Abstract
Signal transducers and activators of transcription (STAT)-induced STAT inhibitor-1 [SSI-1; also known as suppressor of cytokine signaling-1 (SOCS-1)] was identified as a negative feedback regulator of Janus kinase-STAT signaling. We previously generated mice lacking the SSI-1 gene (SSI-1 −/−) and showed that thymocytes and splenocytes in SSI-1 −/− mice underwent accelerated apoptosis. In this paper, we show that murine embryonic fibroblasts lacking the SSI-1 gene are more sensitive than their littermate controls to tumor necrosis factor-α (TNF-α)-induced cell death. In addition, L929 cells forced to express SSI-1 (L929/SSI-1), but not SSI-3 or SOCS-5, are resistant to TNF-α-induced cell death. Furthermore L929/SSI-1 cells treated with TNF-α sustain the activation of p38 mitogen-activated protein (MAP) kinase. In contrast, SSI-1 −/− murine embryonic fibroblasts treated with TNF-α show hardly any activation of p38 MAP kinase. These findings suggest that SSI-1 suppresses TNF-α-induced cell death, which is mediated by p38 MAP kinase signaling.
Cytokines play important roles in controlling homeostasis of organisms through cell differentiation, proliferation, and apoptosis, and these effects are mainly brought about by Janus kinase-signal transducers and activators of transcription (JAK-STAT) signaling.
Recently, several groups independently have cloned negative feedback regulators of JAK-STAT signaling. STAT-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) is known as one of the negative feedback regulators of JAK-STAT signaling (1–3). SSI-1 is induced by various cytokines, such as IL-2, IL-3, IL-4, IL-6, IL-13, granulocyte–macrophage colony-stimulating factor, erythropoietin, IFN-γ, and leukemia inhibitory factor, can bind to all four members of the JAKs, and inhibits signals of various cytokines by suppressing the activation of the JAKs (4). However, the specificity of SSI-1 for the JAKs in vitro is not well defined. SSI-1 has two characteristic domains, an Src homology 2 domain and a carboxyl-terminal conserved domain, which we have called the SC-motif (also referred to as the SOCS box). So far the cloned SSI families (5–6) comprise at least eight members on the basis of the two characteristic domains, even if overlapping is excluded.
Our previous study revealed that mice lacking the SSI-1 gene (SSI-1 −/−) were healthy and normal at birth, but exhibited growth retardation with aging and died within 3 weeks after birth (7). In addition, lymphocytes underwent accelerated apoptosis in lymphoid organs such as thymus and spleen of SSI-1 −/− mice at 10 days after birth. Because apoptosis generally is brought about by death signals, which are produced from tumor necrosis factor-α (TNF-α) or FasL signaling, it was thought that accelerated apoptosis of thymocytes and splenocytes in SSI-1 −/− mice might result from dysregulated TNF-α or FasL signaling. In this study, we observe an increase in the serum TNF-α of SSI-1 −/− mice. Moreover, murine embryonic fibroblasts (MEFs) lacking the SSI-1 gene become sensitive to TNF-α-induced cell death. In contrast, L929 cells forced to express SSI-1 (L929/SSI-1) show resistance to TNF-α-induced cell death. In addition, SB203580, a highly specific inhibitor of p38 mitogen-activated protein (MAP) kinase, eliminates resistance to TNF-α-induced cell death in L929/SSI-1 cells. Also, SSI-1 is revealed to relate to the activation of p38 MAP kinase. Other families of SSI, however, such as SSI-3 and SOCS-5, do not show these effects. The primary nature of SSI-1 is a negative feedback regulator of JAK-STAT signaling. However, the experiment using STAT1 −/− or STAT6 −/− MEFs reveals that JAK-STAT signaling is not related to TNF-α-induced cell death signals. Thus, our results show that SSI-1 suppresses TNF-α-induced cell death through regulation of p38 MAP kinase instead of through regulation of JAK-STAT signaling, and they reveal an important role of SSI-1 on TNF-α signaling.
Materials and Methods
Measurement of Serum TNF-α, IFN-γ, and IL-1β.
Whole blood was collected from SSI-1 −/− mice or SSI-1 +/+ mice under anesthesia at 10 days after birth, and then was centrifuged. Serum of SSI-1 +/+ mice injected intravenously with Con A at a dose of 10 μg/g of body weight was used as positive control. The serum was stored at −80°C. TNF-α serum samples were measured by using a mouse TNF-α ELISA kit (Genzyme) according to the manufacturer's instructions. IFN-γ or IL-1β was measured with an ELISA kit (BioSource International, Camarillo, CA).
Plasmid Construction and DNA Transfection.
Mouse SSI-1 cDNA was subcloned into the mammalian vector pEF-BOS and expressed under the control of an elongation factor gene promoter. An Arg-105 point mutation of SSI-1 yielding Gln was introduced by using a quick-change site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions and designated as RQ. Delta49 was generated by introducing two SalI sites at two predetermined points of the sequence and excising the SalI-SalI fragment. All constructs were cloned into the pEF-BOS vector and confirmed by DNA sequencing. The mutants were transfected into L929 cells by electroporation in combination with pSV2 neo, and neomycin-resistant clones were selected in DMEM supplemented with 500 μg/ml G418 (Nacalai Tesque, Kyoto) and 10% FCS.
Cytotoxicity Assays.
MEFs were derived from embryonic day 16 embryos by the fibroblast-migration method. MEFs were grown in DMEM supplemented with 10% FCS, 100 μg/ml streptomycin sulfate, 100 units/ml penicillin G potassium, and MEM nonessential amino acid solution. Cells were seeded 24 h before the assay in 96-well plates at a density of 2.5 × 104 cells per well in 5% FCS growth medium. Mouse recombinant TNF-α (Peprotech, London) or mouse recombinant IFN-γ (Peprotech) at the indicated concentration was applied to the cells in the presence of cycloheximide at 10 μg/ml (Sigma). After treatment for 24 h, cell viability was assessed with a Cell Counting Kit (Dojin Laboratories, Kumamoto, Japan) to count living cells by combining 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl )-2H-tetrazolium (WST-8) and 1-methoxyphenazine methosulfate (1-methoxy-PMS).
L929 cells were seeded 24 h before the assay in 96-well plates at a density of 2.5 × 104 cells per well in DMEM supplemented with 10% FCS, high glucose, pyridoxine hydrochloride, and 500 μg/ml G418, without phenol red, sodium pyruvate, or l-glutamine (GIBCO/BRL). Mouse recombinant TNF-α at the indicated concentrations was applied to the cells in the absence of cycloheximide. The rest of the treatment was similar to that described above.
Wortmannin (Nacalai Tesque) and U0126, AG490, and SB20580 (Calbiochem) were dissolved in dimethyl sulfoxide. Various inhibitors were preincubated 2 h before TNF-α stimulation in L929 cells.
Measurement of Caspase-8 and Caspase-3.
Cells were seeded 24 h before the assay in a 100 × 20 mm polystyrene nonpyrogenic dish [PRIMARIA tissue culture dish (Falcon)] at a density of 5 × 106 cells per dish. Mouse recombinant TNF-α at 1 ng/ml was applied to the cells in the absence of cycloheximide. Cells were harvested at the indicated time periods. Caspase-8 activity was measured with an ICE/caspase-8 Colorimetric Protease Assay kit (Medical & Biological Laboratories, Nagoya, Japan), and caspase-3 activity was measured with a CPP32/caspase-3 Colorimetric Protease Assay kit (Medical & Biological Laboratories).
Measurement of p38 MAP Kinase Activation.
Cells were seeded 24 h before the assay in a 100 × 20 mm polystyrene nonpyrogenic dish [PRIMARIA tissue culture dish (Falcon)] at a density of 5 × 106 cells per dish. Mouse recombinant TNF-α at 10 ng/ml or mouse recombinant IL-1β (Peprotech) at 10 ng/ml was applied to the cells in the absence of cycloheximide. Cells were harvested at the indicated time periods and lysed with lysis buffer (20 mM Tris, pH 7.5/150 mM NaCl/1 mM EDTA/1% Triton X-100/1 mM Na3VO4/1 μg/ml leupeptin/1 mM PMSF). After they had been kept on ice for 10 min, lysates were clarified by centrifugation. Half of each lysate was used for the expression level check of p38 MAP kinase, and the other half was used for the in vitro kinase assay of p38 MAP kinase. The activated form of p38 MAP kinase was immunoprecipitated with phospho-specific p38 MAP kinase (Thr 180/Tyr 182) antibody (NEB, Beverly, MA). The immune complex was washed two times with a lysis buffer and two more times with a kinase buffer (25 mM Tris, pH 7.5/10 mM MgCl2/2 mM DTT/0.1 mM Na3VO4). Kinase reactions were carried out in the kinase buffer supplemented with 100 μM ATP and 2 μg/ml glutathione S-transferase/activating transcription factor 2 (ATF2) fusion protein as a substrate at 30°C for 30 min. Reactions were stopped by adding a 5× SDS/PAGE sample buffer, and the samples were run on an SDS/PAGE gel, followed by Western blotting. Incorporation of the phosphorus residue into the glutathione S-transferase/ATF2 protein was detected with the phospho-specific ATF2 (Thr71) antibody (NEB).
Results
We previously reported that thymocytes and splenocytes lacking the SSI-1 gene (SSI-1 −/−) underwent accelerated apoptosis (7). To elucidate the causes of this apoptosis in SSI-1 −/− mice, various cytokines in the serum of SSI-1 −/− mice at 10 days after birth were examined by ELISA. The result of the experiment was that serum TNF-α of SSI-1 −/− mice was slightly increased in comparison with that of SSI-1 +/+ mice (Table 1). However, serum IFN-γ and serum IL-1β were not detected by ELISA (Table 1). The elevations of serum TNF-α may be caused either by dysregulation of cytokines to induce TNF-α or by dysregulation of TNF-α production.
Table 1.
Serum TNF-α protein levels, but not IFN-γ or IL-1β, of SSI-1 −/− mice increase in comparison with those of SSI-1 +/+ mice
| Mice | Cytokine conc., pg/ml
|
||
|---|---|---|---|
| TNF-α | IFN-γ | IL-1β | |
| SSI-1 −/− | 42.8 ± 10.2* | <1.0 | <7.0 |
| SSI-1 +/+ | 12.7 ± 4.3 | <1.0 | <7.0 |
| Con A-injected (positive control) | ND | 220.1 | 59.7 |
Serum TNF-α, IFN-γ, and IL-1β concentrations were determined in ten pairs of SSI-1 −/− mice and their littermate controls, SSI-1 +/+ mice, by ELISA. Mice were sacrificed at the age of 10 days. Cytokine protein levels are shown as mean ± SE. ND, not determined.
Serum TNF-α levels of SSI-1 −/− mice show significant difference at P < 0.1 as compared with those of SSI-1 +/+ mice.
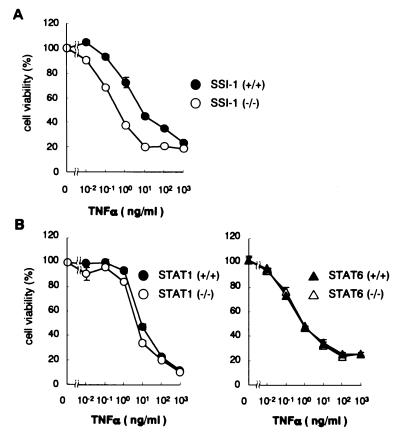
To assess whether TNF-α signaling dysregulates in SSI-1 −/− mice, we examined TNF-α-induced death signals in SSI-1 −/− MEFs. SSI-1 −/− or SSI-1 +/+ MEFs, which were derived from embryonic day 16 embryos by the fibroblast-migration method, were treated with TNF-α for 24 h in the presence of the protein synthesis inhibitor cycloheximide at 10 μg/ml. The result showed that SSI-1 +/+ MEFs were resistant with ≈75% viability at 1 ng/ml TNF-α, whereas the viability of SSI-1 −/− MEFs was dramatically reduced to ≈40% (Fig. 1A).
Figure 1.
SSI-1 −/− MEFs but not STAT1 −/− MEFs or STAT6 −/− MEFs become sensitive to TNF-α-induced cell death. (A) MEFs of SSI-1 −/− or SSI-1 +/+ were treated with the indicated TNF-α concentrations in the presence of cycloheximide (10 μg/ml) for 24 h. Cell viability was determined by WST-8 and 1-Methoxy PMS and is shown as a percentage of living cells and as mean ± SE. (B) STAT1 −/− or STAT6 −/− MEFs were treated with the indicated TNF-α concentrations and cycloheximide (10 μg/ml) for 24 h, and cell viability was determined as described above.
It was recently reported that TNF-α-induced cell death was defective in cells lacking STAT1 (8), which suggested that STATs were required unexpectedly for apoptosis caused by TNF-α stimulation. In addition, Guo et al. (9) reported that TNF-α induced tyrosine phosphorylation and activation of JAK1, JAK2, and Tyk2 in 3T3-L1 adipocytes. Furthermore, the effect of TNF-α on JAK2 phosphorylation was most evident, whereas TNF-α also strongly induced tyrosine phosphorylation of STAT1, STAT3, STAT5, and STAT6. Therefore, we investigated by using STAT1 −/− MEFs or STAT6 −/− MEFs to elucidate whether JAK-STAT signaling is related to TNF-α-induced cell death. The results of this examination showed that sensitivity to TNF-α did not change, even if the STAT1 or STAT6 gene was lacking (Fig. 1B). Taken together, these findings suggest that a lack of the SSI-1 gene results in supersensitivity to TNF-α, which is not caused by the STAT1 or STAT6 signaling.
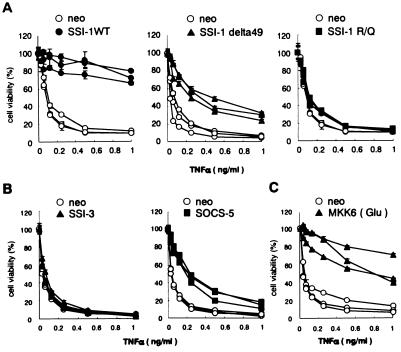
To determine whether SSI-1 suppress TNF-α-induced cell death, we established murine fibroblast cell lines (L929 cells) that constitutively expressed wild-type SSI-1 (L929/SSI-1) or mutant SSI-1 (L929/RQ or L929/delta49), whose cell lines are sensitive against TNF-α-induced cell death in the absence of cycloheximide. The SSI-1 RQ mutant is a point mutant at the Src homology 2 domain, whereas the delta49 mutant is a deletion mutant of the amino-terminal region (Δ2–52) of the Src homology 2 domain (10). These mutants cannot suppress the activation of the JAKs. When treated with TNF-α for 24 h, L929/SSI-1 showed ≈70% viability at 1 ng/ml TNF-α, but L929/neo and L929/RQ cells had only 10% viability at 1 ng/ml TNF-α (Fig. 2A). In contrast, L929/delta49 cells showed ≈60% viability at 0.25 ng/ml TNF-α, but ≈30% at 1 ng/ml TNF-α.
Figure 2.
SSI-1, but not SSI-1 mutant, SSI-3, or SOCS-5, inhibits TNF-α-induced cell death in L929 cells. (A) L929 cells were stably transfected by electroporation with the pEF-BOS-SSI-1 or SSI-1 mutant in combination with pSV2 neo. These cells were then treated with the indicated TNF-α concentrations in the absence of cycloheximide for 24 h. Cell viability was determined by WST-8 and 1-Methoxy PMS and is shown as percentage of living cells and as mean ± SE. This experiment used three independent clones. (B) L929 cells were stably transfected with pEF-BOS-SSI-3 or SOCS-5. (C) L929 cells were stably transfected with MKK6 (Glu)/pcDNA3.1.
Likewise, to determine whether other members of the SSI family have a similar effect on SSI-1 in suppressing TNF-α-induced cell death, cDNA encoding SSI-3 or SOCS-5 was stably transfected into L929 cells. However, SSI-3 did not provide any resistance at all to L929 cells against TNF-α-induced cell death (Fig. 2B). SOCS-5 suppressed TNF-α-induced cell death at a low dose of TNF-α, but had practically no effect at a high dose (Fig. 2B).
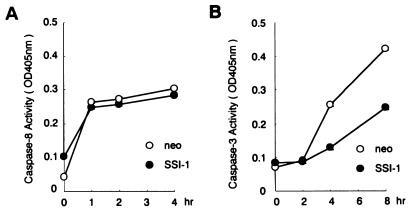
The activation of the mediator of apoptosis caused by TNF-α stimulation, caspase-8, or caspase-3 was examined to determine whether SSI-1 is associated with TNF-α-induced death signals. The activity of caspase-8 peaked at 1 h after TNF-α stimulation in L929/SSI-1 cells as well as in L929/neo cells (Fig. 3A) cells, and an increase in the activity showed no difference in effect between L929/SSI-1 cells and L929/neo cells. On the other hand, the activity of caspase-3 peaked at 8 h after TNF-α stimulation in L929/neo cells and increased about 5 times more than that without TNF-α stimulation, but the activity in L929/SSI-1 cells was lower than that in L929/neo cells and increased only 2.5 times at 8 h (Fig. 3B). These findings suggest that SSI-1 suppresses the activation of caspase-3 and apoptosis, but not of caspase-8.
Figure 3.
SSI-1 inhibits the activation of caspase-3 but not of caspase-8. (A) The activation of caspase-8 in L929/SSI-1 or L929/neo after TNF-α treatment. L929/SSI-1 or L929/neo was treated with TNF-α at 1 ng/ml for the indicated time periods. Caspase activity is seen when OD at 405 nm increases and is indicated as mean ± SE. (B) Activation of caspase-3.
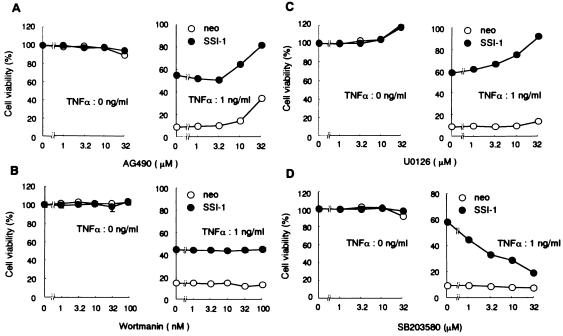
To elucidate mechanisms by which SSI-1 suppresses TNF-α-induced cell death, we coincubated various inhibitors with TNF-α in L929/SSI-1 or L929/neo cells, and observed changes of sensitivity to TNF-α-induced death in these cells. Because it was reported that the cytoplasmic domain of TNF receptor-1 directly interacts with JAK2 (9), we used AG490, which is a selective JAK2 inhibitor. However, L929/neo cells treated with AG490 did not become resistant to TNF-α-induced cell death to the same extent as did L929/SSI-1 cells (Fig. 4A). This finding suggests that JAK2 does not relate to TNF-α-induced cell death in L929 cells.
Figure 4.
SSI-1 relates to p38 MAP kinase, and not to JAK2, phosphatidylinositol 3-kinase–Akt, or MEK-ERK on TNF-α signaling. Sensitizing effect of various inhibitors on TNF-α-induced cell death in L929/SSI-1 or L929/neo. (A) L929/SSI-1 or L929/neo was pretreated for 2 h with AG490, an inhibitor of JAK2, at the indicated doses. Cells were then treated with TNF-α (0 or 1 ng/ml) for 24 h, at which time cell viability was determined by WST-8 and 1-Methoxy PMS and is shown as percentage of living cells and as mean ± SE. (B) Effects of wortmannin, an inhibitor of phosphatidylinositol 3-kinase. (C) Effects of U0126, an inhibitor of MEK1 and MEK2. (D) This histogram shows the effects of SB203580, an inhibitor of p38 MAP kinase.
Recently, it was reported that TNF-α activated phosphatidylinositol 3-kinase–Akt signaling, which is known to provide protection from apoptosis (11). We investigated the effect of wortmannin, which is a selective inhibitor of phosphatidylinositol 3-kinase (IC50 = 5 nM) to elucidate whether SSI-1 causes constitutive activation of phosphatidylinositol 3-kinase–Akt signaling in L929 cells. However, even 100 nM wortmannin did not have any effect (Fig. 4B), indicating that SSI-1 does not relate to survival signals on phosphatidylinositol 3-kinase–Akt signaling.
We also investigated extracellular signal-regulated kinase (ERK) and p38 MAP kinase signaling by using U0126, which is a specific inhibitor of MEK1 and MEK2, and SB203580, which is a highly specific inhibitor of p38 MAP kinase. When treated with U0126, there was no difference in effect between L929/SSI-1 and L929/neo cells (Fig. 4C). On the other hand, when treated with SB203580, resistance to TNF-α-induced cell death in L929/SSI-1 cells was eliminated dose-dependently (Fig. 4D Right). Moreover, this effect also was shown not to result from the nonspecific cytotoxicity of SB203580 (Fig. 4D Left). Therefore, it was conceivable that p38 MAP kinase might function as survival signals in L929 cells treated with TNF-α. In fact, as in the case of overexpression of SSI-1, overexpression of constitutively active MAP kinase kinase (MKK)6, MKK6 (Glu) (12), which selectively activates p38 MAP kinase, protected L929 cells from TNF-α-induced cell death (Fig. 2C).
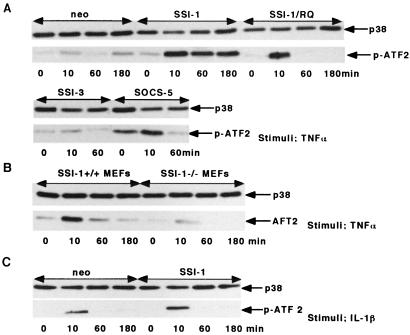
Next, the activity of p38 MAP kinase was examined by means of in vitro kinase assay. The results showed that strong phosphorylation of ATF2 was observed 10 min after TNF-α stimulation in L929/SSI-1 cells, and this phosphorylation was maintained until 3 h after stimulation (Fig. 5A). On the other hand, in L929/neo, RQ, SSI-3, or SOCS-5 cells, which did not provide resistance against TNF-α-induced cell death, the phosphorylation of ATF2 was observed 10 min after TNF-α-stimulation, but their phosphorylation could barely be confirmed at 1 h (Fig. 5A). Consistent with studies using cell lines forced to express SSI-1, strong phosphorylation of ATF2 was observed 10 and 60 min after TNF-α stimulation in SSI-1 +/+ MEFs, whereas the phosphorylation of ATF2 could barely be observed in SSI-1 −/− MEFs (Fig. 5B).
Figure 5.
SSI-1 sustains p38 MAP kinase signaling on TNF-α signals. (A) The phosphorylation of the recombinant activating transcription factor 2 (ATF2) substrate of p38 MAP kinase by in vitro kinase assay. L929/neo, SSI-1, SSI-3, SOCS-5, or SSI-1 R/Q mutant was treated with TNF-α (10 ng/ml) for the indicated time periods. Cells were harvested, and activated p38 MAP kinase was immunoprecipitated with anti-phosphorylated p38 MAP kinase. The activity of p38 MAP kinase was measured by means of immune complex kinase assays with ATF2 as the substrate (Lower). Control Western blots for levels of p38 MAP kinase protein are also shown (Upper). (B) In vitro kinase assay of p38 MAP kinase. SSI-1 +/+ MEFs or SSI-1 −/− MEFs were treated with TNF-α (10 ng/ml) for the indicated time periods. (C) In vitro kinase assay of p38 MAP kinase. L929/SSI-1 or L929/neo cells were treated with IL-1β (10 ng/ml) for the indicated time periods.
Because it is known that p38 MAP kinase is also activated by cytokines other than TNF-α, for example, IL-1β, the activity of p38 MAP kinase after IL-1β stimulation was examined to elucidate whether the activation of the kinase sustained by SSI-1 is specific for TNF-α stimulation. Unlike in the case of TNF-α stimulation, strong phosphorylation of ATF2 was observed 10 min after IL-1β stimulation in both L929/SSI-1 and L929/neo cells, but their phosphorylation could no longer be observed by 1 h after stimulation (Fig. 5C). These findings suggest that SSI-1 sustains the activity of p38 MAP kinase on TNF-α signaling, but not on IL-1β signaling.
Discussion
In this study, we investigated the causes of apoptosis of thymocytes and splenocytes in SSI-1 −/− mice. Initially, we assumed that TNF-α was a candidate for the death factors that cause apoptosis of thymocytes and splenocytes in SSI-1 −/− mice, because it is reported that TNF-α transgenic mice, which drive transgene expression in the T cell lineage, exhibit hypoplastic atrophy with a markedly reduced cortex and reduced numbers of thymocytes (13). Especially, SSI-1 −/− mice are similar to TNF-α transgenic mice in terms of atrophy of the thymus and reduction in the number of thymocytes. In fact, serum TNF-α of SSI-1 −/− mice increased in comparison with that of SSI-1 +/+ mice as expected. Interestingly, it is reported that TNF receptor-associated factor-2 (TRAF2) −/− mice are normal at birth, but exhibit growth retardation with aging and perinatal lethality (14). Also, atrophy of the thymus and spleen and depletion of B cell precursors are observed in TRAF2 −/− mice. Moreover, TRAF2 −/− thymocytes and other hematopoietic progenitors are highly sensitive to TNF-α-induced cell death, and serum TNF-α levels increase in TRAF2 −/− mice. Because of the reasons stated above, it was thought that the phenotype of SSI-1 −/− mice was similar to that of TRAF2 −/− mice, particularly in terms of atrophy of the thymus and spleen and an increase in serum TNF-α. Therefore, we predicted that cells in SSI-1 −/− mice would be highly sensitive to TNF-α-induced cell death as are cells in TRAF2 −/− mice. In fact, SSI-1 −/− MEFs exhibited sensitivity to TNF-α-induced cell death like TRAF2 −/− MEFs, as expected. Moreover, TNF-α-induced cell death was suppressed by transfection of SSI-1 in L929 cells. Taking these results together, we considered that SSI-1 was involved in regulating TNF-α signaling.
We next examined the activation of NFκB on TNF-α signaling in L929/SSI-1 cells. However, SSI-1 did not affect the degradation of IκBα, the activation of NFκB, or the nuclear localization of NFκB after TNF-α stimulation (data not shown). Consistent with this result, normal NFκB activation is clearly demonstrable in TRAF2 −/− MEFs (14), and the proportion of activated NFκB in TRAF2 −/− MEFs is equivalent to that in TRAF2 +/+ MEFs. On the other hand, TRAF2 −/− cells show significantly reduced c-Jun amino-terminal kinase (JNK) activity, and this defect in JNK activation is specific for TNF-α signaling. These results suggest that the MAP kinase signaling containing JNK might function as the survival signals on TNF-α signaling. However, other MAP kinase signaling, such as ERK and p38 MAP kinase in TRAF2 −/− cells, has not yet been investigated.
To test whether the activation of ERK, JNK, and p38 MAP kinase are sustained by SSI-1, the activation of these MAP kinases was examined in L929/SSI-1, L929/neo, and L929/RQ cells mutant not to suppress the activation of the JAKs, L929/SSI-3, or L929/SOCS-5 cells treated with TNF-α. The results showed that their phosphorylation of ERK and JNK was not sustained in L929/SSI-1 cells treated with TNF-α (data not shown), but sustained activation of p38 MAP kinase was observed in L929/SSI-1 cells treated with TNF-α. Moreover, SSI-1 −/− MEFs treated with TNF-α barely activated p38 MAP kinase. In addition, SB203580, which is a highly specific inhibitor of p38 MAP kinase, eliminated resistance to TNF-α-induced cell death in L929/SSI-1 cells. These findings suggest that SSI-1 suppresses TNF-α-induced cell death through sustained activation of p38 MAP kinase in fibroblasts.
Several previous reports have demonstrated that p38 MAP kinase might function as death signals when cells are treated with osmotic stress (15), heat shock stress (15), UV irradiation (16), or antineoplasic agents such as cisplatin (17), or when deprived of neurotrophic factors in primary neurons (18). However, it has been reported recently that the activation of p38 MAP kinase prevented apoptosis as follows. (i) Overexpression of constitutively active MKK6, which selectively activates p38 in cardiac myocytes, protects cells from apoptosis (19); (ii) the basal activity of p38 MAP kinase regulates the survival of cytokine-deprived eosinophils through inhibition of apoptosis (20); (iii) the activation of p38 MAP kinase protects HeLa cells from apoptosis with hypericin, a photosensitizing anticancer drug (21); (iv) expression of p38β attenuates the apoptotic effect of a p38 MAP kinase inhibitor and Fas ligand-induced cell death (22); and (v) early activation of p38 MAP kinase by TNF-α stimulation protects cells from TNF-α-induced cell death (23). These studies clearly show that p38 MAP kinase functions as survival signals to prevent apoptosis induced by death factors in fibroblasts, cardiac myocytes, and lymphocytes. Therefore, we consider that p38 MAP kinase functions as a survival signal in some organs and as a death signal in others. At least, overexpression of constitutively active MKK6 provided protection from TNF-α-induced cell death in L929 cells. Thus, this finding reveals that p38 MAP kinase functions as a cellular survival signal on TNF-α signaling in L929 fibroblast cell lines.
It is known that MKK4 in addition to MKK3 and MKK6 (24–25) can activate p38 MAP kinase in vitro. MKK4 −/− MEFs exhibit defects in p38 MAP kinase phosphorylation (26). This finding indicates that MKK4 is an important factor in the activation of p38 MAP kinase in vivo. Moreover, MKK4 −/− T cells fail to induce expression of the death suppressor Bcl-xL in response to antigen receptor activation (27), and MKK4 −/− liver cells undergo massive apoptosis (28). These findings suggest that MKK4 transduces cellular survival signals, and p38 MAP kinase may function as cellular survival signals in vivo. We previously reported that apoptosis of thymocytes and splenocytes in SSI-1 −/− mice was accelerated by the augmented expression of Bax (7). It seems possible that expression of Bax may indirectly increase because of failure to induce expression of antiapoptotic molecules, such as the Bcl-2 family to neutralize the death effector Bax of thymocytes and splenocytes in SSI-1 −/− mice.
The activation of heat shock protein 27, which serve as downstream targets of p38 MAP kinase, is known to protect cells from apoptosis (29, 30). Therefore, p38 MAP kinase may function as a survival signal by activating heat shock protein 27. On the other hand, it was reported that a dynamic balance among the MAP kinase signaling critically determined cellular fate in response to differentiating, proliferating, or apoptogenic stimuli (18). SSI-1 may therefore augment survival signals as a whole by means of activating p38 MAP kinase.
Recently, Marine et al. (31) reported that introducing an IFN-γ deficiency rescued SSI-1 −/− mice from a loss of lymphocytes and perinatal lethality, which suggests that SSI-1 is essential for inhibition of IFN-γ signaling in vivo. However, when we generated SSI-1 transgenic mice using the lck proximal promoter, which drives transgene expression in the T cell lineage (our unpublished data), and although we conducted forced expression experiments, we could not obtain evidence that SSI-1 possesses specificity for cytokine signals in lymphocytes of lck-SSI-1 transgenic mice. In fact, the mechanisms by which SSI-1 specifically inhibits IFN-γ signaling remain obscure. Also, IFN-γ is not directly able to transmit death signals, hence these effects may be the result of the TNF-α-induced death signal produced from IFN-γ. Furthermore, introducing STAT6 deficiency rescued SSI-1 −/− mice from a loss of splenocytes and perinatal lethality (T.N., unpublished data). Therefore, the cause of several phenotypes of SSI-1 −/− mice may be the result of complex effects of several cytokine signals, such as IFN-γ, IL-4, and TNF-α.
Acknowledgments
We thank Dr. E. Nishida (Department of Genetics and Molecular Biology, Institute for Virus Research, Kyoto University) for providing the MKK6 (Glu)/pcDNA3.1, and Dr. S. Akira (Department of Host Defense, Research Institute for Microbial Diseases, Osaka University) for reading and editing the manuscript. We thank K. Satoh for her technical assistance and R. Harada for her secretarial assistance. This study was supported by a Grant-in-Aid from the Ministry of Education, Science, and Culture, Japan.
Abbreviations
- STAT
signal transducers and activators of transcription
- SSI-1
STAT-induced STAT inhibitor-1
- SOCS-1
suppressor of cytokine signaling-1
- TNF-α
tumor necrosis factor-α
- JAK
Janus kinase
- MEFs
murine embryonic fibroblasts
- RQ
an Arg-105 point mutation of SSI-1 yielding Gln
- TRAF
TNF receptor-associated factor
- MAP kinase
mitogen-activated protein kinase
- MKK
MAP kinase kinase
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun amino-terminal kinase
- ATF2
activating transcription factor 2
- WST-8
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
- 1-methoxy-PMS
1-methoxyphenazine methosulfate
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090084797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090084797
References
- 1.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Nature (London) 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 2.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, et al. Nature (London) 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 3.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. Nature (London) 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 4.Naka T, Fujimoto M, Kishimoto T. Trends Biochem Sci. 1999;24:394–398. doi: 10.1016/s0968-0004(99)01454-1. [DOI] [PubMed] [Google Scholar]
- 5.Minamoto S, Ikegame K, Ueno K, Narazaki M, Naka T, Yamamoto H, Matsumoto T, Saito H, Hosoe S, Kishimoto T. Biochem Biophys Res Commun. 1997;237:79–83. doi: 10.1006/bbrc.1997.7080. [DOI] [PubMed] [Google Scholar]
- 6.Hilton D J, Richardson R T, Alexander W S, Viney E M, Willson T A, Sprigg N S, Starr S R, Nicholson S E, Metcalf D, Nicola N A. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto K. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Commane M, Flickinger T W, Horvath C M, Stark G R. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 9.Guo D, Dunbar J D, Yang C H, Pfeffer L M, Donner D B. J Immunol. 1998;160:2742–2750. [PubMed] [Google Scholar]
- 10.Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto K. Proc Natl Acad Sci USA. 1998;95:13130–13134. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. Nature (London) 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 12.Zechner D, Thuerauf D J, Hanford D S, McDonough P M, Glembotski C C. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probert L, Keffer J, Corbella P, Cazlaris H, Patsavoudi E, Stephens S, Kaslaris E, Kioussis D, Kollias G. J Immunol. 1993;151:1894–1906. [PubMed] [Google Scholar]
- 14.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 15.Nagata Y, Todokoro K. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- 16.Shimizu H, Banno Y, Sumi N, Naganawa T, Kitajima Y, Nozawa Y. J Invest Dermatol. 1999;112:769–774. doi: 10.1046/j.1523-1747.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Perez I, Perona R. FEBS Lett. 1999;453:151–158. doi: 10.1016/s0014-5793(99)00690-0. [DOI] [PubMed] [Google Scholar]
- 18.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 19.Zechner D, Craig R, Hanford D S, McDonough P M, Sabbadini R A, Glembotski C C. J Biol Chem. 1998;273:8232–8239. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]
- 20.Kankaanranta H, De Souza P M, Barnes P J, Salmon M, Giembycz M A, Lindsay M A. J Pharmacol Exp Ther. 1999;290:621–628. [PubMed] [Google Scholar]
- 21.Assefa Z, Vantieghem A, Declercq W, Vandenabeele P, Vandenheede J R, Merlevede W, de Witte P, Agostinis P. J Biol Chem. 1999;274:8788–8796. doi: 10.1074/jbc.274.13.8788. [DOI] [PubMed] [Google Scholar]
- 22.Emoto S, Xiang J, Huang S, Lin A. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 23.Roulston A, Reinhard C, Amiri P, Williams L T. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 24.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, et al. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. J Biol Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- 26.Ganiatsas S, Kwee L, Fujiwara Y, Perkins A, Ikeda T, Labow M A, Zon L I. Proc Natl Acad Sci USA. 1998;95:6881–6886. doi: 10.1073/pnas.95.12.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishina H, Radvanyi L, Raju K, Sasaki T, Kozieradzki I, Penninger J M. J Immunol. 1998;161:3416–3420. [PubMed] [Google Scholar]
- 28.Nishina H, Vaz C, Billia P, Nghiem M, Sasaki T, De la Pompa J L, Furlonger K, Paige C, Hui C, Fischer K D, et al. Development (Cambridge, UK) 1999;126:505–516. doi: 10.1242/dev.126.3.505. [DOI] [PubMed] [Google Scholar]
- 29.Samali A, Cotter T G. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 30.Mehlen P, Kretz-Remy C, Preville X, Arrigo A P. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 31.Marine J-C, Topham D J, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle J N. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]