Abstract
OBJECTIVE—White adipose tissue is a critical regulator of whole-body glucose metabolism. Preadipocyte factor-1 (Pref-1) is a secreted protein that inhibits adipocyte differentiation, both in vitro and in vivo. In this study, we have investigated the effects of Pref-1 overexpression on whole-body glucose homeostasis and its contribution to the development of insulin resistance.
RESEARCH DESIGN AND METHODS—To gain insight into the role of Pref-1 on the onset of insulin resistance and type 2 diabetes, we measured body composition and whole-body insulin-stimulated glucose metabolism during a hyperinsulinemic-euglycemic clamp in Pref-1 transgenic and wild-type control mice fed a high-fat diet.
RESULTS—Mice overexpressing Pref-1 were resistant to high-fat diet–induced obesity, as reflected by a marked reduction in adipose tissue mass. However, Pref-1–overexpressing mice were severely insulin resistant, mainly because of a reduction in insulin-stimulated glucose uptake in skeletal muscle and adipose tissue. The aggravated insulin resistance was associated with impaired insulin signaling and increased diacylglycerol content in skeletal muscle.
CONCLUSIONS—Mice overexpressing Pref-1 are insulin resistant despite being protected from diet-induced obesity and may provide a new rodent model for the study of lipodystrophic disorders.
The prevalence of type 2 diabetes is rapidly increasing worldwide, and it has been predicted that >366 million people will be affected by the year 2030 (1). Although the primary cause of type 2 diabetes is not well defined, it is believed that insulin resistance plays a key role in the development of the disease (2). The mechanisms that trigger insulin resistance remain poorly understood, but evidence points to alterations in adipose tissue function and a concomitant ectopic lipid accumulation in muscle and liver as one of the principal underlying causes.
White adipose tissue (WAT) serves as the main energy storage depot of the organism. Energy excess stored in the form of triglycerides is released in periods of scarcity to supply the energy needs of other tissues. However, adipose tissue also functions as an active endocrine organ by secreting a variety of biologically active molecules such as leptin, adiponectin, adipocyte-specific secretory factor/resistin, tumor necrosis factor-α, interleukin (IL)-6, and plasma activator inhibitor (rev. in 3 and 4). These adipokines regulate multiple and crucial aspects of the organism's physiology, including appetite, energy metabolism, immune function, and reproduction. Hence, adipose tissue is now recognized as a key player in the regulation of energy balance and glucose homeostasis (5). Indeed, alterations in the capacity of adipose tissue to store triglycerides or to synthesize/secrete adipokines have been linked to the appearance of metabolic disease, particularly insulin resistance and type 2 diabetes (6).
Lipodystrophies, a family of congenital or acquired disorders characterized by total or partial loss of adipose mass (rev. in 7), represent one of the best paradigms of adipose tissue dysfunction. Patients affected by lipodystrophy exhibit metabolic complications that include insulin resistance, hyperlipidemia, or diabetes. The pathogenic basis of most of the lipodystrophies remain still unknown, and only mutations in a few genes, such as lamin A/C (8), AGPAT2 (9), seipin (10), or peroxisome proliferator–activated receptor (PPAR)-γ (11), have been identified as a cause of congenital lipodystrophies. These genes regulate different aspects of adipose cell biology, particularly metabolism, differentiation, and survival of adipocytes, underscoring that terminal maturation and proper functionality of adipocytes are essential requirements for appropriate whole-body lipid and glucose homeostasis.
The mechanisms that control adipocyte differentiation are complex. However, several critical transcriptional regulators and extracellular signals that regulate adipocyte differentiation have been identified (rev. in 12 and 13). Among them, our laboratory identified preadipocyte factor-1 (Pref-1) (14) as an inhibitor of adipocyte differentiation, both in vitro and in vivo (rev. in 4). Pref-1 is synthesized as a transmembrane protein whose epidermal growth factor repeat-containing ectodomain is cleaved by tumor necrosis factor-α–converting enzyme to release a biologically active 50-kDa soluble form (15). Soluble Pref-1 functions in a paracrine/endocrine manner to prevent preadipocyte differentiation through MEK/ERK activation (16,17). Mouse models of loss or gain of function have unequivocally demonstrated the important role of Pref-1 in adipogenesis. Mice lacking Pref-1 show growth retardation and skeletal abnormalities as well as increased adiposity when fed a high-fat diet (18), supporting the role of Pref-1 on the regulation of adipocyte differentiation. Accordingly, young adult mice that overexpress soluble Pref-1 exhibited a marked reduction in WAT mass as a result of impaired adipocyte differentiation (19). Interestingly, these mice also showed skeletal malformations, impaired whole-body insulin sensitivity, and reduced glucose tolerance. These reports suggest that alterations in circulating Pref-1 levels can affect whole-body glucose homeostasis. However, the effect of Pref-1 on glucose homeostasis, particularly in individual tissues, or the underlying mechanisms of such metabolic alterations have not been explored.
Here, we examined the effects of Pref-1 overexpression on insulin action and glucose and lipid metabolism in mice that have been chronically fed a high-fat diet. We found that mice overexpressing Pref-1 were insulin resistant despite a decrease in fat mass. Therefore, Pref-1 transgenic mice may provide a new rodent model of partial lipodystrophy.
RESEARCH DESIGN AND METHODS
Animals.
Generation of transgenic mice (Tg) overexpressing the Pref-1/hFc fusion protein driven by the adipose-specific aP2 promoter has been previously described (19). Wild-type (Wt) and transgenic littermates were fed a high-fat diet (45% kcal fat, 35% kcal carbohydrate, 20% kcal protein) (Research Diets, NB, NJ) ad libitum for a period of 17 weeks after weaning. Food intake was measured every 2 days over a 10-day period in 15-week-old male mice. All procedures involving animals were conducted in accordance with the institutional animal use and care guidelines of the University of California–Berkeley and the Yale University School of Medicine.
Body composition.
Fat and lean body mass was assessed by 1H magnetic resonance spectroscopy (Bruker BioSpin, Billerica, MA). The mass of major adipose depots (gonadal, inguinal, and retroperitoneal depots) was directly measured by weighing the tissues after dissection.
Adipose tissue histology.
Inguinal WAT from 20-week-old Pref-1 Tg mice and Wt littermates was isolated and fixed overnight in Bouin's solution. After dehydration, the tissue was embedded in paraffin for subsequent sectioning. The 8-μm sections were stained with hematoxylin-eosin.
Serum metabolites.
Blood from Pref-1 Tg mice and Wt littermates random-fed or fasted for 5 h was collected from tail and centrifuged at 3,000 rpm for 5 min to obtain serum. Triglyceride levels were measured with the Infinity triglyceride reagent (Sigma, St. Louis, MO). Free fatty acids were determined with the NEFA-C kit (Wako, Richmond, VA). Circulating insulin and leptin levels were measured using a rat insulin and mouse leptin enzyme-linked immunosorbent assay (ELISA) kit (Crystal Chem, Downers Grove, IL), respectively. For adiponectin, a rat/mouse ELISA kit (B-Bridge, Sunnyvale, CA) was used. Blood glucose was measured with a blood glucose meter (AccuCheck; Roche, Indianapolis, IN).
Glucose tolerance test.
A glucose tolerance test was performed on 12-h fasted mice. Blood glucose was measured at 0, 30, 60, 90, and 120 min after an intraperitoneal injection of glucose (1 g/kg body wt).
Hyperinsulinemic-euglycemic clamp study.
Seven days before the hyperinsulinemic-euglycemic clamp studies, indwelling catheters were placed into the right internal jugular vein. After an overnight fast, [3-3H]glucose (HPLC purified; Perkin Elmer, Boston, MA) was infused at a rate of 0.05 μCi/min for 2 h to assess the basal glucose turnover. After the basal period, a hyperinsulinemic-euglycemic clamp was conducted for 120 min with a primed/continuous infusion of human insulin (105 pmol/kg prime, 15 pmol · kg−1 · min−1 infusion) (Novo Nordisk, Princeton, NJ). Blood samples (10 μl) were collected at 10- to 20-min intervals for the immediate measurement of plasma glucose, and 20% dextrose was infused at variable rates to maintain plasma glucose at basal concentrations (∼6.7 mmol/l). To estimate insulin-stimulated whole-body glucose fluxes, [3-3H]glucose was infused at a rate of 0.1 μCi/min throughout the clamps, and 2-deoxy-d-[1-14C]glucose (Perkin Elmer, Boston, MA) was injected as a bolus 75 min into the clamp to estimate the rate of insulin-stimulated tissue glucose uptake as previously described (20). Blood samples (10 μl) for the measurement of plasma 3H and 14C activities were taken at the end of the basal period and during the last 45 min of the clamp. At the end of the clamp, mice were killed, and tissues were removed for biochemical measurements.
Analysis of insulin signaling.
After an overnight fast, Wt and Pref-1 Tg mice were intraperitoneally injected with saline or 0.85 units/kg insulin and killed 10 min after. Lysates from WAT, liver, and skeletal muscle were obtained in homogenization buffer (150 mmol/l NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10 mmol/l Tris, pH 7.4) containing a protease and phosphatase inhibitor cocktail (Sigma). Protein (30–60 μg) was subjected to SDS-PAGE and immunoblotted with specific antibodies for total Akt or Akt phosphorylated at residue Ser473 (Cell Signaling, Danvers, MA). For insulin receptor substrate (IRS) phosphorylation, lysates (1 mg protein) were first immunoprecipitated with specific antibodies against IRS-1 or IRS-2 (Millipore, Billerica, MA). Immunoprecipitates were then subjected to SDS-PAGE and blotted with 4G10 antibody (Millipore) that detects phosphorylated tyrosine residues or antibodies that detect total IRS-1 or IRS-2.
Akt activity.
Tissue lysates (500 μg protein) were subjected to immunoprecipitation for 4 h at 4°C with 5 μl of a polyclonal Akt antibody (Upstate Biotechnology), coupled to protein A-sepharose beads. The immune complex was washed, and Akt activity was determined as described (21).
Lipid metabolites.
Tissue triglycerides were extracted using the method of Bligh and Dyer (22), and content was measured using a DCL Triglyceride Reagent (Diagnostic Chemicals, Oxford, CT). Fatty acyl-CoA, diacylglycerol, and ceramide extraction and measurement by liquid chromatography/tandem mass spectrometry have been described previously (23).
Gene expression.
RNA was isolated from skeletal muscle, WAT, and liver, and cDNAs were synthesized with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo dT. Gene expression was assessed by real-time quantitative PCR using specific primers and TaqMan probes for fatty acid transport protein-1, -2, -4, and -5 and CD36 (Applied Biosystems). Quantification was performed by the ΔΔCT threshold cycle method, and relative gene expression was normalized to GAPDH levels.
Statistical analysis.
Results are expressed as means ± SE. Statistical significance of differences between experimental groups was assessed using the unpaired Student's t test.
RESULTS
Pref-1 overexpressing mice are resistant to diet-induced obesity.
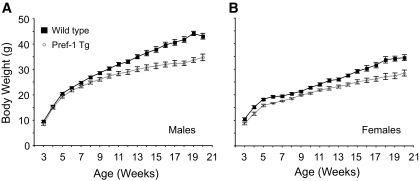
We recently reported that overexpression of a Pref-1/hFc fusion protein in mice impaired adipocyte differentiation (19). Interestingly, these mice exhibited a mild degree of glucose intolerance and insulin resistance at young age (∼10 weeks old). To further investigate the effects of Pref-1 overexpression on tissue-specific insulin sensitivity, we carried out comparative studies on Pref-1 Tg mice and Wt littermates fed a high-fat diet for 17 weeks. High-fat diets are known to induce obesity and to promote insulin resistance and diabetes in mice and humans, particularly if individuals are subject to such diets for a long period of time. At weaning, Pref-1 transgenic male mice weighed slightly less than Wt littermates (Wt = 9.5 ± 0.5 g, n = 17, vs. Tg = 8.4 ± 0.5 g, n = 15; P = 0.074) (Fig. 1A). After 17 weeks of high-fat diet feeding, Wt mice became evidently obese, exhibiting an average of >8 g in body weight above Pref-1 transgenic mice, which remained considerably leaner (Wt = 43.1 ± 1.1 g, n = 17, vs. Tg = 34.8 ± 1.3 g, n = 15; P < 0.01). Similarly, female transgenic mice were also resistant to diet-induced obesity (Wt = 34.2 ± 1.0 g, n = 10, vs. Tg = 28.5 ± 1.5 g, n = 10; P < 0.01) (Fig. 1B). The resistance to high-fat diet–induced obesity occurred despite similar food intake (Wt = 0.420 ± 0.04 kcal · g−1 · day−1, n = 6, vs. Tg = 0.417 ± 0.03 kcal · g−1 · day−1, n = 7).
FIG. 1.
Body weight of wild-type (▪) and Pref-1 transgenic (○) mice fed a high-fat diet for 17 weeks. A: Males (n = 15–17/group). B: Females (n = 10/group).
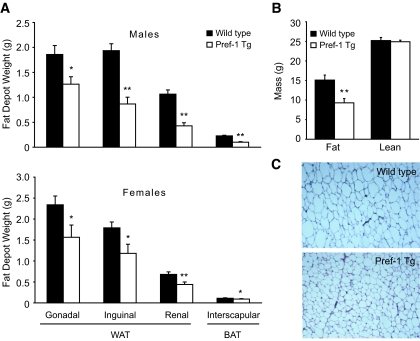
Compared with Wt, Pref-1 Tg mice exhibited a significant reduction in the mass of the major WAT depots (Fig. 2A), such as gonadal, inguinal, and renal fat. The interscapular brown adipose tissue was also significantly decreased. The reduction in adipose tissue mass was accountable for most of the decrease observed in body weight, since 1H magnetic resonance spectroscopy revealed no differences in lean mass between Wt and Pref-1 Tg mice (Fig. 2B). Fat depots of Pref-1 transgenic mice appeared normal by gross morphological examination, although adipocytes were significantly smaller than those of Wt mice (Fig. 2C). In addition, we have previously shown that expression levels for adipocyte markers including PPARγ, C/EBPα, and FAS are lower in adipose tissue of Pref-1 transgenic mice, indicating impairment of adipocyte differentiation (19). The reduction in adipose tissue mass and adipocyte size in Pref-1 transgenic mice was accompanied by an increase in the circulating levels of free fatty acids and triglycerides, in both fasted and fed states (Table 1), reflecting the fact that Pref-1 transgenic mice have reduced capacity to store triglycerides in adipose tissue.
FIG. 2.
Protection from high-fat diet–induced obesity in Pref-1 transgenic mice. A: Weight of major adipose tissue depots from Wt (▪) and Pref-1 Tg (□) male (upper panel) and female (lower panel) mice. B: Fat mass and lean mass in Wt and Pref-1 Tg male mice measured by 1H magnetic resonance spectroscopy (n = 9/group). C: Paraffin-embedded sections stained with hematoxilin-eosin of inguinal WAT of Wt and Pref-1 Tg mice. BAT, brown adipose tissue. (Please see http://dx.doi.org for a high-quality digital representation of this figure.)
TABLE 1.
Serum parameters in fed and 5-h fasted Pref-1 Tg and Wt littermates
| Fed |
Fasted |
|||
|---|---|---|---|---|
| Wt | Pref-1 Tg | Wt | Pref-1 Tg | |
| Glucose (mg/dl) | ND | ND | 202 ± 11 | 214 ± 7 |
| FFA (mEq/l) | 0.406 ± 0.03 | 0.560 ± 0.03† | 0.710 ± 0.08 | 0.950 ± 0.08 (P = 0.06) |
| Triglycerides (mg/dl) | 54.9 ± 3.7 | 64.4 ± 7.7 | 73.5 ± 7 | 90.2 ± 8.9 |
| Insulin (pg/ml) | 5,010 ± 1,041 | 3,721 ± 819 | 3,734 ± 525 | 3,107 ± 554 |
| Adiponectin (μg/ml) | 24.0 ± 2.7 | 10.5 ± 0.8† | 27.0 ± 3.1 | 10.1 ± 1.0† |
| Leptin (ng/ml) | 28.0 ± 2.4 | 5.8 ± 0.9† | ND | ND |
P < 0.01, n = 6–7. ND, not determined.
Dysregulated glucose homeostasis in Pref-1 transgenic mice.
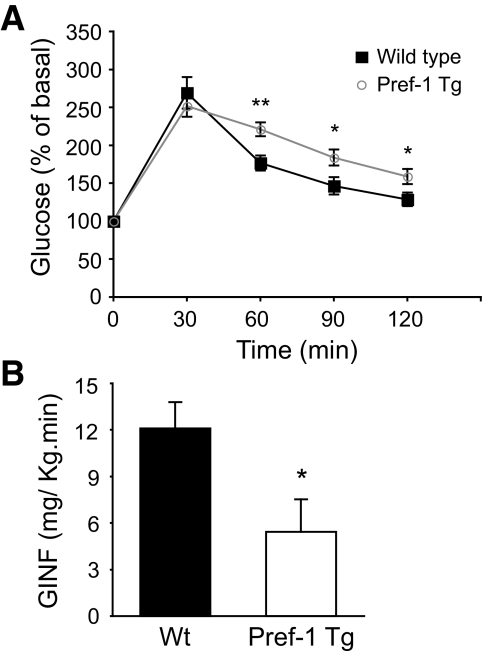
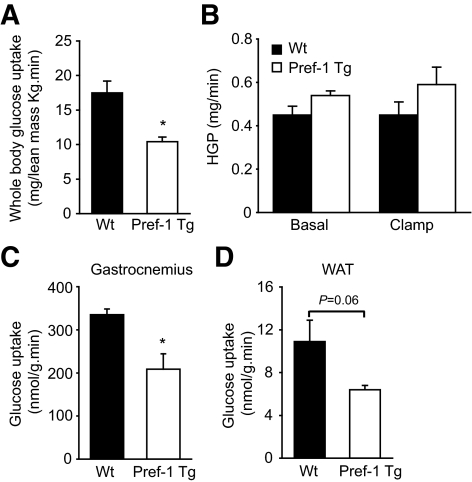
The inability of adipocytes to properly store fat is frequently linked to alterations in glucose homeostasis and development of insulin resistance. Although fasting plasma glucose levels were similar in Pref-1 Tg and Wt mice (Table 1), the glucose tolerance test showed that transgenic mice have an impaired capacity to clear glucose from circulation compared with Wt mice (Fig. 3A). To investigate the mechanisms of glucose intolerance, a 2-h hyperinsulinemic-euglycemic clamp assay was conducted on awake Wt and Pref-1 transgenic littermates. During the clamp, plasma insulin level was increased to ∼330 pmol and glucose levels were maintained at ∼120 mg/dl by variable infusion of glucose. The glucose infusion rate necessary to maintain euglycemia in Pref-1 Tg mice was 50% lower than in Wt mice, indicating that insulin-stimulated glucose uptake and metabolism was severely blunted in transgenic mice (Fig. 3B). This could be attributed to a 40% decrease in insulin-stimulated whole-body glucose uptake in Pref-1 transgenic mice (Fig. 4A). Because hepatic glucose production (HGP) was similar in Pref-1 Tg and Wt mice during the clamp as well as in basal conditions, the inability of insulin to suppress HGP during the clamp in either Wt or Pref-1 Tg mice suggests that both experimental groups of mice developed severe but similar hepatic insulin resistance (Fig. 4B). Supporting a similar HGP, no differences in the expression of key gluconeogenic genes Pepck and G6pc3 between Pref-1 transgenic mice and wild-type littermates were found (data not shown). On the other hand, consistent with the differences observed in whole-body glucose uptake between Wt and Pref-1 Tg mice, insulin-stimulated glucose uptake was significantly reduced in skeletal muscle and WAT of Pref-1 transgenic mice compared with Wt mice (Fig. 4C and D).
FIG. 3.
Glucose intolerance and insulin resistance in Pref-1 transgenic mice fed a high-fat diet. A: Glucose tolerance test on Wt (▪) and Pref-1 Tg (○) mice, after 17 weeks on a high-fat diet (n = 6–7; *P < 0.05; **P < 0.01). B: Average glucose infusion rate (GINF) during the last 30 min of the hyperinsulinemic-euglycemic clamp assay in Wt (▪) and Pref-1 Tg (□) mice (n = 5–7/group; *P < 0.05).
FIG. 4.
Glucose metabolism in Pref-1 transgenic mice (□) and wild-type littermates (▪) during hyperinsulinemic-euglycemic clamps. A: Insulin-stimulated whole-body glucose uptake. B: Hepatic glucose production (HGP) before and during the clamp. C: Glucose uptake by skeletal muscle (gastrocnemius). D: Glucose uptake by WAT.
Impaired insulin signaling and increased lipid metabolites in skeletal muscle of Pref-1 transgenic mice.
The action of insulin on glucose metabolism in peripheral tissues relies on the proper activation of the insulin-signaling pathway and the correct elicitation of responses by molecular targets that eventually lead to glucose transport and metabolism. To investigate whether the exacerbated insulin resistance present in Pref-1 transgenic mice is due to defects in insulin signaling, we analyzed the insulin-stimulated IRS and Akt phosphorylation in a variety of insulin-sensitive tissues (Fig. 5A and B). Insulin administration, although at a reduced degree, similarly increased phosphorylation of IRS-2 and Akt in liver of Wt and Tg mice, indicating a comparable degree of hepatic insulin sensitivity. In WAT, insulin-stimulated phosphorylation of IRS-1 and IRS-2, as well as Akt, was ∼50% lower in Pref-1 Tg mice compared with Wt littermates. More significantly, phosphorylation of IRS-1 and Akt upon injection of insulin was severely blunted by 80% in skeletal muscle of Pref-1 Tg mice compared with Wt mice (Fig. 5A and B). Consistent with these observations, a 40% reduction in Akt activity was observed in gastrocnemius muscle of Pref-1 Tg mice compared with Wt mice (Fig. 5C). Similarly, Akt activity in WAT tended to be 40% lower in Tg mice. On the other hand, there was no difference in liver Akt activity in Pref-1 Tg and Wt mice (Fig. 5C). Together, these results demonstrate that, in Pref-1 Tg mice, insulin action in WAT and skeletal muscle, the latter being the major contributor to glucose utilization in the organism, is strongly impaired.
FIG. 5.
Insulin-signaling pathway analysis. Mice were fasted overnight, injected with saline or insulin (0.85 units/kg), and killed 10 min after injection. A: For analysis of insulin-induced phosphorylation of IRS, 1 mg protein lysates from liver, WAT, or skeletal muscle was first immunoprecipitated with anti–IRS-1 or anti–IRS-2 antibodies. Immunoprecipitates were then subjected to SDS-PAGE and blotted with anti–IRS-1 in liver and WAT and anti–IRS-2 in WAT and skeletal muscle to detect total levels of IRS-1 or IRS-2. Phosphorylation of IRS was detected in all tissues with an antibody specifically detecting phosphotyrosine residues. B: Total Akt and phosphorylated Akt were also detected by Western blot in protein lysates of liver, WAT, and skeletal muscle using specific antibodies against total Akt or phosphorylated Akt. C: Akt activity in skeletal muscle, WAT, and liver. Tissue lysates (500 mg protein) were subjected to immunoprecipitation (IP) for 4 h at 4°C with an Akt polyclonal antibody. Precipitated complexes were assayed for Akt activity as described previously (21). Results show mean ± SE of four to six animals per group. *P < 0.05, #P < 0.09.
Evidence suggests that increased lipid accumulation in nonadipose tissues plays a major role in the development of insulin resistance associated with obesity and lipodystrophy. As shown in Table 1, circulating free fatty acid and triglyceride levels were higher in Pref-1 transgenic mice, presumably due to the significant reduction in lipid storage capacity of adipose tissue in these mice. To better understand how the metabolic alterations observed in mice overexpressing Pref-1 can inhibit insulin signaling and induce insulin resistance, we analyzed lipid metabolites content in liver and gastrocnemius muscle of Wt and Pref-1 transgenic mice. In liver, no significant difference in diacylglycerols (DAG) or fatty acyl-CoAs were found (Fig. 6A), although triacylglycerols and ceramides content was somewhat reduced (25 and 17%, respectively). In skeletal muscle, we did not observe any significant difference in triacylglycerol, fatty acyl-CoA, or ceramide content (Fig. 6B) between Wt and Pref-1Tg mice. However, we detected an increase of nearly 60% in total DAG content in muscle of Pref-1 transgenic mice (Fig. 6C). Elevated DAG level in skeletal muscle has previously been shown to cause insulin resistance after lipid infusion or fat feeding in rodents (24–26) as well as humans (27). This suggests that the increased levels of DAG observed in the skeletal muscle of Pref-1 transgenic mice could be a contributing factor for the aggravated insulin resistance associated with the lipodystrophy present in Pref-1 transgenic mice.
FIG. 6.
Lipid metabolite levels were measured in liver (A) and skeletal muscle (B) of Pref-1 transgenic (□) and wild-type littermates (▪) by liquid chromatography/tandem mass spectrometry. Results are expressed as means ± SE of seven to eight animals per group. *P < 0.05.
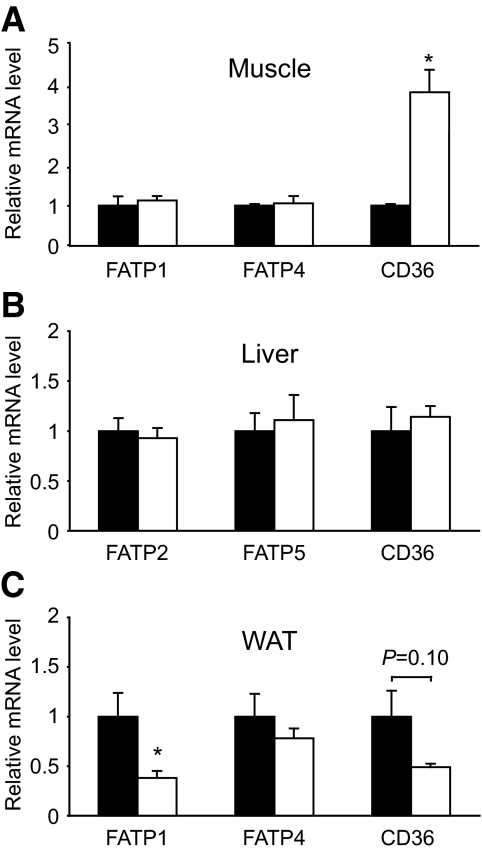
We next examined whether changes in the expression of proteins involved in flux of fatty acids into the cell, including FATPs and CD36, contribute to lipid accumulation in tissues of Pref-1 Tg mice. Interestingly, we found a fourfold increase in CD36 expression in muscle, but not in liver, whereas no difference was observed in the expression of FATP family members known to be expressed in each of these tissues (Fig. 7A and B). In addition, a substantial decrease in FATP1 and CD36 mRNA was found in WAT of Pref-1 Tg mice (Fig. 7C), probably due to the impairment in adipocyte differentiation and lipid accumulation observed in Pref-1 Tg mice. These results suggest that higher CD36 expression in muscle of Pref-1 Tg mice, together with increased lipid availability, may contribute to the preferential lipid, namely DAG, accumulation observed in the skeletal muscle of Pref-1 Tg mice.
FIG. 7.
Expression levels of fatty acid transporters CD36 and FATPs in skeletal muscle (A), liver (B), and WAT (C) were assessed by real-time quantitative PCR using specific primers and TaqMan probes. Fold-changes in comparison to the levels in Wt mice are shown and represent the mean ± SE of four to eight animals per group. *P < 0.05.
DISCUSSION
In this study, we show that high levels of circulating Pref-1 prevent the body weight gain and adipose tissue accumulation that are normally associated with high-fat diets. However, similar to other models of lipodystrophy, the resistance to diet-induced obesity exhibited by Pref-1 transgenic mice did not prevent the deleterious effects associated with feeding of a high-fat diet, such as hyperlipidemia and insulin resistance. Indeed, compared with Wt littermates, Pref-1 transgenic mice showed an aggravated degree of whole-body insulin resistance with higher circulating lipid levels. A generalized decrease in adipose tissue mass together with insulin resistance are defining traits of lipodystrophy (28). In this sense, Pref-1 transgenic mice may represent a novel rodent model of partial lipodystrophy.
It is evident that chronic feeding of a high-fat diet promoted development of glucose intolerance and insulin resistance in both Wt and Pref-1 transgenic animals. This is illustrated, for instance, by the very low overall glucose infusion rate required to maintain euglycemia during hyperinsulinemic-euglycemic clamp in Wt and Pref-1 transgenic mice fed a high-fat diet, compared with Wt mice fed a standard chow diet (10% kcal fat) (data not shown). Indeed, in mice fed a high-fat diet, glucose infusion rate oscillated between 12.1 and 5.4 mg · kg−1 · min−1 in Wt and Pref-1 Tg mice, respectively (Fig. 3B), whereas the glucose infusion rate required for maintaining euglycemia in a cohort of Wt mice fed a normal chow diet for the same period was approximately three- to sixfold higher (data not shown). In this sense, the lack of effect of insulin in inhibiting hepatic glucose production in both Wt and Pref-1 Tg mice indicates the presence of severe hepatic insulin resistance in both groups. This is supported by a weak phosphorylation of hepatic IRS-2 and Akt upon insulin stimulation and similar Akt activity in liver of both groups, compared with those observed in other tissues. Also, the similar degree of activation of the insulin-signaling pathway in liver of Wt and Tg mice, together with similar levels of gluconeogenic gene expression in fed Wt and Tg mice, suggests a comparable degree of hepatic insulin resistance with a minor contribution of liver to the differential whole-body insulin sensitivity exhibited by Pref-1 Tg mice. On the other hand, overexpression of Pref-1 clearly worsens insulin sensitivity in skeletal muscle, which is considered to be the primary tissue for insulin-mediated glucose disposal, as well as in adipose tissue, as demonstrated by a decrease in glucose uptake, decreased insulin-stimulated IRS and Akt phosphorylation, and decreased Akt activity. Thus, the differential degree of insulin resistance in these two insulin-sensitive tissues of Pref-1 transgenic mice could account for the observed impairment in whole-body glucose tolerance and insulin sensitivity in Pref-1 transgenic mice compared with Wt mice when fed a high-fat diet.
Alterations in adipocyte functions, such as the capacity to store lipids and to secrete adipokines, have been proven to have major repercussions in the ability of the organism to regulate substrate fluxes in tissues and undoubtedly have impacts on the onset of insulin resistance. Ectopic accumulation of lipids in insulin-sensitive tissues other than adipose tissue is considered a major cause of the development of insulin resistance (2). There is a strong correlation between intramyocellular triglyceride accumulation and insulin resistance (29,30). However, recent studies have shown that lipid metabolism intermediates other than triglycerides, including diacylglycerols (26,27,31), fatty acyl-CoAs (24,32), and ceramides (33,34), could be directly responsible for the inhibition of insulin signaling in the insulin-resistant state. In this regard, compared with Wt mice, Pref-1 transgenic mice show a significant accumulation of diacylglycerols, but not fatty acyl-CoAs, triacylglycerols, or ceramides, in gastrocnemius muscle, identifying DAG as a potential candidate for the inhibition of insulin signaling in muscle of Pref-1 Tg mice. The mechanisms by which mice overexpressing Pref-1 accumulate DAG preferentially in muscle but not in other insulin-sensitive tissues are still unclear, although the high expression of the fatty acid transporter CD36 in muscle could be a factor contributing to lipid accumulation and insulin resistance in this tissue (35,36). Nevertheless, a causative relationship between increased DAG accumulation and the development of insulin resistance in muscle of Pref-1 Tg mice cannot be unequivocally established, and other possible factors may need to be taken into account. For instance, the inability of adipocytes from Pref-1 Tg mice to secrete adequate levels of insulin-sensitizing adipokines, such as leptin and adiponectin, could also contribute to the insulin resistance observed in Pref-1 lipodystrophic mice. In this regard, future experiments of leptin and/or adiponectin replacement are needed to elucidate the precise role that alteration in leptin/adiponectin secretion plays in the development of insulin resistance in Pref-1 Tg mice. In addition, we have observed an increase in the expression of inflammatory cytokines, such as IL-6 and IL-1β but not tumor necrosis factor-α, in WAT of Pref-1 Tg mice, although such an increase does not seem to be accompanied by macrophage infiltration (assessed by expression of F4/80) (Supplemental Fig. 1, available in an online appendix at http://dx.doi.org/10.2337/db07-1739). Hyperlipidemia is often associated with the activation of inflammatory pathways and the appearance of insulin resistance (rev. in 37). Therefore, besides ectopic lipid accumulation or decreased endocrine function, a contribution of inflammatory pathways to the insulin resistance of Pref-1 Tg mice cannot be excluded.
The dysregulated lipid metabolism and the resulting alterations in glucose homeostasis in Pref-1 transgenic mice are attributable to the effects that Pref-1 has on the adipose tissue development, which is the major target of Pref-1 action. Our previous studies have unequivocally demonstrated the critical role of Pref-1 in repressing preadipocyte differentiation into adipocytes (14,17,38). In vivo, repression of adipocyte differentiation by Pref-1 is manifested by reduced expression of mature adipocyte markers in WAT (19), including C/EBPα, aFABP, or SCD, and the consequent reduction in the ability to store triglycerides and to secrete adipokines such as leptin and adiponectin (Fig. 2 and Table 1). The reduction in fat mass associated with high circulating levels of Pref-1 is not constrained only to our transgenic model, but naturally occurring mutations that affect the expression of Pref-1 result in a similar phenotype in other species. Indeed, in sheep, a mutation of the intergenic region of chromosome 18 located between genes encoding for Pref-1—also known as dlk1 (39)—and the noncoding gene Gtl2 increases the expression of the Pref-1/dlk1 gene. The mutation results in the callipyge phenotype, which is characterized by pronounced muscle hypertrophy and reduction of fat mass (40,41). Also, in pigs, a polymorphism in the Pref-1/dlk1 gene resulting in increased Pref-1 expression causes a decrease in fat deposition as well as an increase in lean muscle mass (42). Although we have not observed muscle hypertrophy in Pref-1 transgenic mice, our studies clearly suggest that the decreased fat mass observed in these models could be due to the inhibitory effect of Pref-1 on adipocyte differentiation. Similar phenotype, although more severe, has been observed in different rodent models for total or partial lipodystrophy, including PPARγ2-KO (43), conditional PPARγldi KO (44), FAT-ATTAC mouse (45), aP2-DTA mouse (46,47), aP2-nSREBP-1c (48), and aP2 A-ZIP/F1 fatless (49). These rodent models underscore the role of adipose tissue as an integrator and critical regulator of energy and glucose homeostasis in the organism. Most of these genetically engineered mice are good models for the study of severe or total lipodystrophy, but to date, only PPARγ2 KO and aP2-nSREBP-1c constitute acceptable models for partial lipodystrophy. The analogy between Pref-1 transgenic mice and the rodent models that are totally or partially devoid of adipose tissue allows us to propose Pref-1 transgenic mice as a new additional model for partial lipodystrophy.
In humans, a strong correlation between the severity of insulin resistance and the extent of loss of adipose tissue has also been observed. So far, few genes responsible for human lipodystrophies have been identified. These include BSCL2/seipin and AGPAT2, which are associated with the development of generalized lipodystrophy, as well as lamin A/C and PPARγ, which have been found to cause partial lipodystrophy. Yet, no direct association has been established between the expression of dlk1, the human homolog of Pref-1, and the appearance of congenital lipodystrophies. Interestingly, Pref-1 expression is increased in adipose tissue of HIV-1–infected patients that suffer from highly active antiretroviral treatment (HAART)-associated lipodystrophy (50), a syndrome that has a high incidence (between 25 and 50%) and is characterized not only by wasting of subcutaneous fat, but also by hyperlipidemia and insulin resistance (51). Although the possible implication of Pref-1/dlk1 in human lipodystrophic syndromes is very suggestive, further studies will be necessary to firmly establish the function of Pref-1 on the development of adipose tissue disorders in humans.
In conclusion, our present study shows that high circulating levels of the soluble form of Pref-1 prevents high-fat diet–induced obesity but exacerbates insulin resistance, which is associated with accumulation of DAG and decreased insulin sensitivity in skeletal muscle and WAT. Mice overexpressing Pref-1 exhibit all the characteristics of other lipodystrophic models, including reduced adipose tissue mass, dyslipidemia, and insulin resistance and therefore could be considered as a model for the study of partial lipodystrophies.
Supplementary Material
Acknowledgments
This research was supported by grants RO1 DK-50828 and DK-75682 to H.S.S. and grants RO1 DK-40936, U24 DK-76169, and P30 DK-45735 to G.I.S. G.I.S. is an investigator of The Howard Hughes Medical Institute and the recipient of a Distinguished Clinical Investigator Award from the American Diabetes Association.
Published ahead of print at http://diabetes.diabetesjournals.org on 3 October 2008.
J.A.V., C.S.C., and Y.W. contributed equally to this work.
J.A.V. is currently affiliated with the Metabolism and Obesity Group, Hospital Universitari Vall d’Hebron Research Institute, Barcelona, Spain. C.S.C. is currently affiliated with the Laboratory of Cellular and Molecular Physiology and Metabolism, Lee Gil Ya Cancer and Diabetes Institute, Gachon University of Medicine and Science, Incheon, Korea.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Smyth S, Heron A: Diabetes and obesity: the twin epidemics. Nat Med 12 :75 –80,2006 [DOI] [PubMed] [Google Scholar]
- 2.Shulman GI: Cellular mechanisms of insulin resistance. J Clin Invest 106 :171 –176,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kershaw EE, Flier JS: Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89 :2548 –2556,2004 [DOI] [PubMed] [Google Scholar]
- 4.Villena JA, Kim KH, Sul HS: Pref-1 and ADSF/resistin: two secreted factors inhibiting adipose tissue development. Horm Metab Res 34 :664 –670,2002 [DOI] [PubMed] [Google Scholar]
- 5.Rosen ED, Spiegelman BM: Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444 :847 –853,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerre-Millo M: Adipose tissue and adipokines: for better or worse. Diabete Metab 30 :13 –19,2004 [DOI] [PubMed] [Google Scholar]
- 7.Garg A: Acquired and inherited lipodystrophies. N Engl J Med 350 :1220 –1234,2004 [DOI] [PubMed] [Google Scholar]
- 8.Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O’Rahilly S, Trembath RC: LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 24 :153 –156,2000 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A: AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31 :21 –23,2002 [DOI] [PubMed] [Google Scholar]
- 10.Magre J, Delepine M, Khallouf E, Gedde-Dahl T Jr, Van Maldergem L, Sobel E, Papp J, Meier M, Megarbane A, Bachy A, Verloes A, d’Abronzo FH, Seemanova E, Assan R, Baudic N, Bourut C, Czernichow P, Huet F, Grigorescu F, de Kerdanet M, Lacombe D, Labrune P, Lanza M, Loret H, Matsuda F, Navarro J, Nivelon-Chevalier A, Polak M, Robert JJ, Tric P, Tubiana-Rufi N, Vigouroux C, Weissenbach J, Savasta S, Maassen JA, Trygstad O, Bogalho P, Freitas P, Medina JL, Bonnicci F, Joffe BI, Loyson G, Panz VR, Raal FJ, O’Rahilly S, Stephenson T, Kahn CR, Lathrop M, Capeau J: Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28 :365 –370,2001 [DOI] [PubMed] [Google Scholar]
- 11.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S: Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402 :880 –883,1999 [DOI] [PubMed] [Google Scholar]
- 12.Gregoire FM, Smas CM, Sul HS: Understanding adipocyte differentiation. Physiol Rev 78 :783 –809,1998 [DOI] [PubMed] [Google Scholar]
- 13.Rosen ED, Spiegelman BM: Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16 :145 –171,2000 [DOI] [PubMed] [Google Scholar]
- 14.Smas CM, Sul HS: Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73 :725 –734,1993 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Sul HS: Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol Cell Biol 26 :5421 –5435,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KA, Kim JH, Wang Y, Sul HS: Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol 27 :2294 –2308,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mei B, Zhao L, Chen L, Sul HS: Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem J 364 :137 –144,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, Sul HS: Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol 22 :5585 –5592,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS: Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1). J Clin Invest 111 :453 –461,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM: Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55 :2042 –2050,2006 [DOI] [PubMed] [Google Scholar]
- 21.Kim YB, Shulman GI, Kahn BB: Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C lambda/zeta but not on glycogen synthase kinase-3. J Biol Chem 277 :32915 –32922,2002 [DOI] [PubMed] [Google Scholar]
- 22.Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37 :911 –917,1959 [DOI] [PubMed] [Google Scholar]
- 23.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI: Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2 :55 –65,2005 [DOI] [PubMed] [Google Scholar]
- 24.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277 :50230 –50236,2002 [DOI] [PubMed] [Google Scholar]
- 25.Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, Biden TJ: Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes 46 :169 –178,1997 [DOI] [PubMed] [Google Scholar]
- 26.Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, Chen Y, Yu C, Moore IK, Reznick RM, Higashimori T, Shulman GI: Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest 117 :1995 –2003,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51 :2005 –2011,2002 [DOI] [PubMed] [Google Scholar]
- 28.Agarwal AK, Garg A: Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med 57 :297 –311,2006 [DOI] [PubMed] [Google Scholar]
- 29.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH: Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46 :983 –988,1997 [DOI] [PubMed] [Google Scholar]
- 30.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L: Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48 :1600 –1606,1999 [DOI] [PubMed] [Google Scholar]
- 31.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Khan M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI: Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282 :22678 –22688,2007 [DOI] [PubMed] [Google Scholar]
- 32.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI: Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103 :253 –259,1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ: Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53 :25 –31,2004 [DOI] [PubMed] [Google Scholar]
- 34.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5 :167 –179,2007 [DOI] [PubMed] [Google Scholar]
- 35.Hajri T, Han XX, Bonen A, Abumrad NA: Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109 :1381 –1389,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ: Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. Faseb J 18 :1144 –1146,2004 [DOI] [PubMed] [Google Scholar]
- 37.Wellen KE, Hotamisligil GS: Inflammation, stress, and diabetes. J Clin Invest 115 :1111 –1119,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smas CM, Chen L, Sul HS: Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol 17 :977 –988,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laborda J, Sausville EA, Hoffman T, Notario V: dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem 268 :3817 –3820,1993 [PubMed] [Google Scholar]
- 40.Freking BA, Murphy SK, Wylie AA, Rhodes SJ, Keele JW, Leymaster KA, Jirtle RL, Smith TP: Identification of the single base change causing the callipyge muscle hypertrophy phenotype, the only known example of polar overdominance in mammals. Genome Res 12 :1496 –1506,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smit M, Segers K, Carrascosa LG, Shay T, Baraldi F, Gyapay G, Snowder G, Georges M, Cockett N, Charlier C: Mosaicism of solid gold supports the causality of a noncoding A-to-G transition in the determinism of the callipyge phenotype. Genetics 163 :453 –456,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KS, Kim JJ, Dekkers JC, Rothschild MF: Polar overdominant inheritance of a DLK1 polymorphism is associated with growth and fatness in pigs. Mamm Genome 15 :552 –559,2004 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE: Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci U S A 101 :10703 –10708,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S, Huang LW, Snow KJ, Ablamunits V, Hasham MG, Young TH, Paulk AC, Richardson JE, Affourtit JP, Shalom-Barak T, Bult CJ, Barak Y: A mouse model of conditional lipodystrophy. Proc Natl Acad Sci U S A 104 :16627 –16632,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE: Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 11 :797 –803,2005 [DOI] [PubMed] [Google Scholar]
- 46.Ross SR, Graves RA, Spiegelman BM: Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev 7 :1318 –1324,1993 [DOI] [PubMed] [Google Scholar]
- 47.Burant CF, Sreenan S, Hirano K, Tai TA, Lohmiller J, Lukens J, Davidson NO, Ross S, Graves RA: Troglitazone action is independent of adipose tissue. J Clin Invest 100 :2900 –2908,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS: Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 12 :3182 –3194,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C: Life without white fat: a transgenic mouse. Genes Dev 12 :3168 –3181,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giralt M, Domingo P, Guallar JP, Rodriguez de la Concepcion ML, Alegre M, Domingo JC, Villarroya F: HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV-1/HAART-associated lipodystrophy. Antivir Ther 11 :729 –740,2006 [PubMed] [Google Scholar]
- 51.Koutkia P, Grinspoon S: HIV-associated lipodystrophy: pathogenesis, prognosis, treatment, and controversies. Annu Rev Med 55 :303 –317,2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.