Abstract
OBJECTIVE—The expansion of adipose tissue is linked to the development of its vasculature. However, the regulation of adipose tissue angiogenesis in humans has not been extensively studied. Our aim was to compare the angiogenesis associated with subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) from the same obese patients in an in vivo model.
RESEARCH DESIGN AND METHODS—Adipose tissue samples from visceral (VAT) and subcutaneous (SAT) sites, obtained from 36 obese patients (mean BMI 46.5 kg/m2) during bariatric surgery, were layered on chick chorioallantoïc membrane (CAM).
RESULTS—Both SAT and VAT expressed angiogenic factors without significant difference for vascular endothelial growth factor (VEGF) expression. Adipose tissue layered on CAM stimulated angiogenesis. Angiogenic stimulation was macroscopically detectable, with engulfment of the samples, in 39% and was evidenced by angiography in 59% of the samples. A connection between CAM and adipose tissue vessels was evidenced by immunohistochemistry, with recruitment of both avian and human endothelial cells. The angiogenic potency of adipose tissue was not related to its localization (with an angiogenic stimulation in 60% of SAT samples and 61% of VAT samples) or to adipocyte size or inflammatory infiltrate assessed in adipose samples before the graft on CAM. Stimulation of angiogenesis by adipose tissue was nearly abolished by bevacizumab, which specifically targets human VEGF.
CONCLUSIONS—We have established a model to study the regulation of angiogenesis by human adipose tissue. This model highlighted the role of VEGF in angiogenesis in both SAT and VAT.
Adipose tissue retains substantial plasticity in adulthood. Its mass can increase or decrease up to 10-fold throughout life. The prevalence of obesity has doubled over the last 20 years, and current pharmacotherapy is relatively ineffective in maintaining long-term weight loss (1). A better understanding of the development of adipose tissue is required to identify new therapeutic approaches to obesity.
Angiogenesis and adipogenesis are linked functionally (2). During embryogenesis, the development of adipose tissue and its vascularization are temporally and spatially related (3). In animal models of genetic and induced obesity, the expansion of adipose tissue is associated with active angiogenesis, whereas inhibition of angiogenesis prevents adipose tissue development (4–6). Angiogenesis induced by adipose cells in animal models increases along with adipocyte differentiation (7–9), and conversely, angiogenic factors, such as vascular endothelial growth factor (VEGF), can modulate adipocyte differentiation (8). The cross-talk between adipocytes and endothelial cells involves numerous paracrine factors associated with angiogenesis and/or adipose tissue differentiation. It also involves direct cell-to-cell interactions, and it should be noted that human adipose tissue–derived stem cells can differentiate into either adipocyte or endothelial cells (10–12).
Numerous studies have shown that adipose tissue can stimulate angiogenesis in physiological models, such as the chick chorioallantoïc membrane (CAM) and the rabbit cornea (7,13,14), or in pathophysiological models, such as wound healing and revascularization of ischemic tissues (2,11,12,15–17). Adipose tissue produces several factors involved in angiogenesis, and local or circulating levels of tumor necrosis factor-α (TNF-α), VEGF, plasminogen activator inhibitor-1 (PAI-1), and angiopoïetin-2 are increased in animal and human obesity (2,18–20). Conversely, factors involved in adipocyte regulation, such as leptin, adiponectin, visfatin, or peroxisome proliferator–activated receptor γ (PPARγ), for example (2,3,8,21–24), also regulate angiogenesis.
However, there have been few studies of the mechanisms of stimulation of adipose tissue angiogenesis in obesity. The characteristics of adipose tissue that could influence angiogenesis in humans, such as whether the tissue is of subcutaneous or visceral origin, the presence of infiltrating macrophages, and the profile of expression of angiogenic factors are still unknown. Very few studies (8,24,25) have tried to inhibit angiogenic factors to identify their role in adipose tissue angiogenesis.
Our main objectives were to compare the angiogenic potency of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) from obese patients and to assess whether the characteristics of adipose tissue and the phenotype of the patients influence angiogenesis associated with adipose tissue.
RESEARCH DESIGN AND METHODS
Thirty-six consecutive patients attending our institution for bariatric surgery were included in the study between October 2005 and June 2007. The bariatric surgery was performed in accordance with the recommendations of international committees and consensus conferences, as previously described (26). Informed consent was required and obtained before inclusion, and the local ethics committee approved the protocol. All obese patients underwent routine physical examination and systematic fasting biological analyses (26) before surgery (mean 3 ± 2 months). A two-point assessment of fasting plasma insulin and blood glucose concentrations was performed only in nondiabetic patients to calculate the homeostasis minimal assessment (HOMA) index of insulin resistance. Treatments for hypertension, diabetes, dyslipidemia, and obstructive sleep apnea were systematically recorded.
Adipose tissues samples.
SAT from abdominal wall and VAT from an omental site were collected during the surgical procedure and were immediately transported at 20°C to the laboratory in Dulbecco's modified Eagle's medium (DMEM)/F12 (Invitrogen, Cergy pontoise, France) containing 2% BSA (Sigma, Saint-quentin Fallavier, France). Aliquots of the samples were fixed in 4% paraformaldehyde (PFA) or frozen in liquid nitrogen and stored at −80°C. The remaining samples were rinsed in DMEM/F12 without BSA, centrifuged for 30 s at 250g to separate from erythrocytes (11), cut in small pieces of 3–50 mg, and placed onto the CAM (see below). Adipose tissue fractions were isolated as previously described (11).
In vivo angiogenesis.
Fertilized White Leghorn chick eggs were incubated as previously described (27). SAT and VAT samples were layered on the chick or quail CAM after 8 days of incubation. One to five samples were placed on each CAM. Angiogenesis was quantified on CAM 5–7 days later (days 13–15 of incubation) with a MZ FLIII Leica stereomicroscope (×8). Angiographies were performed as described previously (28). For VEGF inhibition, PTK 787/ZK222584 (Novartis Pharma, Basel, Switzerland), an inhibitor of the tyrosine kinase of the VEGF receptor active in human and chicken (27), was added to the adipose tissue samples at 200 μmol/l twice daily for 48 h. Bevacizumab (Genentech, San Francisco, CA), a monoclonal antibody that specifically inhibits human VEGF with no cross-reaction with other species (29), was added at 320 μg/ml with the same protocol.
Histochemistry.
For immunohistochemistry, the primary antibodies used are detailed below. Staining was performed as previously described (28) except for the quail hematopoietic and endothelial cells (QH1) antibody for which the peroxidase-linked secondary antibody (1/200) was revealed by incubation with HistoGreen (AbCys, Paris, France) (30).
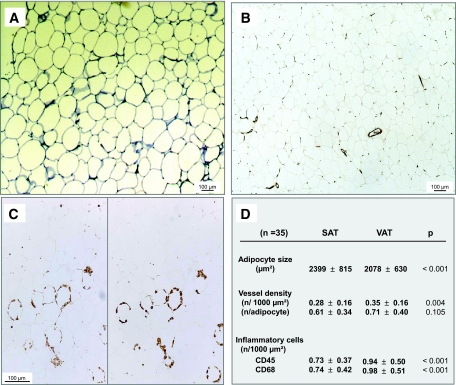
Freshly collected adipose tissue samples were fixed for 5 h in 4% PFA and embedded in paraffin (27). Samples were stained with 0.2% toluidine blue to measure surface areas of individual adipocytes. The vascular density was measured on slides that were stained with anti–von Willebrand antibody (1:600; Dako, Trappes, France). The inflammatory infiltrate was assessed using either anti-CD45 or anti-CD68 antibodies (1:50; Dako). Adipocyte area and vessel density were determined on at least three fields of 2,059 × 1,544 μm2, and CD45 and CD68 were measured on at least five fields of 1,029 × 772 μm2, using the IPLab program (Scanalytics, Fairfax, VA). The coefficient of correlation between the density of CD45+ and CD68+ cells was r = 0.86 (P < 0.0001).
Adipose tissue samples that were grafted on CAM were fixed for 2 h in 4% PFA, embedded in paraffin, and stained with hematoxylin-eosin for morphological studies. Chick erythrocytes were stained with an antibody specific for chicken (1:1,500; QMS, Biovalley, Marne la vallée, France). Human endothelial cells were detected by staining with an anti-CD34 primary antibody (1/100; Immunotech, Marseille, France). For double staining of human and quail endothelial cells, adipose tissue samples grafted on quail CAM were stained with anti-CD34 antibody and then incubated with an anti-QH1 antibody (1/2) (30).
The level of hypoxia within adipose tissue samples that were grafted on CAM was assessed by Hypoxyprobe (Chemicon, Millipore, Molsheim, France) according to the manufacturer's instructions. Hypoxyprobe was incubated for 3 h at 21% O2, either layered in a siliconized ring around adipose tissue samples (1 mg/ml) or injected intravenously (100 μl at 10 mg/ml). Bromo-deoxy-uridine (BrdU) incorporation was performed with the BrdU in situ detection kit II (BD Biosciences Pharmingen, San Diego, CA) as previously described (27).
Chick cells inside human adipose tissue samples were stained after injection of avian replication competent avian sarcoma-leucosis (RCAS) virus–green fluorescent protein (GFP) (31) into the vitellin anterior vein of the embryo on day 3 of incubation in a volume of 1–2 μl. RCAS-infected CAM vessels around adipose tissue were observed on day 15 of incubation under fluorescent light. Samples were then stained with a biotinylated anti-GFP antibody (1:200; Vector, Burlingame, CA).
Real-time quantitative RT-PCR.
Angiogenic factor mRNAs were quantified by real-time quantitative RT-PCR. Total RNA was extracted with TriZol (2.5 ml/500 mg tissue) and purified using the RNeasy Lipid Tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions (12). Angiogenic factor mRNAs were assayed with RT2 profiler PCR array plates and RT2 real-time SYBR Green fluorescein PCR master mix (Bioscience, Frederick, MD) following the manufacturer's instructions. SYBR green fluorescence was quantified with icycler (Bio-Rad).
We selected the genes of angiogenic factors previously implicated in adipose tissue angiogenesis (2,8,9,18,32,33) and factors that were differentially expressed in VAT and SAT in our preliminary experiments. Five genes of which the expression increases with adipocyte differentiation were also included as controls. The genes were analyzed in triplicate, and results are reported relative to the values for three housekeeping genes (RPL13A, GAPDH, and actin B). Using this method, one can compare different samples for the expression of a given gene but not different genes in the same sample. The difference of ΔCt (Ct for the gene − Ct for the mean of three housekeeping genes) between SAT and VAT for each gene of interest is expressed as fold difference (2ΔCt2−ΔCt1). The difference of mRNA expression between SAT and VAT was considered significant when fold difference was >2 (34).
Statistical analysis.
Data are expressed as means ± SD or SE as indicated for continuous data and percentages for categorical data. They were analyzed by ANOVA and regression analysis. We tested that first and second moments of models are correctly specified (homoscedasticity). Statistical analysis used the SAS System Package version 8.02 (SAS Institute, Cary, NC). All tests of significance were two-tailed. Differences were considered significant when the P value was <0.05. Associations were first considered statistically significant at a two-tailed value of 0.05.
RESULTS
Characterization of VAT and SAT.
The main clinical characteristics of the patients are available in Table 1. These patients (men/women = 7/29) presented with severe obesity (BMI 46.5 ± 7.2 kg/m2), and 86% had severe comorbidities.
TABLE 1.
Clinical characteristics of the patients
| n | 36 |
| Men/women | 7/29 |
| Age (years) | 46 ± 10 |
| Weight (kg) | 127 ± 23 |
| BMI (kg/m2) | 46.5 ± 7.2 |
| Waist circumference (cm) | 126 ± 16 |
| Systolic blood pressure (mmHg) | 131 ± 13 |
| Diastolic blood pressure (mmHg) | 68 ± 13 |
| Apnea-hypopnea index (n/h) | 32 ± 25 |
| Current smokers (n [%]) | 9 (25) |
| Women with oral contraceptive | 8 (28) |
| Menopause (n [%]) | 6 (21) |
| Patients receiving treatment for | |
| Hypertension (n [%]) | 17 (47) |
| Beta-blocker | 6 |
| Angiotensin inhibitors | 9 |
| Calcium channel blockers | 3 |
| Diuretics | 7 |
| Diabetes (n [%]) | 8 (22) |
| Metformin | 5 |
| Sulfamides | 4 |
| Thiazolidinediones | 1 |
| Insulin | 2 |
| Dyslipidemia (n [%]) | 7 (19) |
| Statins | 5 |
| Fibrates | 2 |
| Obstructive sleep apnea (n [%]) | 25 (69) |
| Continuous positive airway pressure | 25 |
| Asthma (n [%]) | 5 (14) |
Data are means ± SD or n (%) of patients. Apnea-hypopnea index >10 indicates obstructive sleep apnea syndrome. Blood pressure was measured at rest with an automatic device. Apnea-hypopnea index was measured by ambulatory polysomnography.
Histological characteristics of both abdominal SAT and omental VAT before graft are illustrated in Fig. 1. The average adipocyte area of SAT exceeded that of VAT (Fig. 1A). The vessel density (Fig. 1B) was higher in VAT than in SAT. However, when normalized to the mature adipocyte density, the vessel density was similar in VAT and SAT (Fig. 1D). More inflammatory cells, both CD45+ and CD68+ cells, were detected in VAT than in SAT (Fig. 1C). After normalization for the adipocyte density, the difference persisted in paired t tests for CD68+ cells (2.13 ± 1.17 vs. 1.87 ± 1.05 n/adipocyte for VAT and SAT, respectively; P < 0. 05).
FIG. 1.
Histological characteristics of SAT and VAT. Slides of adipose tissue samples were stained with toluidine blue for the measurement of adipocyte area (A), anti–von Willebrand antibody for the evaluation of vessel density (magnification ×50) (B), and anti-CD45 (left) and anti-CD68 (right) antibodies for the quantification of inflammatory infiltrate (magnification ×100) (C). D: Results obtained in 35 patients (n) are expressed as means ± SD and were compared by paired t tests. All significant differences between SAT and VAT were also significant in unpaired t tests (P < 0.05). (Please see http://dx.doi.org/10.2337/db07-1812 for a high-quality digital representation of this figure.)
mRNA of genes involved in angiogenesis.
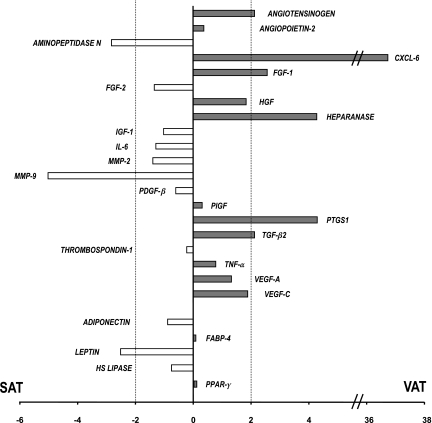
Figure 2 summarizes the semiquantitative assessment of the level of mRNA expression of a set of genes involved in the regulation of angiogenesis and that of five genes of adipocyte differentiation in VAT and SAT samples from 10 of the obese subjects. Details are available in an online appendix at http://dx.doi.org/10.2337/db07-1812 (supplementary Table). There was no difference in the expression of markers of adipocyte differentiation, according to the visceral or subcutaneous origin, except for leptin, which was expressed at a higher level in SAT than in VAT (Fig. 2), as expected (33). The expression level of the genes of growth factors involved in adipose tissue angiogenesis, including VEGF-A and -C, was similar in SAT and VAT. Also, the expression of cytokines, including TNF-α and interleukin 6 (IL-6), did not significantly differ between VAT and SAT. However, several genes were expressed more strongly in VAT than in SAT: the chemokine CXCL6, the cyclooxygenase PTGS1, heparanase, and fibroblast growth factor (FGF-1). Conversely, mRNA of the metalloproteases MMP9 and aminopeptidase N were more abundant in SAT than in VAT (Fig. 2).
FIG. 2.
Ratio of mRNA expression of angiogenesis genes in adipose tissue. Results are expressed as fold differences between SAT and VAT and are the means of the data of mRNA abundance in whole adipose tissue from 10 obese patients of a set of genes involved in the regulation of angiogenesis and that of five genes of adipocyte differentiation (adiponectin, fatty acid binding protein-4 [FABP-4], hormone-sensible lipase [HS Lipase], leptin, and PPARγ). MMP, matrix metallo proteinase; PDGF, platelet-derived growth factor; PLGF, placental growth factor; TGF, transforming growth factor. Positive values indicate a higher expression in VAT than in SAT. The difference of mRNA expression between SAT and VAT was considered significant when fold difference was >2. This threshold is indicated by a dotted line. Details of the data are available in the online appendix (supplementary Table).
Preliminary results of expression of the same set of genes in adipose tissue fractions, i.e., mature adipocytes and stromavascular fraction from two patients, are shown in the online appendix (supplementary Table). One important finding was that both VEGF-A and -C were expressed at similar levels in stromavascular fraction and mature adipocyte fractions.
In vivo angiogenesis.
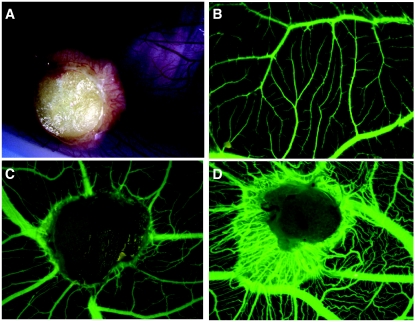
Adipose tissue samples were collected from 29 of the 36 patients and were layered on CAM. They induced a strong angiogenic response (Fig. 3). A macroscopic angiogenic response of the CAM, including engulfment of the samples with the formation of a dense surrounding vascular network (Fig. 3A), was observed in 39% of the adipose tissue samples from the 29 obese subjects after 7 days (n = 913 samples). Angiogenic stimulation by adipose tissue from 11 of the obese subjects was angiographically assessed: vascular attraction (Fig. 3C) or engulfment (Fig. 3D), scored as positive responses, was observed in 59% of the samples (n = 117).
FIG. 3.
In vivo angiogenesis induced by adipose tissue on CAM. Adipose tissue samples were layered on the CAM on day 8 of incubation and were observed between days 13 and 15. A: Macroscopic engulfment of adipose tissue sample. B: Normal angiographic aspect of CAM. C: Vascular attraction by adipose tissue on angiography. D: Angiographic aspect of covered sample with strong angiogenic stimulation. (Please see http://dx.doi.org/10.2337/db07-1812 for a high-quality digital representation of this figure.)
Viability of adipose tissue samples layered on CAM.
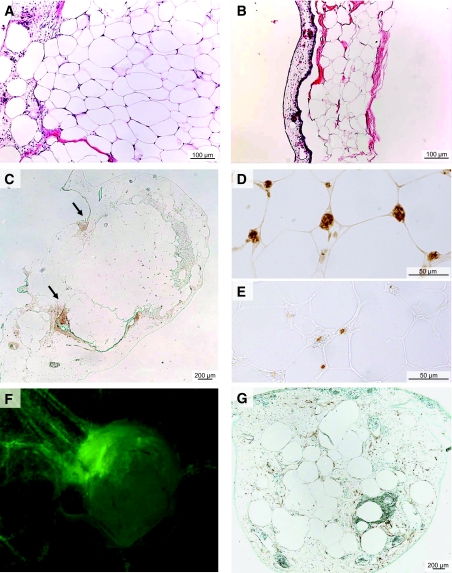
After 7 days of incubation on CAM, adipose tissue samples that had induced angiogenic response were viable and had a normal histological aspect (Fig. 4A). In contrast, samples that did not induce angiogenic response were dry and flat and showed degenerative features (Fig. 4B). No evidence of hypoxia was detected between days 9 and 15 within the successful adipose tissue grafts, after an intravenous Hypoxyprobe injection (Fig. 4C), or after local delivery (data not shown).
FIG. 4.
Connection between CAM vascular network and adipose tissue vessels. Viability of adipose tissue samples layered on the CAM was assessed by hematoxylin-eosin coloration of adipose tissue samples layered on CAM. Successfully grafted samples showing an angiogenic response had normal adipose tissue architecture (A), whereas adipose tissue degeneration was observed in samples without an angiogenic response (B). C: Staining with Hypoxyprobe at day 15 (incubated for 3 h at 21% O2 after an intravenous injection) showed a weak staining at the border between CAM and adipose tissue sample predominantly at the tip of the engulfment zone (see arrows) without staining inside the grafts. D: Adipose tissue samples layered on CAM from days 8 to 15 were stained with an antibody specific for chick erythrocytes. E: BrdU inside the samples was detected with an anti-BrdU antibody using samples that were collected 18 h after injection of BrdU in a CAM vein. An avian RCAS retrovirus-GFP was injected into the vitellin anterior vein of chick embryos on day 3. Adipose tissue samples were layered on the CAM on day 8. F: On day 15, fluorescence was observed in vessels formed around the samples, indicating that avian vessels had been the site of active angiogenesis. G: RCAS-GFP was detected in fixed adipose tissue samples stained with an anti-GFP antibody, indicating that cells of avian origin had infiltrated the vessels undergoing active angiogenesis inside the adipose tissue graft. (Please see http://dx.doi.org/10.2337/db07-1812 for a high-quality digital representation of this figure.)
Vascular connections between the vascular network of the CAM and that of viable adipose tissue grafts were evidenced first by the detection of chick erythrocytes within human adipose tissue (Fig. 4D). In a second experiment, BrdU was injected into a vein of the CAM, at distance from the adipose tissue grafts. BrdU+ cells were detected inside the grafts, confirming a connection between adipose tissue and CAM vascular network and also showing active replication of vascular cells inside the graft (Fig. 4E).
Finally, a RCAS avian retrovirus tagged with GFP was injected into the vitellin anterior vein of chick embryos. Fluorescent, RCAS-infected chick cells were present in the wall of vessels attracted by the samples (Fig. 4F). The presence of RCAS-infected cells inside adipose tissue samples was confirmed by immunohistochemistry using an anti-GFP antibody (Fig. 4G).
Origin of the vessels inside adipose tissue.
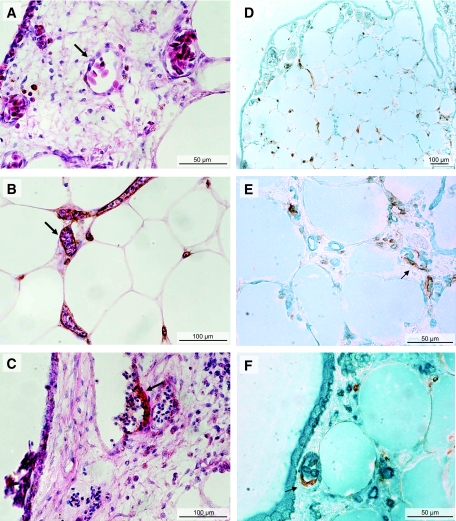
Experiments with RCAS suggested the presence of chicken vessels inside adipose tissue grafts. To determine the chick or human origin of the vessels that developed in adipose tissue, endothelial cells in sections of successfully grafted adipose tissue were stained with an anti–human CD34 antibody. The species specificity of this antibody was confirmed by the absence of staining of CAM vessels distant from grafts (Fig. 5A). In contrast, vessels inside the grafted adipose tissue samples were stained by the anti-CD34 antibody (Fig. 5B). The presence of nucleated chick erythrocytes in the lumen of the stained vessels (Fig. 5B) indicated that these vessels were functional. Furthermore, the detection of CD34+ human vessels budding from CD34− CAM vessels (Fig. 5C) showed that the interaction of adipose tissue with CAM induced the recruitment of human endothelial cells. Finally, in experiments performed with quail embryos, double staining with anti-QH1 and anti-CD34 antibodies confirmed the presence of avian endothelial cells inside the adipose tissue vessels and the mobilization of human endothelial cells into quail CAM bordering adipose tissue samples (Fig. 5D and E). Furthermore, the double staining evidenced chimeric vessels made up of endothelial cells of both avian and human origin (Fig. 5E and F).
FIG. 5.
Recruitment of human and avian endothelial cells. A–C: Adipose tissue samples layered on chick CAM from days 8 to 15 were stained with hematoxylin-eosin and an anti–human CD34 antibody. The arrows point to a CD34− vessel of the CAM (A) and to a CD34+ vessel in adipose tissue (B). B: See the nucleated chick erythrocytes inside the CD34+ vessels around adipocytes of successfully grafted adipose tissue. C: A CD34+ vessel buds from a CAM, CD34− vessel. D–F: Adipose tissue samples were layered on quail CAM for double staining of endothelial cells with anti–human CD34 antibody (in brown) and anti–quail-QH1 (in green) antibody that stains quail endothelial cells but does not cross-react with human endothelial cells. See the coexistence of human and quail vessels within the adipose tissue (D and E) and the presence (arrows) of both quail and human endothelial cells in the same vessels (E and F). (Please see http://dx.doi.org/10.2337/db07-1812 for a high-quality digital representation of this figure.)
Comparison of the angiogenic response in SAT and VAT.
The angiogenic response of the CAM to adipose tissue was not influenced by the origin of the samples: The percentage of macroscopically detectable angiogenic response was similar for SAT (38%, n = 467) and VAT (40%, n = 446). Also, the percentage of positive angiogenic responses assessed by angiography for samples from 11 patients was 60% for SAT (n = 63) and 61% for VAT (n = 54). To confirm this finding, we studied the kinetics of angiogenesis induction in SAT and VAT. The time of apparition of covered samples did not differ between samples of the two origins: in both cases, it started on day 10 (48 h after graft) and increased up to day 15 (supplementary Figure available in the online appendix). The angiogenic stimulation did not correlate with the weight of the samples in the range of 3–50 mg. The “dose-response” profile did not differ between SAT and VAT. Overall, these findings are consistent with the similar abundance of mRNAs of the main angiogenic factors in VAT and SAT.
Role of VEGF.
VEGF inhibitors were used to determine the role of VEGF, the main angiogenic growth factor, in the angiogenic response induced by adipose tissue (Fig. 6). First, PTK added at days 9 and 10 substantially inhibited angiogenesis around almost all of the samples (n = 8). PTK also inhibited angiogenesis on the CAM, such that vessels were absent from large areas as shown by angiography (Fig. 6B). Neutralization of human VEGF with bevacizumab added at days 9 and 10 strongly inhibited the angiogenic response of the CAM to the adipose tissue samples; it caused a large decrease of the vascular network around the samples compared with vehicle (Fig. 6A) but had no effect on the CAM vessel density (Fig. 6C). The effect of bevacizumab did not differ between SAT and VAT samples (Fig. 6D). These findings highlighted the major role of VEGF in both SAT and VAT angiogenesis.
FIG. 6.
Effect of VEGF inhibitors on angiogenesis associated with adipose tissue. SAT (n = 38) and VAT (n = 32) samples were layered on CAM on day 8. Angiographic observation was performed on day 15. Adipose tissue samples were incubated on days 9 and 10 with: vehicle (A), resulting in a typical angiogenic response with engulfment; 200 μmol/l PTK (B), resulting in inhibition of the angiogenic response both around the sample and on the CAM; or 300 μg/ml bevacizumab (C), resulting in inhibition of the angiogenic response around the sample. D: The percentages of covered SAT and VAT samples in controls incubated with vehicle (CT, □) and samples incubated with bevacizumab (BV, ▪). Bar graphs are 95% CI. *P < 0.05, **P < 0.01. (Please see http://dx.doi.org/10.2337/db07-1812 for a high-quality digital representation of this figure.)
Relation with phenotype.
We did not find any direct correlation between the angiogenic potency of adipose tissue samples, measured as the percentage of covered samples, and histological characteristics, such as adipocyte area and the presence of inflammatory infiltrate in adipose tissue before graft, either in SAT or in VAT.
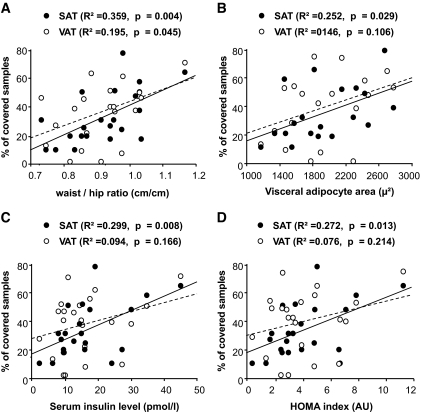
No correlation was found between SAT and VAT angiogenic responses and age, weight, or BMI. However, we found a positive correlation between the SAT angiogenic response and waist-to-hip ratio (r2 = 0.243, P = 0.0078). SAT angiogenic response was also correlated with the mean area of visceral adipocytes and with serum insulin levels and the HOMA index measured in the 22 nondiabetic patients (Fig. 7). There was no correlation with systolic blood pressure and other markers of the metabolic syndrome: triglyceride concentrations and HDL cholesterol concentrations (data not shown). In multivariate analysis, only mean area of visceral adipocytes and serum insulin level remained significantly associated with SAT angiogenesis. In contrast, there was no correlation between the VAT angiogenic response and markers of the metabolic syndrome, except for a weak correlation with waist-to-hip ratio (Fig. 7).
FIG. 7.
Correlation of adipose tissue angiogenesis with patient phenotype. Angiogenic response to adipose tissue samples was assessed as the percentage of covered SAT (•) or VAT (○) samples. Correlation between the angiogenic response and waist-to-hip ratio (A), average visceral adipocyte surface (B), serum insulin levels (C), and HOMA index (D) was assessed by regression analysis in the 22 nondiabetic patients. AU, arbitrary units.
DISCUSSION
There has been little work on the regulation of adipose tissue angiogenesis in obese subjects, although evidence implicates angiogenesis in the development of adipose tissue. Our study shows that adipose tissue from obese adult subjects grafted on CAM was able to induce angiogenesis with recruitment of vascular cells of both human and host (avian) origin. Although the expression of several angiogenic genes differed slightly between SAT and VAT, the expression of the main angiogenic factors, and notably VEGF, was similar in SAT and VAT. SAT and VAT also had similar angiogenic potencies on CAM, and inhibition of VEGF strongly inhibited angiogenesis in both tissues.
Adipose tissue is highly vascularized (2,3,5,9), and many angiogenic factors are secreted by adipose tissue, including VEGF, hepatocyte growth factor, placental growth factor (PlGF), angiopoietins, FGFs, TNF-α, PAI-1, and metalloproteases (2,8,9,18,32,33). Adipose tissue contains mature adipocytes and many other cell types known as the stromavascular fraction, which includes preadipocytes, endothelial cells, inflammatory cells, and progenitor cells (35). The respective contributions of stromavascular fraction and mature adipocytes to the secretion of angiogenic factors are unclear (16,18,32,33,36,37). Our preliminary results suggest that VEGF, one main angiogenic factor, was expressed by both stromavascular fraction cells and mature adipocytes, as described in both animals and humans by some authors (2,36,38). Most in vivo studies of adipose tissue angiogenesis focused on stromavascular fraction cells isolated from adipose tissue (12,15,17). Few studies have tested the angiogenic properties of whole intact adipose tissue (13,14,25,39). Early studies described a stimulation of angiogenesis by adipose tissue in animal models, such as grafts of rabbit adipose tissue onto cornea (13) or onto the brain surface (14). A more recent study showed de novo formation of vessels from human adipose tissue embedded in fibrin thrombin clot (25). The adipose tissue samples that we obtained from obese subjects induced a powerful angiogenic response when layered on CAM, a well-known and robust model of angiogenesis (27). We observed successful connection of adipose tissue and CAM vascularization in most of the samples.
In a model of subcutaneous injection of 3T3 preadipocytes in nude mice, it was shown by fluorescent staining that the vessels induced by the grafted fat pads originate only from the host, not from the adipose cells (32). Here, we show that after grafting of human adipose tissue on CAM, the new vessels, both inside and around the grafts, were of both avian and human origin. These findings demonstrate that when the blood supply becomes deficient, human adult whole adipose tissue can induce angiogenesis from its own cells and also recruit distant endothelial cells. It would be interesting to determine the nature of the human endothelial precursors because adipose stem cells can differentiate into endothelial cells (10,12).
To our knowledge, little is known about vessel density with respect to adipose tissue localization (5), and no study has compared the angiogenic potencies of SAT and VAT in humans. In rabbit, corneal neovascularization induced by rabbit VAT and SAT were similar in both rate and intensity (13). In the present study, the vessel densities in VAT and SAT from obese human subjects did not differ after adjustment for adipocyte densities. Moreover, SAT and VAT had the same angiogenic potency on CAM. Our results are supported by the similar expression of the major angiogenic factors in the two tissues, in contrast with observations in animal models, which indicate higher expression of VEGF in VAT than in SAT (18,19,36). These converging results suggest that despite metabolic differences between VAT and SAT (33), the main factors of angiogenesis regulation are conserved in human adipose tissue at different sites to maintain appropriate vessel density for the number of adipocytes.
The angiogenic property of adipose tissue was not correlated with BMI or adipocyte size. This may be because all of the patients had long-term, stable obesity. It has been suggested that the expression of angiogenic factors is related to variations of weight rather than to the absolute weight (2,20,40). Another explanation could be that, whereas both hyperplasia and hypertrophy of adipose tissue influence BMI, only hyperplasia is linked to adipose tissue angiogenesis (39).
Hypoxia and inflammation strongly promote angiogenesis (2,3,25,32). It was shown in mice that oxygen supply is decreased in hypertrophic adipose tissue (41), and expression and macrophage density of cytokines in adipose tissue are increased in obesity (37,42). However, we did not observe stigma of hypoxia in the core of successful grafts, although active angiogenesis was present. Angiogenic potency of adipose tissue was not correlated with inflammatory infiltrate. Although we found that the inflammatory cell density assessed before graft was higher in VAT than in SAT, as previously described (42), angiogenesis did not differ between the two sites in our study.
An unexpected and interesting finding was that angiogenic potency of SAT correlated with stigma of insulin resistance, including waist-to-hip ratio, visceral adipocyte hypertrophy, insulin levels, and HOMA index, in nondiabetic patients (43). Insulin resistance and visceral adiposity are linked (43), and we found an association between VAT adipocyte area and insulin resistance in our patients (44). In patients with abdominal obesity, stimulation of SAT angiogenesis associated with insulin resistance may promote SAT hyperplasia, thus enhancing the storage capability of adipose tissue (45). The protective effect of SAT against metabolic disturbances is now well demonstrated (46). Insulin has angiogenic properties, in part through VEGF stimulation (3,38,47,48), notably in adipose tissue (49). Given the greater insulin sensitivity of SAT than VAT (33,35), one can assume that insulin may directly enhance angiogenesis in SAT, in parallel to adipogenesis. However, we cannot rule out the involvement of other factors associated with both insulin resistance and angiogenesis, such as resistin and visfatin (22,50,51).
VEGF inhibitors strongly inhibited angiogenesis of both SAT and VAT layered on CAM, clearly demonstrating a major role for VEGF in angiogenesis associated with adipose tissue. However, inhibition of angiogenesis was observed when VEGF inhibitors were added at day 9 to 10, but later on, other factors may be involved in the angiogenic process. VEGF inhibitors are used clinically for the treatment of cancers and retinal vascular proliferative diseases (28), but their effects on adipose tissue of treated patients have not been studied. In animal studies, inhibition of VEGF (8,36,40) or PlGF (24) reduced not only adipose tissue angiogenesis but also adipose tissue expansion. Better knowledge of angiogenesis regulation in adipose tissue may allow angiogenesis inhibition to be focused on adipose tissue.
Our study has several limitations. First, we used the model of CAM to study angiogenesis, and the results need to be confirmed in vivo in murine models and in vitro using human endothelial cells, because species-specific responses may exist (52). We only investigated severely obese patients, and it would be interesting to compare angiogenesis of adipose tissue from lean and obese subjects. Furthermore, comorbidities, notably diabetes, and medications taken by the patients could have influenced angiogenesis, but the number of patients included in this study did not allow subgroup analyses.
In conclusion, we have shown that human adipose tissue from severely obese adults is able to induce angiogenesis involving recruitment of its own endothelial cells. There was no difference in the angiogenic potency of SAT and VAT. VEGF, which was expressed at similar levels in SAT and VAT, seemed to play a major role in adipose tissue angiogenesis. Further studies are needed to document the link between VEGF expression, insulin resistance, adipogenesis, and angiogenesis.
Supplementary Material
Acknowledgments
This study was supported by a grant from the European vascular genomics network.
We thank Judith Favier, Jean Galitzky, and Luc Pardanaud for their scientific advice and their technical assistance. We thank Stefano Scarengi, Benjamin Castel, and the staff of the surgical unit and of the pathology department of Hôpital Louis Mourier for their help with the management of surgical samples. We thank Anne Mabille for her careful reading of the manuscript.
Published ahead of print at http://diabetes.diabetesjournals.org on 3 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bessesen DH: Update on obesity. J Clin Endocrinol Metab 93 :2027 –2034,2008 [DOI] [PubMed] [Google Scholar]
- 2.Cao Y: Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117 :2362 –2368,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausman GJ, Richardson RL: Adipose tissue angiogenesis. J Anim Sci 82 :925 –934,2004 [DOI] [PubMed] [Google Scholar]
- 4.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ: Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 99 :10730 –10735,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y: Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 94 :1579 –1588,2004 [DOI] [PubMed] [Google Scholar]
- 6.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W: Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10 :625 –632,2004 [DOI] [PubMed] [Google Scholar]
- 7.Castellot JJ Jr, Karnovsky MJ, Spiegelman BM: Differentiation-dependent stimulation of neovascularization and endothelial cell chemotaxis by 3T3 adipocytes. Proc Natl Acad Sci U S A 79 :5597 –5560,1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK: Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93 :e88 –e97,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouloumie A, Lolmede K, Sengenes C, Galitzky J, Lafontan M: Angiogenesis in adipose tissue. Ann Endocrinol 63 :91 –95,2002 [PubMed] [Google Scholar]
- 10.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC: Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332 :370 –379,2005 [DOI] [PubMed] [Google Scholar]
- 11.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A: Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110 :349 –355,2004 [DOI] [PubMed] [Google Scholar]
- 12.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L: Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109 :656 –663,2004 [DOI] [PubMed] [Google Scholar]
- 13.Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC: Angiogenic activity of adipose tissue. Biochem Biophys Res Commun 153 :347 –352,1988 [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith HS, Griffith AL, Kupferman A, Catsimpoolas N: Lipid angiogenic factor from omentum. JAMA 252 :2034 –2036,1984 [PubMed] [Google Scholar]
- 15.Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z: Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis 18 :221 –227,2007 [DOI] [PubMed] [Google Scholar]
- 16.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL: Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109 :1292 –1298,2004 [DOI] [PubMed] [Google Scholar]
- 17.Sumi M, Sata M, Toya N, Yanaga K, Ohki T, Nagai R: Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci 80 :559 –565,2007 [DOI] [PubMed] [Google Scholar]
- 18.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW: Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145 :2273 –2282,2004 [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y: Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab 288 :E1128 –E1136,2005 [DOI] [PubMed] [Google Scholar]
- 20.Silha JV, Krsek M, Sucharda P, Murphy LJ: Angiogenic factors are elevated in overweight and obese individuals. Int J Obes 29 :1308 –1314,2005 [DOI] [PubMed] [Google Scholar]
- 21.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y: Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A 98 :6390 –6395,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SR, Bae SK, Choi KS, Park SY, Jun HO, Lee JY, Jang HO, Yun I, Yoon KH, Kim YJ, Yoo MA, Kim KW, Bae MK: Visfatin promotes angiogenesis by activation of extracellular signal-regulated kinase 1/2. Biochem Biophys Res Commun 357 :150 –156,2007 [DOI] [PubMed] [Google Scholar]
- 23.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y: Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A 10 :2476 –2481,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lijnen HR, Christiaens V, Scroyen I, Voros G, Tjwa M, Carmeliet P, Collen D: Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes 55 :2698 –2704,2006 [DOI] [PubMed] [Google Scholar]
- 25.Greenway FL, Liu Z, Yu Y, Caruso MK, Roberts AT, Lyons J, Schwimer JE, Gupta AK, Bellanger DE, Guillot TS, Woltering EA: An assay to measure angiogenesis in human fat tissue. Obes Surg 17 :510 –515,2007 [DOI] [PubMed] [Google Scholar]
- 26.Ledoux S, Msika S, Moussa F, Larger E, Boudou P, Salomon L, Roy C, Clerici C: Comparison of nutritional consequences of conventional therapy of obesity, adjustable gastric banding, and gastric bypass. Obes Surg 16 :1041 –1049,2006 [DOI] [PubMed] [Google Scholar]
- 27.Cruz A, Parnot C, Ribatti D, Corvol P, Gasc JM: Endothelin-1, a regulator of angiogenesis in the chick chorioallantoic membrane. J Vasc Res 38 :536 –545,2001 [DOI] [PubMed] [Google Scholar]
- 28.Larger E, Marre M, Corvol P, Gasc JM: Hyperglycemia-induced defects in angiogenesis in the chicken chorioallantoic membrane model. Diabetes 53 :752 –761,2004 [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN: Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66 :7843 –7848,2006 [DOI] [PubMed] [Google Scholar]
- 30.Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA: Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100 :339 –349,1987 [DOI] [PubMed] [Google Scholar]
- 31.Hartmann C, Tabin CJ: Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127 :3141 –3159,2000 [DOI] [PubMed] [Google Scholar]
- 32.Neels JG, Thinnes T, Loskutoff DJ: Angiogenesis in an in vivo model of adipose tissue development. FASEB J 18 :983 –985,2004 [DOI] [PubMed] [Google Scholar]
- 33.Lafontan M: Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol 45 :119 –146,2005 [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Kumar Dorairaj S, Prabhakaran VC, Prakash DR, Chakraborty S: Identification of genes associated with tumorigenesis of meibomian cell carcinoma by microarray analysis. Genomics 90 :559 –566,2007 [DOI] [PubMed] [Google Scholar]
- 35.Trujillo ME, Scherer PE: Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27 :762 –778,2006 [DOI] [PubMed] [Google Scholar]
- 36.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK: Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res 67 :147 –154,1997 [DOI] [PubMed] [Google Scholar]
- 37.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112 :1796 –1808,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fain JN, Madan AK: Insulin enhances vascular endothelial growth factor, interleukin-8, and plasminogen activator inhibitor 1 but not interleukin-6 release by human adipocytes. Metabolism 54 :220 –226,2005 [DOI] [PubMed] [Google Scholar]
- 39.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S: Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56 :1517 –1526,2007 [DOI] [PubMed] [Google Scholar]
- 40.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR: Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146 :4545 –4554,2005 [DOI] [PubMed] [Google Scholar]
- 41.Ye J, Gao Z, Yin J, He Q: Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293 :E1118 –E1128,2007 [DOI] [PubMed] [Google Scholar]
- 42.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A: Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92 :2240 –2247,2007 [DOI] [PubMed] [Google Scholar]
- 43.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM: Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 91 :4459 –4466,2006 [DOI] [PubMed] [Google Scholar]
- 44.Ledoux S, Queguiner I, Msika S, Clerici C, Gasc JM, Corvol P, Larger E: Connection between metabolic syndrome and size of visceral and subcutaneous adipocytes. Diabetes Metab 33 :S72 –S72,2007 [Google Scholar]
- 45.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE: Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117 :2621 –2637,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran TT, Yamamoto Y, Gesta S, Kahn CR: Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7 :410 –420,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP: Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109 :805 –815,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M, Kahn CR: Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest 111 :1835 –1842,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mick GJ, Wang X, McCormick K: White adipocyte vascular endothelial growth factor: regulation by insulin. Endocrinology 143 :948 –953,2002 [DOI] [PubMed] [Google Scholar]
- 50.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A: Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49 :744 –747,2006 [DOI] [PubMed] [Google Scholar]
- 51.Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D’Asta M, Caforio L, Caruso A: Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol 189 :691 –699,2006 [DOI] [PubMed] [Google Scholar]
- 52.Jung SP, Siegrist B, Wang YZ, Wade MR, Anthony CT, Hornick C, Woltering EA: Effect of human angiostatin protein on human angiogenesis in vitro. Angiogenesis 6 :233 –240,2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.