Glycemic control, a worthwhile goal for people with diabetes, is limited by the barrier of iatrogenic hypoglycemia (1). Iatrogenic hypoglycemia 1) causes recurrent morbidity in most people with type 1 diabetes and many with advanced type 2 diabetes and is sometimes fatal, 2) compromises physiological and behavioral defenses against subsequent falling plasma glucose concentrations and thus causes a vicious cycle of recurrent hypoglycemia, and 3) precludes maintenance of euglycemia over a lifetime of diabetes and therefore full realization of the vascular benefits of glycemic control. The premise of this “Perspective in Diabetes” is that insight into the pathophysiology of glucose counterregulation in diabetes leads to understanding of the frequency and impact of, risk factors for, and prevention of iatrogenic hypoglycemia.
Partial glycemic control reduces, but does not eliminate, the development of microvascular complications of diabetes (retinopathy, nephropathy, and neuropathy) in type 1 (2) and type 2 (3,4) diabetes. Extrapolation of the Diabetes Control and Complications Trial (DCCT) retinopathy data suggests that long-term maintenance of euglycemia might eliminate those complications (5). Follow-up of the DCCT patients seemingly indicates that a period of earlier partial glycemic control reduces the development of macrovascular complications in type 1 diabetes (6). Aside from the metformin subset of the U.K. Prospective Diabetes Study (4), randomized controlled trials of intensive glycemic therapy have not documented a cardiovascular mortality benefit in type 2 diabetes (3,7,8). However, those trials do not exclude a cardiovascular benefit if glycemic control, or even partial glycemic control, could be maintained over a longer period of time. In any event, given its documented microvascular benefit, maintenance of euglycemia over a lifetime of diabetes would be in the best interest of people with diabetes if that could be accomplished safely.
Unfortunately, maintenance of euglycemia over a lifetime of diabetes cannot be accomplished safely with currently available treatment methods because of the barrier of hypoglycemia (1). Even if they are effective initially, medications that should not, and probably do not, cause hypoglycemia (a biguanide [metformin], thiazolidinediones, α-glucosidase inhibitors, glucagon-like peptide-1 receptor agonists, and dipeptidyl dipeptidase-IV inhibitors) seldom maintain euglycemia in the long-term in type 2 diabetes. The same is true of insulin secretagogues (sulfonylureas or glinides), which can cause hypoglycemia in type 2 diabetes. Therapy with insulin causes hypoglycemia progressively more frequently over time in type 2 diabetes and throughout the course of established type 1 diabetes (1,9). Elimination of iatrogenic hypoglycemia from the lives of people with diabetes will require new treatment methods that provide plasma glucose–regulated insulin replacement or secretion.
Frequency of hypoglycemia.
Hypoglycemia is a fact of life for most people with type 1 diabetes (1). The average patient has untold numbers of episodes of asymptomatic hypoglycemia and suffers two episodes of symptomatic hypoglycemia per week (thousands of such episodes over a lifetime of diabetes). He or she suffers one or more episodes of severe, temporarily disabling hypoglycemia, often with seizure or coma, per year. There is no evidence that this problem has abated over the decade and a half since it was highlighted by the report of the DCCT (2) in 1993. For example, in 2007 the U.K. Hypoglycemia Study Group (9) reported an incidence of severe hypoglycemia of 110 episodes per 100 patient-years (nearly twice that in the DCCT) in patients with type 1 diabetes, who were necessarily treated with insulin, for <5 years and an incidence of 320 episodes per 100 patient-years in those with type 1 diabetes for >15 years.
Overall, hypoglycemia is less frequent in type 2 diabetes (1). However, for pathophysiological reasons that will be discussed shortly, hypoglycemia becomes progressively more frequent and limiting to glycemic control later in the course of type 2 diabetes (1). For example, when the U.K. Hypoglycemia Study Group (9) contrasted patients with type 2 diabetes treated with insulin for <2 years with those treated with insulin for >5 years, they found severe hypoglycemia prevalences of 7 and 25% and incidences of 10 and 70 episodes per 100 patient-years, respectively. The corresponding values for mild hypoglycemia were 51 and 64% and 410 and ∼1,020 episodes per 100 patient-years, respectively. Thus, while the risk of hypoglycemia is relatively low in the first few years of insulin treatment of type 2 diabetes (at least with current glycemic goals that are above the euglycemic range), the risk increases substantially (approaching that in type 1 diabetes) in advanced type 2 diabetes.
Although they represent only a small fraction of the total hypoglycemia experience, estimates of the frequency of severe hypoglycemia, particularly if determined in prospective, population-based studies, are the most reliable because they are dramatic events that are more likely to be reported (by the patient or an associate) (1). The prospective, population-based data of Donnelly et al. (10) indicate that the overall incidence of hypoglycemia in insulin-treated type 2 diabetes is approximately one-third of that in type 1 diabetes. The incidence of any and of severe hypoglycemia was ∼4,300 and 115 episodes per 100 patient-years, respectively, in type 1 diabetes and ∼1,600 and 35 episodes per 100 patient-years, respectively, in insulin-treated type 2 diabetes. In addition, in population-based studies the incidence of severe hypoglycemia requiring emergency treatment in insulin-treated type 2 diabetes was ∼40% (11) and ∼100% (12) of that in type 1 diabetes. Since the prevalence of type 2 diabetes is ∼20-fold greater than that of type 1 diabetes, and most people with type 2 diabetes ultimately require treatment with insulin, these data suggest that most episodes of iatrogenic hypoglycemia, including severe hypoglycemia, occur in people with type 2 diabetes.
Impact of hypoglycemia.
Iatrogenic hypoglycemia causes recurrent physical and psychological morbidity and some mortality, impairs defenses against subsequent hypoglycemia, and precludes maintenance of euglycemia over a lifetime of diabetes (1). Hypoglycemia causes brain fuel deprivation that, if unchecked, results in functional brain failure that is typically corrected after the plasma glucose concentration is raised (13). Rarely, it causes sudden, presumably cardiac arrhythmic death or, if it is profound and prolonged, brain death (13). To the extent that there is a macrovascular benefit of glycemic control (6), the barrier of hypoglycemia also contributes to cardiovascular morbidity and mortality.
The physical morbidity of an episode of hypoglycemia ranges from unpleasant symptoms to seizure and coma (1). Hypoglycemia can impair judgment, behavior, and performance of physical tasks. Permanent neurological damage is rare. While there is concern that recurrent hypoglycemia might cause chronic cognitive impairment, long-term follow-up of the DCCT patients is largely reassuring in that regard (14). Nonetheless, the possibility that it might do so in young children or the elderly remains (1). The psychological morbidity includes fear of hypoglycemia, which can be a barrier to glycemic control (1).
Three early reports indicated that 2–4% of people with diabetes die from hypoglycemia (1). More recent reports indicated that 6% (14), 7% (15), and 10% (16) of deaths of people with type 1 diabetes were the result of hypoglycemia. Up to 10% of episodes of severe sulfonylurea-induced hypoglycemia in type 2 diabetes may be fatal (17).
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, 10,251 patients with type 2 diabetes at high cardiovascular risk (but with no history of frequent or recent serious hypoglycemic events) were randomized to either intensive glycemic therapy with an A1C goal of <6.0% or to standard glycemic therapy (7). After a median follow-up of 3.4 years, with stable median A1C levels of 6.4 and 7.5%, respectively, intensive glycemic therapy was discontinued because 5.0% of the patients in the intensive therapy group, compared with 4.0% of those in the standard therapy group, had died. The cause of excess mortality during intensive glycemic therapy in the ACCORD study is not known and likely will not be known with certainty (7). It could have been chance; excess mortality during intensive glycemic therapy was not observed in the ADVANCE trial, although there was less glycemic separation between the groups (median A1C levels of 6.4 and 7.0%) (8). It could have been the result of a nonglycemic effect of the intensive therapy regimen (e.g., an adverse effect of one or more of the drugs, weight gain, or something else), although none was apparent. Nonetheless, the most plausible cause of excess mortality during intensive therapy in the ACCORD study is iatrogenic hypoglycemia: 1) Median glycemia (A1C) was intentionally and demonstrably lower in the intensive glycemic therapy group. 2) Lower A1C levels are known to be associated with a higher frequency of hypoglycemia in type 2 diabetes (7,8,18). Indeed, the prevalence of severe hypoglycemia was more than threefold higher in the intensive therapy group in the ACCORD study (7). 3) Hypoglycemia can be fatal in type 2 diabetes (13,17). That includes sudden, presumably cardiac arrhythmic death (13). 4) More patients died in the intensive glycemic therapy group (7).
Physiology of glucose counterregulation
Hypoglycemia and the brain.
Glucose is an obligate oxidative fuel for the brain under physiological conditions (1). The brain accounts for >50% of whole-body glucose utilization. The brain can oxidize alternative fuels, such as ketones, if their circulating levels rise high enough to enter the brain in quantity, but that is seldom the case. Because it cannot synthesize glucose, utilize physiological levels of circulating nonglucose fuels effectively, or store more than a few minutes supply of glucose as glycogen, the brain requires a virtually continuous supply of glucose from circulation. Since facilitated blood-to-brain glucose transport is a direct function of the arterial plasma glucose concentration, that supply requires maintenance of plasma glucose concentration. At some level of hypoglycemia (perhaps ∼50–55 mg/dl [2.8–3.1 mmol/l] since symptoms normally occur at that level [19–21]), blood-to-brain glucose transport becomes limiting to brain glucose metabolism and, therefore, function.
Clinical manifestations of hypoglycemia.
The symptoms and signs of hypoglycemia are not specific (1,19,20). Thus, clinical hypoglycemia is most convincingly documented by Whipple's triad: symptoms, signs, or both consistent with hypoglycemia, a low measured plasma glucose concentration, and resolution of those symptoms and signs after the plasma glucose concentration is raised.
Symptoms of hypoglycemia (19) are categorized as neuroglycopenic (those that are the direct result of brain glucose deprivation per se) and neurogenic (or autonomic), those that are largely the result of the perception of physiological changes caused by the sympathoadrenal (largely the sympathetic neural) (20) discharge triggered by hypoglycemia. Neuroglycopenic manifestations include cognitive impairments, behavioral changes and psychomotor abnormalities, and, at lower plasma glucose concentrations, seizure and coma. Adrenergic neurogenic symptoms include palpitations, tremor, and anxiety/arousal (19). Cholinergic neurogenic symptoms include sweating, hunger, and paresthesias (19). Central, as well as peripheral, mechanisms may be involved in the generation of some symptoms such as hunger (1). Awareness of hypoglycemia is largely the result of the perception of neurogenic symptoms (19). Pallor and diaphoresis (the result of adrenergic cutaneous vasoconstriction and cholinergic stimulation of sweat glands, respectively) are common signs of hypoglycemia (1). Neuroglycopenic manifestations are often observable.
Maintenance of systemic glucose balance.
Falling plasma glucose concentrations elicit a sequence of responses (Table 1) that normally prevent or rapidly correct hypoglycemia (Fig. 1) (1,21). Because obligatory glucose utilization by the brain is fixed and exogenous glucose delivery from food is intermittent, systemic glucose balance is maintained and hypoglycemia (as well as hyperglycemia) is prevented by dynamic regulation of endogenous glucose production by the liver (and the kidneys) and of glucose utilization by nonneural tissues such as muscle.
TABLE 1.
Physiologic responses to decreasing plasma glucose concentrations
| Response | Glycemic threshold (mg/dl [mmol/l])* | Physiologic effects | Role in prevention or correction of hypoglycemia (glucose counterregulation) |
|---|---|---|---|
| ↓ Insulin | 80–85 [4.4–4.7] | ↑Ra (↓Rd)† | Primary glucose regulatory factor, first defense against hypoglycemia |
| ↑ Glucagon | 65–70 [3.6–3.9] | ↑Ra | Primary glucose counterregulatory factor, second defense against hypoglycemia |
| ↑ Epinephrine | 65–70 [3.6–3.9] | ↑Ra, ↓Rd | Involved, critical when glucagon is deficient, third defense against hypoglycemia |
| ↑ Cortisol and growth hormone | 65–70 [3.6–3.9] | ↑Ra, ↓Rd | Involved, not critical |
| Symptoms | 50–55 [2.8–3.1] | ↑Exogenous glucose | Prompt behavioral defense (food ingestion) |
| ↓ Cognition | <50 [<2.8] | — | (Compromises behavioral defense) |
Arterialized venous, not venous, plasma glucose concentrations.
Ra, rate of glucose appearance, glucose production by the liver and kidneys; Rd, rate of glucose disappearance, glucose utilization by insulin-sensitive tissues such as skeletal muscle (no direct effect on central nervous system glucose utilization). This table was prepared initially for Cryer PE: Glucose homeostasis and hypoglycemia. In Williams Textbook of Endocrinology, 11th Edition. Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, Eds. Saunders, Philadelphia, 2008, p. 1503–1533.
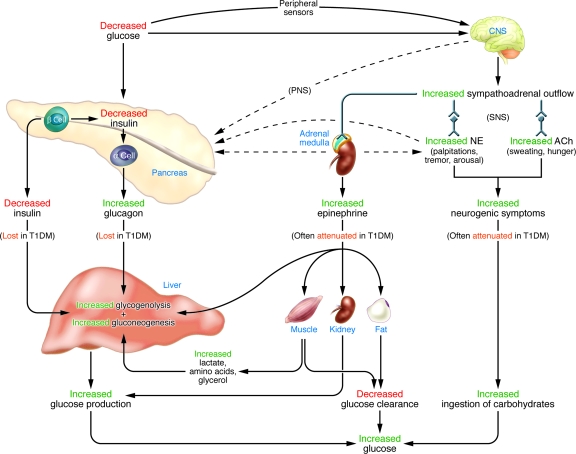
FIG. 1.
Physiological and behavioral defenses against hypoglycemia in humans. ACh, acetylcholine; NE, norepinephrine; PNS, parasympathetic nervous system; SNS, sympathetic nervous system. From Cryer PE: Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest 116:14701–473, 2006. © 2006, American Society for Clinical Investigation. All rights reserved.
The physiological defenses against declining plasma glucose concentrations include 1) a decrease in insulin secretion, 2) an increase in glucagon secretion, and, absent the latter, an increase in epinephrine secretion (Table 1, Fig. 1) (1,21). The behavioral defense is the ingestion of carbohydrates prompted by awareness of hypoglycemia (Table 1) (Fig. 1) (1,21). The first physiological defense against hypoglycemia is a decrease in pancreatic islet β-cell insulin secretion. That occurs as plasma glucose concentrations decline within the physiological range (Table 1) and increases hepatic (and renal) glucose production with virtual cessation of glucose utilization by insulin-sensitive nonneural tissues (Fig. 1). The second physiological defense is an increase in pancreatic islet α-cell glucagon secretion. That occurs as plasma glucose concentrations fall just below the physiological range (Table 1) and increases hepatic glucose production (largely by stimulating glycogenolysis) (Fig. 1). Increased glucagon secretion is signaled by a decrease in intraislet insulin, perhaps among other β-cell secretory products, in the setting of low plasma glucose concentrations (1,22). The third physiological defense, which becomes critical when glucagon secretion is deficient, is an increase in adrenomedullary epinephrine secretion. That, too, occurs as plasma glucose concentrations fall just below the physiological range (Table 1) and raises plasma glucose concentrations through an array of mechanisms (Fig. 1). Those include direct stimulation of hepatic (and renal) glucose production, limitation of glucose clearance by insulin-sensitive tissues, mobilization of gluconeogenic substrates such as lactate and amino acids from muscle and glycerol from fat, and limitation of insulin secretion (1,22). Unlike insulin and glucagon secretion, which are regulated primarily by changes in glucose concentrations within the pancreatic islets and only secondarily by central nervous system–mediated autonomic inputs, sympathoadrenal activity, including epinephrine secretion, is regulated within the central nervous system (1,21).
If these physiological defenses fail to abort developing hypoglycemia, lower plasma glucose concentrations cause a more intense sympathoadrenal response that causes neurogenic symptoms (Fig. 1) (1,21). Those, in turn, lead to awareness of hypoglycemia that prompts the behavioral defense: the ingestion of carbohydrates (1,21). All of these defenses against developing hypoglycemia, not just insulin secretion, are typically compromised in people with type 1 diabetes and advanced (i.e., absolutely endogenous insulin deficient) type 2 diabetes.
Pathophysiology of glucose counterregulation in diabetes
Insulin excess and compromised defenses.
While insulin excess of sufficient magnitude can cause hypoglycemia, iatrogenic hypoglycemia in people with diabetes is typically the result of the interplay of relative or absolute therapeutic hyperinsulinemia and compromised physiological and behavioral defenses against falling plasma glucose concentrations (1,23,24). Because of their pharmacokinetic imperfections, insulin secretagogues or insulin result in episodes of hyperinsulinemia and falling plasma glucose concentrations. It is the integrity of the defenses against falling plasma glucose concentrations that determines whether those episodes result in clinical hypoglycemia.
Defective glucose counterregulation and hypoglycemia unawareness.
In fully developed (i.e., C-peptide negative) type 1 diabetes, circulating insulin concentrations do not decrease as plasma glucose concentrations decline in response to therapeutic (exogenous) hyperinsulinemia (1,23). That is the result of β-cell failure, which also causes loss of the α-cell glucagon secretory response (22). Thus, both the first and the second physiological defenses against hypoglycemia are lost. In that setting, an attenuated increase in adrenomedullary epinephrine secretion, the third physiological defense, causes the clinical syndrome of defective glucose counterregulation (1,23), which is associated with a 25-fold (25) or greater (26) increased risk of severe iatrogenic hypoglycemia. Furthermore, the attenuated epinephrine response is a marker for an attenuated sympathoadrenal, including sympathetic neural, response, which is largely responsible for the development of the clinical syndrome of hypoglycemia unawareness (or impaired awareness of hypoglycemia since there is a spectrum ranging from normal to reduced to absent awareness) (1,23,27). Hypoglycemia unawareness is associated with a sixfold increased risk of severe iatrogenic hypoglycemia (27).
Albeit with different time courses, the pathophysiology of glucose counterregulation is the same in type 1 diabetes and advanced (i.e., absolutely endogenous insulin deficient) type 2 diabetes (1,23,24). Because absolute β-cell failure (which causes loss of both the insulin and the glucagon responses) occurs rapidly in type 1 diabetes but slowly in type 2 diabetes, the syndromes of defective glucose counterregulation and hypoglycemia unawareness develop early in type 1 diabetes but later in type 2 diabetes. That explains why iatrogenic hypoglycemia becomes progressively more frequent and limiting to glycemic control as patients approach the insulin-deficient end of the spectrum of type 2 diabetes, as discussed earlier.
Hypoglycemia-associated autonomic failure in diabetes.
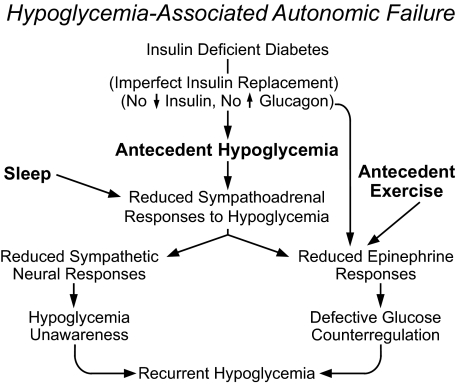
The concept of hypoglycemia-associated autonomic failure (HAAF) in diabetes (Fig. 2) posits that recent antecedent hypoglycemia (1,8,9), as well as prior exercise (28) or sleep (29), causes both defective glucose counterregulation (by reducing increments in epinephrine in the setting of absent decrements in insulin and absent increments in glucagon during subsequent hypoglycemia) and hypoglycemia unawareness (by reducing sympathoadrenal and the resulting neurogenic symptom responses during subsequent hypoglycemia) and, therefore, a vicious cycle of recurrent iatrogenic hypoglycemia. Perhaps the most compelling support for the concept of HAAF is the finding, in three independent laboratories (30–32), that as little as 2–3 weeks of scrupulous avoidance of hypoglycemia reverses hypoglycemia unawareness and improves the attenuated epinephrine component of defective glucose counterregulation in most affected patients. While HAAF is largely a functional, dynamic disorder that can be induced and reversed, there may also be a structural (neuropathic) contribution to the attenuated sympathoadrenal response (1).
FIG. 2.
Schematic diagram of HAAF in diabetes. Modified from Cryer PE: Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 350:22722–279, 2004. © 2004 Massachusetts Medical Society. All rights reserved.
Diverse causes of HAAF in diabetes.
There are currently three recognized causes of HAAF: recent antecedent hypoglycemia (23,24), prior exercise (28), and sleep (29). Each of these inciting events causes an attenuated sympathoadrenal response to subsequent falling plasma glucose concentrations, the key feature of HAAF (i.e., sympathoadrenal failure associated with the development of iatrogenic hypoglycemia in people with diabetes). Among these recognized causes, hypoglycemia-related HAAF (1,23,24) led to the concept. Exercise-related HAAF is exemplified by late postexercise hypoglycemia that typically occurs 6–15 h after strenuous exercise and is often nocturnal (1,33,34). It is attributable to an attenuated sympathoadrenal response to falling plasma glucose concentrations (35). Sleep-related HAAF (1,29) is the result of a further attenuated sympathoadrenal response to falling plasma glucose concentrations during sleep. Sleeping patients have both reduced epinephrine responses and reduced arousal from sleep.
Mechanisms of HAAF in diabetes.
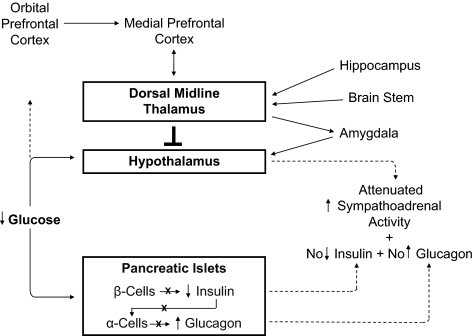
Loss of decrements in insulin secretion and of increments in glucagon secretion as plasma glucose concentrations fall in response to therapeutic hyperinsulinemia, prerequisites for HAAF in type 1 diabetes and advanced type 2 diabetes (1,23,24), are the result of β-cell failure (Fig. 3) (1,22). Since low glucose concentrations cause decreased insulin and increased glucagon secretion from the transplanted human and denervated dog pancreas (as well as from the perfused pancreas and perifused islets), innervation is not required. Therefore, loss of the glucagon response lies at the level of the diseased islets.
FIG. 3.
Pancreatic islet and hypothalamic and cerebral network mechanisms of HAAF in diabetes.
In the setting of absent insulin and glucagon responses, an attenuated sympathoadrenal response to falling plasma glucose concentrations caused by therapeutic hyperinsulinemia causes both defective glucose counterregulation and hypoglycemia unawareness (1,23,24). The mechanism of the attenuated sympathoadrenal response is not known, but it must lie at the level of the brain (or the afferent or efferent components of the sympathoadrenal system) (Fig. 3). Discussion of the various theories of the pathogenesis of this key feature of HAAF (1,36–39) (the systemic mediator, brain fuel transport, and brain metabolism hypotheses) is beyond the scope of this perspective. While much of the neuroscience research into this issue has focused on the hypothalamus (37), recent translational research has raised the possibility that a complex cerebral network normally regulates the hypothalamic (and thus the sympathoadrenal) response to falling plasma glucose concentrations (38,39) and that an inhibitory signal mediated through the thalamus might be involved in the pathogenesis of HAAF (Fig. 3) (39). Clearly, much remains to be learned about the mechanism of HAAF in diabetes.
Risk factors for hypoglycemia in diabetes
Absolute or relative insulin excess.
The conventional risk factors for hypoglycemia in diabetes (Table 2) are based on the premise that absolute or relative therapeutic insulin excess is the sole determinant of risk (1,40). Absolute therapeutic insulin excess occurs when insulin secretagogue or insulin doses are excessive, ill-timed, or of the wrong type or when insulin clearance is decreased, as in renal failure. Relative therapeutic insulin excess occurs when exogenous glucose delivery is decreased (as following missed or low carbohydrate meals and during the overnight fast), when glucose utilization is increased (as during and shortly after exercise), when endogenous glucose production is decreased (as following alcohol ingestion), and when sensitivity to insulin is increased (as in the middle of the night or following weight loss, improved fitness or improved glycemic control). People with diabetes and their caregivers must consider each of these when hypoglycemia is a problem. However, they explain only a minority of episodes of iatrogenic hypoglycemia.
TABLE 2.
Risk factors for hypoglycemia in diabetes
| Absolute or relative therapeutic insulin excess |
|---|
| 1. Insulin or insulin secretagogue doses are excessive, ill-timed, or of the wrong type |
| 2. Exogenous glucose delivery is decreased (e.g., following missed meals and during the overnight fast) |
| 3. Glucose utilization is increased (e.g., during and shortly after exercise) |
| 4. Endogenous glucose production is decreased (e.g., following alcohol ingestion) |
| 5. Sensitivity to insulin is increased (e.g., in the middle of the night and following weight loss, improved fitness or improved glycemic control) |
| 6. Insulin clearance is decreased (e.g., with renal failure) |
| HAAF |
|---|
| 1. Absolute endogenous insulin deficiency |
| 2. A history of severe hypoglycemia, hypoglycemia unawareness, or both as well as recent antecedent hypoglycemia, prior exercise, and sleep |
| 3. Aggressive glycemic therapy per se (lower A1C levels, lower glycemic goals) |
Compromised defenses against hypoglycemia.
The risk factors indicative of HAAF (Table 2) include the degree of endogenous insulin deficiency (1,2,41); a history of severe hypoglycemia, hypoglycemia unawareness, or both as well as recent antecedent hypoglycemia, prior exercise, or sleep (1,2,42); and lower mean glycemia (1,7,8,18,41,42). The degree of endogenous insulin deficiency determines the extent to which insulin levels will not decrease and glucagon levels will not increase as plasma glucose concentrations fall in response to therapeutic hyperinsulinemia. A history of severe hypoglycemia indicates (and that of hypoglycemia unawareness implies) recent antecedent hypoglycemia, which, like prior exercise and sleep, causes attenuated sympathoadrenal and symptomatic responses to subsequent hypoglycemia, the key feature of HAAF. Studies with a control group treated to higher mean glycemia consistently document higher rates of hypoglycemia in the group treated to lower mean glycemia (2,7,8,18). The latter does not mean that one cannot both improve glycemic control and reduce the risk of hypoglycemia in individual patients (40).
Definition and classification of hypoglycemia.
The American Diabetes Association Workgroup on Hypoglycemia (43) defined hypoglycemia in diabetes as “all episodes of abnormally low plasma glucose concentration that expose the individual to potential harm.” The American Diabetes Association Workgroup recommended that people with drug-treated diabetes (implicitly those treated with an insulin secretagogue or insulin) become concerned about the possibility of developing hypoglycemia at a plasma glucose concentration of ≤70 mg/dl (3.9 mmol/l) (43). Within the error of self-blood glucose monitoring (or continuous glucose sensing), that conservative alert level approximates the lower limit of the physiological postabsorptive plasma glucose concentration range and the glycemic thresholds for activation of physiological glucose counterregulatory systems and is low enough to reduce glycemic defenses against subsequent hypoglycemia in nondiabetic individuals. The recommended alert value does not mean that people with diabetes should always treat for hypoglycemia at an estimated plasma glucose concentration of ≤70 mg/dl. Rather, it indicates that they should consider actions ranging from repeating the measurement in the near term through behavioral changes such as avoiding exercise or driving without treatment to carbohydrate ingestion and subsequent regimen adjustments. The intent of the use of the alert value is to prevent clinical hypoglycemia and not to estimate the frequency of clinically important hypoglycemia. The Workgroup also suggested the following classification of hypoglycemia in diabetes: 1) severe hypoglycemia, 2) documented symptomatic hypoglycemia, 3) asymptomatic hypoglycemia, 4) probable symptomatic hypoglycemia, and 5) relative hypoglycemia. Those require 1) an episode requiring the assistance of another person, 2) symptoms and a plasma glucose concentration ≤70 mg/dl, 3) a plasma glucose concentration ≤70 mg/dl without symptoms, 4) symptoms attributed to hypoglycemia without a plasma glucose measurement, and 5) symptoms attributed to hypoglycemia with a plasma glucose concentration >70 mg/dl but falling toward that level, respectively.
Hypoglycemia risk factor reduction.
It is, of course, preferable to prevent rather than treat iatrogenic hypoglycemia. The prevention of hypoglycemia requires the practice of hypoglycemia risk factor reduction (1,40). That involves 1) acknowledging and addressing the problem, 2) applying the principles of intensive glycemic therapy (diabetes self-management based on patient education and empowerment, frequent self blood glucose monitoring [and in some instances continuous glucose sensing], appropriate and flexible insulin [and other drug] regimens including use of insulin analogues, individualized glycemic goals, and ongoing professional guidance and support), 3) considering the conventional risk factors and adjusting the regimen appropriately, and 4) considering the risk factors indicative of HAAF in diabetes. With respect to the latter, a history of severe hypoglycemia should prompt consideration of a substantial change in the treatment regimen and a history of hypoglycemia unawareness should prompt consideration of a 2- to 3-week period of scrupulous avoidance of hypoglycemia with the anticipation that awareness of hypoglycemia will return (30–32). Minimizing the risk of hypoglycemia while maintaining meaningful glycemic control is a challenge for people with diabetes and their caregivers, which is addressed in detail separately (44).
Perspective on hypoglycemia in diabetes.
Diabetes is a common chronic disease. Its human and economic costs are large and, despite therapeutic advances, growing because of the increasing prevalence of diabetes. Glycemic control is only one aspect of the management of diabetes. It is now possible to drive plasma LDL cholesterol concentrations to subphysiological levels and to normalize blood pressure pharmacologically, usually without major side effects, in most people with diabetes. Weight reduction and smoking cessation are more challenging, but worthy, goals in obese patients or those who smoke. However, it is not possible to maintain euglycemia over a lifetime of diabetes because of the barrier of hypoglycemia.
Nonetheless, people with diabetes and their caregivers should keep the problem of iatrogenic hypoglycemia in perspective. The principle of the glycemic management of diabetes is that maintenance of glycemia as close to the nondiabetic range as can be accomplished safely over time is generally in the patient's best interests. The extent to which that goal can be met is a function of many factors, including the type of diabetes and the stage in the progression of the disease in the individual patient. Early in the course of type 2 diabetes, by far the most common type of diabetes, hyperglycemia may respond to lifestyle changes, specifically weight loss, or to plasma glucose–lowering drugs that should not, and probably do not, cause hypoglycemia. In theory, when such drugs are effective in the absence of side effects, there is no reason not to accelerate their dosing until euglycemia is achieved. Over time, however, as people with type 2 diabetes become progressively more insulin deficient, these drugs, even in combination, fail to maintain glycemic control. Insulin secretagogues are also effective early in type 2 diabetes, but they can cause hyperinsulinemia and therefore introduce the risk of hypoglycemia. Euglycemia is not an appropriate goal during therapy with an insulin secretagogue or with insulin in people with type 2 diabetes. Nonetheless, as discussed earlier, the frequency of hypoglycemia is relatively low (at least with current glycemic goals that are above the euglycemic range) during treatment with an insulin secretagogue, or even with insulin, early in type 2 diabetes (9) when defenses against hypoglycemia are intact. Thus, over much of the course of the most common type of diabetes it is possible to achieve a meaningful degree of glycemic control with no risk or relatively low risk of hypoglycemia. The challenge is greater in people with advanced type 2 diabetes or type 1 diabetes because of compromised defenses against hypoglycemia. In such patients, therapy with insulin is demonstrably effective, but it is not demonstrably safe. Nonetheless, concerns about hypoglycemia should not be used as an excuse for poor glycemic control by patients or their caregivers. Both should strive to achieve and maintain the greatest degree of glycemic control that can be accomplished safely in a given person with diabetes at a given stage of the progression of his or her diabetes. It should be recalled that the relationship between mean glycemia and microvascular complications is curvilinear (2,5). While near euglycemia is desirable, some degree of long-term glycemic control puts the patient at lower risk than little or no glycemic control (5).
Diabetes will someday be cured and prevented. Pending that, elimination of hypoglycemia from the lives of people with diabetes will likely be accomplished by new treatment methods that provide plasma glucose–regulated insulin replacement or secretion. In the meantime, innovative research is needed if we are to improve the lives of all people affected by diabetes by lowering the barrier of hypoglycemia.
Acknowledgments
The author's original work cited has been supported in part by the U.S. Public Health Service, National Institutes of Health grants (R37 DK27085, MO1 RR00036 [now UL1 RR24992], P60 DK20579, and T32 DK07120), and by a fellowship award from the American Diabetes Association.
P.E.C. is grateful for the contributions of his mentors, collaborators, and colleagues; the efforts of the postdoctoral fellows who did the bulk of the work and made the work better by their conceptual input; and the skilled nursing, technical, dietary, and data management/statistical assistance of the staff of the Washington University General Clinical Research Center (now Intensive Research Unit). Janet Dedeke prepared this manuscript.
P.E.C. has served as a consultant to Amgen, Johnson & Johnson, MannKind, Marcadia Biotech, Medtronic MiniMed, Merck, Novo Nordisk, Takeda Pharmaceuticals North America, and TolerRx in recent years.
REFERENCES
- 1.Cryer PE: Hypoglycemia in Diabetes: Pathophysiology, Prevalence, and Prevention. American Diabetes Association, Alexandria, VA. In press
- 2.The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. N Engl J Med 329 :977 –986,1993 [DOI] [PubMed] [Google Scholar]
- 3.U.K. Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352 :837 –853,1998 [PubMed] [Google Scholar]
- 4.U.K. Prospective Diabetes Study (UKPDS) Group: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352 :854 –865,1998 [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group: The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 44 :968 –983,1995 [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353 :2643 –2653,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Action to Control Cardiovascular Risk in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358 :2545 –2559,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358 :2560 :2572,2008 [DOI] [PubMed] [Google Scholar]
- 9.U.K. Hypoglycaemia Study Group: Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 50 :1140 –1147,2007 [DOI] [PubMed] [Google Scholar]
- 10.Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, Band MM, Reekie G, Leese GP, the DARTS/MEMO Collaboration: Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med 22 :749 –755,2005 [DOI] [PubMed] [Google Scholar]
- 11.Holstein A, Plaschke A, Egberts E-H: Clinical characterization of severe hypoglycaemia: a prospective population-based study. Exp Clin Endocrinol Diabetes 111 :364 –369,2003 [DOI] [PubMed] [Google Scholar]
- 12.Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD, the DARTS/MEMO Collaboration: Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population based study of health service resource use. Diabetes Care 26 :1176 –1180,2003 [DOI] [PubMed] [Google Scholar]
- 13.Cryer PE: Hypoglycemia, functional brain failure, and brain death. J Clin Invest 117 :868 –870,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356 :1842 –1852,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, Reynolds C, McKinney PA: Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes. Diabetes Care 31 :922 –926,2008 [DOI] [PubMed] [Google Scholar]
- 16.Skrivarhaug T, Bangstad H-J, Stene LC, Sandvik L, Hanssen KF, Joner G: Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 49 :298 –305,2006 [DOI] [PubMed] [Google Scholar]
- 17.Gerich JE: Oral hypoglycemic agents. N Engl J Med 34 :1231 –1245,1989 [DOI] [PubMed] [Google Scholar]
- 18.Wright AD, Cull CA, MacLeod KM, Holman RR, the UKPDS Group: Hypoglycemia in type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis (UKPDS 73). J Diabetes Complications 20 :395 –401,2006 [DOI] [PubMed] [Google Scholar]
- 19.Towler DA, Havlin CE, Craft S, Cryer PE: Mechanism of awareness of hypoglycemia: perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 42 :1791 –1798,1993 [DOI] [PubMed] [Google Scholar]
- 20.DeRosa MA, Cryer PE: Hypoglycemia and the sympathoadrenal system: neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. Am J Physiol Endocrinol Metab 287 :E32 –E41,2004 [DOI] [PubMed] [Google Scholar]
- 21.Cryer PE: The prevention and correction of hypoglycemia. In Handbook of Physiology. Section 7, The Endocrine System. Volume II, The Endocrine Pancreas and Regulation of Metabolism. Jefferson LS, Cherrington AD, Eds. Oxford University Press, New York,2001. , p.1057 –1092
- 22.Raju B, Cryer PE: Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes. Diabetes 54 :757 –764,2005 [DOI] [PubMed] [Google Scholar]
- 23.Dagogo-Jack SE, Craft S, Cryer PE: Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. J Clin Invest 91 :819 –828,1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segel SA, Paramore DS, Cryer PE: Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes 51 :724 –733,2002 [DOI] [PubMed] [Google Scholar]
- 25.White NH, Skor DA, Cryer PE, Levandoski LA, Bier DM, Santiago JV: Identification of type 1 diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med 308 :485 –491,1983 [DOI] [PubMed] [Google Scholar]
- 26.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Massi-Benedetti M, Santeusanio F, Gerich JE, Brunetti P: A reliable and reproducible test for adequate glucose counter-regulation in type 1 diabetes mellitus. Diabetes 33 :732 –737,1984 [DOI] [PubMed] [Google Scholar]
- 27.Geddes J, Schopman JE, Zammitt NN, Frier BM: Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med 25 :501 –504,2008 [DOI] [PubMed] [Google Scholar]
- 28.Ertl AC, Davis SN: Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab Res Rev 20 :124 –130,2004 [DOI] [PubMed] [Google Scholar]
- 29.Banarer S, Cryer PE: Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes 52 :1195 –1203,2003 [DOI] [PubMed] [Google Scholar]
- 30.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E, Perriello G, De Feo P, Santeusanio F, Brunetti P, Bolli GB: Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycemia, following institution of rational, intensive therapy in IDDM. Diabetologia 37 :1265 –1276,1994 [DOI] [PubMed] [Google Scholar]
- 31.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA: Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 344 :283 –287,1994 [DOI] [PubMed] [Google Scholar]
- 32.Dagogo-Jack S, Rattarasarn C, Cryer PE: Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 43 :1426 –1434,1994 [DOI] [PubMed] [Google Scholar]
- 33.MacDonald MJ: Post exercise late onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care 10 :584 –588,1987 [DOI] [PubMed] [Google Scholar]
- 34.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, Wysocki T, Weinzimer SA, Buckingham BA, Kollman C, Xing D, Ruedy KJ, the Diabetes Res in Network (DirecNet) Study Group: Impact of exercise on overnight glycemic control in children with type 1 diabetes. J Pediatr 147 :528 –534,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoval DA, Aftab Guy DL, Richardson MA, Ertl AC, Davis SN: Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes. Diabetes 53 :1798 –1806,2004 [DOI] [PubMed] [Google Scholar]
- 36.Cryer PE: Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54 :3592 –3601,2005 [DOI] [PubMed] [Google Scholar]
- 37.McCrimmon R: The mechanisms that underlie glucose sensing during hypoglycaemia in diabetes. Diabet Med 25 :513 –522,2008 [DOI] [PubMed] [Google Scholar]
- 38.Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ: Attenuation of amygdala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes. Diabetes 56 :2766 –2773,2007 [DOI] [PubMed] [Google Scholar]
- 39.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE: Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes 57 :470 –475,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cryer PE, Davis SN, Shamoon H: Hypoglycemia in diabetes. Diabetes Care 26 :1902 –1912,2002 [DOI] [PubMed] [Google Scholar]
- 41.Steffes MW, Sibley S, Jackson M, Thomas W: β-Cell function and the development of diabetes related complications in the Diabetes Control and Complications Trial. Diabetes Care 26 :832 –836,2003 [DOI] [PubMed] [Google Scholar]
- 42.Mühlhauser I, Overmann H, Bender R, Bott U, Berger M: Risk factors for severe 42: hypoglycaemia in adult patients with type 1 diabetes: a prospective population based study. Diabetologia 41 :1274 –1282,1997 [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association Workgroup on Hypoglycemia: Defining and reporting hypoglycemia in diabetes. Diabetes Care 28 :1245 –1249,2005 [DOI] [PubMed] [Google Scholar]
- 44.Heller SR: Minimizing hypoglycemia while maintaining glycemic control in diabetes. Diabetes,57 :3177 –3183,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]