SE, McGregor M, Ducceschi S. Offering HIV prophylaxis to people who have been sexually assaulted: 16 months' experience in a sexual assault service. CMAJ. 2000;11(5):641–645. [PMC free article] [PubMed] [Google Scholar]
- Garcia MT, Figueiredo RM, Moretti ML, Resende MR, Bedoni AJ, Papaiordanou PM. Postexposure prophylaxis after sexual assaults: a prospective cohort study. Sex Transm Dis. 2005;11(4):214–219. doi: 10.1097/01.olq.0000149785.48574.3e. [DOI] [PubMed] [Google Scholar]
- Meel BL. HIV/AIDS post-exposure prophylaxis (PEP) for victims of sexual assault in South Africa. Med Sci Law. 2005;11(3):219–224. doi: 10.1258/rsmmsl.45.3.219. [DOI] [PubMed] [Google Scholar]
- Sonder GJ, Hoek A van den, Regez RM, Brinkman K, Prins JM, Mulder JW, Veenstra J, Claessen FA, Coutinho RA. Trends in HIV postexposure prophylaxis prescription and compliance after sexual exposure in Amsterdam, 2000–2004. Sex Transm Dis. 2007;11(5):288–293. doi: 10.1097/01.olq.0000237838.43716.ee. [DOI] [PubMed] [Google Scholar]
- Cohen MS. HIV Post-exposure prophylaxis following sexual assault: Why is it so hard to accomplish? Sexually Transmitted Diseases. 2008. in press . [DOI] [PubMed]
- Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in resource-limited settings. J Acquir Immune Defic Syndr. 2006;11(5):632–641. doi: 10.1097/01.qai.0000194234.31078.bf. [DOI] [PubMed] [Google Scholar]
- Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, Harrigan PR. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;11:531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- Lima VD, Johnston K, Hogg RS, Levy AR, Harrigan PR, Anema A, Montaner JS. Expanded Access to Highly Active Antiretroviral Therapy: A Potentially Powerful Strategy to Curb the Growth of the HIV Epidemic. J Infect Dis. 2008;11(1):59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- Salomon JA, Hogan DR, Stover J, Stanecki KA, Walker N, Ghys PD, Schwartländer B. Integrating HIV prevention and treatment: from slogans to impact. PLoS Med. 2005;11(1):e16. doi: 10.1371/journal.pmed.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]


