Abstract
OBJECTIVE—Many of the effects of angiotensin (Ang) II are mediated through specific plasma membrane receptors. However, Ang II also elicits biological effects from the interior of the cell (intracrine), some of which are not inhibited by Ang receptor blockers (ARBs). Recent in vitro studies have identified high glucose as a potent stimulus for the intracellular synthesis of Ang II, the production of which is mainly chymase dependent. In the present study, we determined whether hyperglycemia activates the cardiac intracellular renin-Ang system (RAS) in vivo and whether ARBs, ACE, or renin inhibitors block synthesis and effects of intracellular Ang II (iAng II).
RESEARCH DESIGN AND METHODS—Diabetes was induced in adult male rats by streptozotocin. Diabetic rats were treated with insulin, candesartan (ARB), benazepril (ACE inhibitor), or aliskiren (renin inhibitor).
RESULTS—One week of diabetes significantly increased iAng II levels in cardiac myocytes, which were not normalized by candesartan, suggesting that Ang II was synthesized intracellularly, not internalized through AT1 receptor. Increased intracellular levels of Ang II, angiotensinogen, and renin were observed by confocal microscopy. iAng II synthesis was blocked by aliskiren but not by benazepril. Diabetes-induced superoxide production and cardiac fibrosis were partially inhibited by candesartan and benazepril, whereas aliskiren produced complete inhibition. Myocyte apoptosis was partially inhibited by all three agents.
CONCLUSIONS—Diabetes activates the cardiac intracellular RAS, which increases oxidative stress and cardiac fibrosis. Renin inhibition has a more pronounced effect than ARBs and ACE inhibitors on these diabetes complications and may be clinically more efficacious.
Involvement of the renin-angiotensin (Ang) system (RAS) in human pathophysiology has expanded to include several diseases beyond a traditional role in saltwater homeostasis (1). In diabetes, there is significant overactivity of the RAS, which is reversed by treatment with RAS inhibitors, thus decreasing diabetes complications (2). Activation of the RAS in diabetes includes activation of new components, such as the pro(renin) receptor (3), and Ang II–independent effects, mediated through interaction of pro(renin), with the pro(renin) receptor (4). Although circulating renin and Ang II levels are reduced in diabetes, prorenin levels are enhanced severalfold (5,6). Prorenin may have dual effects, providing for generation of Ang I at tissue sites through receptor-mediated nonproteolytic activation and directly through activation of receptor-mediated signaling pathways (4,7,8). Ang II–independent RAS actions suggest that efficacy of RAS inhibitors, Ang receptor blockers (ARBs), and ACE inhibitors would have limitations in hyperglycemic conditions. Recent meta-analyses of clinical trials have suggested that currently used RAS blockers may not provide additional benefits in diabetic compared with nondiabetic patients (9,10).
We recently reported a novel aspect of the RAS, the intracellular RAS, having identified an intracellular or intracrine system (11,12). In cardiac myocytes and fibroblasts, we demonstrated the presence of RAS components and synthesis of Ang II intracellularly (13,14). Hyperglycemia selectively upregulates the intracellular system in cardiac myocytes, vascular smooth muscle cells (VSMCs), and renal mesangial cells, where Ang II synthesis is largely catalyzed by chymase, not ACE (14–18). We and others have previously reported that intracellular Ang II (iAng II) elicits biological effects, some of which are not blocked by ARBs (19–22). These observations further support the speculation that currently available RAS inhibitors may not provide the anticipated cardiovascular benefits in diabetic conditions (23). In this study, we have examined the activation of the cardiac intracellular RAS in a rat model of diabetes. We also determined the role of iAng II in diabetes-induced oxidative stress, cardiac myocyte apoptosis, and cardiac fibrosis and the efficacy of different RAS blockers under hyperglycemic conditions.
RESEARCH DESIGN AND METHODS
All animal use was approved by the Institutional Animal Care and Use Committee of the Texas A&M Health Science Center. The AT1 receptor blocker candesartan was obtained from AstraZeneca (Wilmington, DE); the renin inhibitor aliskiren was from Novartis (Cambridge, MA); the ACE inhibitor benazepril was from Sigma; and insulin (Humulin N) was from Eli Lilly (Indianapolis, IN).
Induction of diabetes and treatment of animals.
Diabetes was induced by a single injection of streptozotocin (STZ, 65 mg/kg body wt i.p.) dissolved in 0.1 mol/l sodium citrate–buffered saline (pH 4.5), in adult male Sprague Dawley rats (250–300 g). Control animals received buffered saline alone. Diabetes was confirmed by sustained blood glucose levels >15 mmol/l, as determined 48 h after STZ injection and on alternate days thereafter. Diabetic rats, in groups of nine animals, were treated with insulin (2–5 units s.c., twice daily), candesartan (1 mg/kg i.p.), aliskiren (30 mg/kg orally), or benazepril (10 mg/kg orally) daily for 7 days beginning 48 h after STZ injection. Twenty-four hours after the last treatment, animals were weighed and anesthetized using ketamine/xylazine (50/5 mg/kg), and hearts were isolated and weighed before perfusion, the latter using the Langendorff methodology.
Isolation of cardiac myocytes and measurement of iAng II.
Hearts were isolated and perfused with Krebs-Henseleit bicarbonate buffer, followed by digestion with 0.1% (wt/vol) collagenase II. Myocytes were separated from nonmyocytes by differential centrifugation at 25g. The purity of the myocyte preparations using this procedure was >90%, as analyzed by fluorescence-activated cell sorting, using anti-sarcomeric myosin (MF-20) and anti-sarcomeric actin antibody. The pellet containing myocytes was processed for Ang II extraction, as described previously (14). Briefly, cells were lysed in ice-cold 1 mol/l acetic acid containing a protease inhibitor cocktail (Sigma) by brief sonication. The lysate was sedimented at 20,000g for 10 min, and the supernatant was dried in a vacufuge, followed by reconstitution in 1% acetic acid. The samples were applied to a conditioned DSC-18 column (Supelco), washed, and eluted with methanol. The eluted samples were dried and reconstituted in PBS for enzyme-linked immunosorbent assay (ELISA). For isolation of Ang II from plasma, an equal volume of 2% acetic acid was added to plasma, followed by filtration through Amicon Ultra-15 filters. The filtrate was applied to DSC-18 columns, and Ang II was eluted as described for the cell lysates. Using the above procedure, we have obtained >90% recovery of exogenously added Ang II. Ang II was measured by quantitative, competitive ELISA, using a specific anti–Ang II antibody (Peninsula Labs), which was previously validated by high-performance liquid chromatography–chip/mass spectrometric analysis (14). ELISA was performed on protein-A and anti–Ang II antibody–coated 96-well dishes. Competitive binding of synthetic biotinylated Ang II, in the presence of the extracted peptide, was detected with streptavidin–horseradish peroxidase conjugate. A standard curve, generated from binding of a constant amount of biotinylated Ang II with increasing concentrations of nonbiotinylated synthetic Ang II, was used to calculate the concentration of the peptide in the sample. The concentration of Ang II in the cell lysates is expressed as femtomoles per milligram protein and in plasma as femtomoles per milliliter.
Immunohistochemistry of RAS components.
Hearts were frozen in OCT compound (Tissue-Tek; Sakura Finetek) at −80°C for immunofluorescence staining of Ang II, renin, and anti-angiotensinogen (AGT). Frozen tissue was cut into 5-μm sections, which were air-dried, fixed with 4% formaldehyde, and permeabilized using 0.2% Triton X-100. Nonspecific binding was blocked by 5% BSA for 1 h at room temperature. The sections were incubated with anti–Ang II antibody (1:100; Peninsula Labs), anti-renin antibody (1:100; gift from Dr. Tadashi Inagami [Vanderbilt University, Nashville, TN]), or AGT antibody (1:500; Swant). The sections were costained for anti-sarcomeric actin and laminin, where indicated. After washings, the sections were incubated with respective secondary antibodies. Specificity of the staining was determined by preadsorption of primary antibodies with the antigen or by using secondary antibody alone. Images were acquired with a confocal fluorescence microscope (Olympus Fluoview 300). Fluorescence intensities in tissue sections were determined by digital microscopy software (Slide Book 4.2) after subtracting background fluorescence.
Cardiac myocyte apoptosis.
Apoptotic cardiac myocytes were detected in paraffin-embedded heart sections using the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay and cleaved caspase-3 staining. TUNEL assay was performed using an assay kit (Millipore, Temecula, CA) per the manufacturer's instructions. Cytoplasm and nuclei from the myocytes were counterstained using anti-sarcomeric actin antibody and DAPI, respectively. For cleaved caspase-3 staining, deparaffinized sections were subjected to antigen retrieval in 0.01 mol/l citrate buffer (pH 6.0) by microwaving. After blocking with 5% BSA, the sections were incubated with rabbit monoclonal anti–cleaved caspase-3 antibody (1:200; Cell Signaling Technology, Danvers, MA) overnight at 4°C, followed by fluorescein isothiocyanate–conjugated goat anti-rabbit IgG (1:200; Molecular Probes). The number of positively stained nuclei was counted from 20 fields per heart (∼25,000 cells) and three hearts per treatment group.
Reactive oxygen species detection in the heart.
Superoxide production in the hearts was detected by dihydroethidium (DHE) staining (Sigma-Aldrich). Frozen heart sections (20 μm thick) were incubated with 10 μmol/l DHE at 37°C for 45 min in a humidified chamber protected from light. Fluorescent images obtained with an Olympus FV300 confocal microscope were analyzed with Slide Book 4.2. The mean DHE fluorescence intensity of myocyte nuclei was calculated by dividing the combined fluorescence value of the pixels by the total number of pixels in 15 randomly selected fields observed with identical laser and photomultiplier settings.
Cardiac fibrosis.
Cardiac interstitial fibrosis was determined by Masson's trichrome staining on 5-μm paraffin-embedded sections. The extent and degree of fibrosis was subjectively graded on a scale of 0–4. Grade 0 signified no apparent collagen fiber proliferation except for small islets of fibrous tissue around the capillaries, as well as an intercellular single layer of collagenous tissue, as in normal myocardium. Focal and minimal fibrosis was graded as 1, mild patchy fibrosis as grade 2, moderate diffuse fibrosis as grade 3, and the most prominent fibrosis, covering a major area of the specimen, was classified as 4. A minimum of three sections per heart with five fields per section and three animals per experimental group were analyzed, and results are presented as an average grade.
Statistical analysis.
Values are expressed as means ± SE. ANOVA with Tukey's post hoc test was used for statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
Hyperglycemia increases intracellular levels of Ang II in cardiac myocytes from diabetic heart.
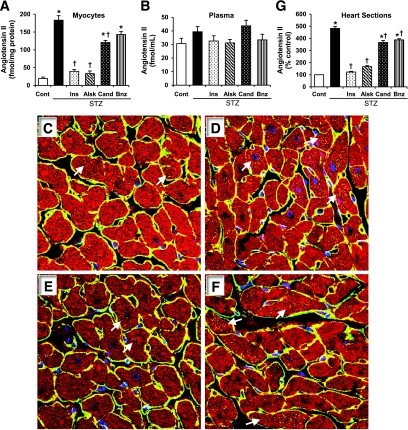
Diabetes was induced in adult male rats by STZ injection. One group of diabetic animals was treated with insulin to confirm that the observed effects in the experimental groups were secondary to hyperglycemia. One week of diabetes significantly reduced body and heart weights, which were normalized by insulin treatment but not by any of the RAS inhibitors (Table 1). However, no significant effect on the heart-to-body weight ratio (Table 1) or plasma Ang II levels (Fig. 1B) was observed, which is consistent with previous reports (24,25). Ang II levels in cardiac myocytes, which were isolated after perfusion of the hearts and enzymatic dispersion, represented Ang II present intracellularly. To determine the source of iAng II, i.e., intracellular synthesis or AT1-mediated internalization, one group of diabetic animals was treated with the AT1 antagonist candesartan to prevent receptor-mediated uptake. As shown in Fig. 1A, cardiac myocytes from diabetic rat hearts demonstrated a 9.4-fold elevation in the levels of iAng II (183 ± 13 fmol/mg protein) compared with cells from control animals (19 ± 4 fmol/mg protein). Normalization of blood glucose levels by insulin in rats administered STZ completely blocked the rise in iAng II levels, indicating that the latter was a specific effect of hyperglycemia. Treatment of diabetic rats with candesartan partially reduced iAng II levels, suggesting that the major source of iAng II was intracellular synthesis, which is consistent with our previous report in neonatal rat ventricular myocytes (NRVMs) (14).
TABLE 1.
Effect of diabetes on body and heart weight
| Control | Diabetic |
|||||
|---|---|---|---|---|---|---|
| No treatment | Insulin | Aliskiren | Candesartan | Benazepril | ||
| Body weight (g) | 337 ± 10 | 263 ± 8.3* | 307 ± 4.7 | 248 ± 8.5* | 285 ± 5.9* | 267 ± 6.9* |
| Heart weight (mg) | 1,168 ± 54 | 933 ± 33* | 1,074 ± 27 | 867 ± 37* | 988 ± 15* | 901 ± 16* |
| Heart weight/body weight (mg/g) | 3.46 ± 0.1 | 3.55 ± 0.1 | 3.49 ± 0.1 | 3.50 ± 0.1 | 3.47 ± 0.1 | 3.37 ± 0.1 |
Data are means ± SE (n = 9) after 1 week of induction of diabetes.
P < 0.05 vs. control.
FIG. 1.
Ang II levels in cardiac myocytes and plasma. Ang II was measured by a competitive ELISA in cardiac myocytes (A) and plasma (B) of control rats (Cont); diabetic rats (STZ); and diabetic rats treated with insulin (Ins), aliskiren (Alsk), candesartan (Cand), or benazepril (Bnz). Values are expressed as means ± SE, n = 6. C–F: Intracellular localization of Ang II (yellow dots, indicated by white arrow), as determined by confocal immunofluorescence microscopy, in heart sections from control rats (C), diabetic rats (D), diabetic rats treated with aliskiren (E), and diabetic rats treated with candesartan (F). Myocyte profiles were identified by costaining with anti-sarcomeric actin (red) and laminin (yellow, peripheral staining). The blue color indicates nuclear staining by DAPI. Magnification ×1,200. G: Quantitative representation of Ang II fluorescence intensity in heart sections (from five images per heart and three hearts per group). Values are expressed as means ± SE, n = 15. *P < 0.05 vs. control, †P < 0.05 vs. diabetic rats without any treatment. (Please see http://dx.doi.org/10.2337/db08-0805 for a high-quality digital representation of this figure.)
Intracellular synthesis of Ang II is not blocked by an ACE inhibitor.
We and others had previously reported that several cell types (NRVMs, VSMCs, and renal mesangial cells) use alternative pathways to synthesize Ang II in high-glucose culture conditions (14–18). To determine the mechanism of hyperglycemia-induced cardiac iAng II synthesis in vivo, diabetic rats were treated with either a renin inhibitor (aliskiren) or an ACE inhibitor (benazepril). As shown in Fig. 1A, aliskiren completely normalized (33 ± 7 fmol/mg protein) iAng II levels in diabetic rat cardiac myocytes, whereas benazepril did not have any effect (143 ± 8 fmol/mg protein). These results indicated that the observed increase in iAng II was not catalyzed by ACE, which is consistent with intracellular synthesis of Ang II. None of the RAS blocking drugs significantly altered plasma Ang II levels, which were measured 24 h after the last dose. The latter observation corroborated with the reported 2- to 6-h period for reactive changes in plasma levels of Ang II, which returned to baseline between 14 and 30 h after drug intake (26–28). The lack of increase in plasma Ang II levels in any of the treatment groups further indicated that the increase in cardiac myocyte Ang II levels was due to local synthesis.
Immunohistochemical localization of Ang II in rat heart.
To further confirm elevation of Ang II in cardiac myocytes and intracellular localization, frozen heart sections were immunostained with anti–Ang II antibody and visualized using confocal microscopy. Sections were counterstained for laminin to mark cell boundaries (peripheral green/yellow staining in merged images), anti-sarcomeric actin to identify cardiac myocytes (red), and DAPI to identify nuclei (blue). Significantly increased levels of Ang II staining, which colocalized with anti-sarcomeric actin, were observed in diabetic rat hearts (Fig. 1C–F). Quantification of fluorescence intensity revealed about a fivefold increase in iAng II levels (Fig. 1G), consistent with the Ang II measurement by ELISA (Fig. 1A). Immunohistochemistry also confirmed that candesartan partially reduced iAng II levels, benazepril did not have any effect, and aliskiren completely prevented the increase in iAng II in diabetic hearts (Fig. 1G).
Intracellular localization of AGT and renin in cardiac myocytes.
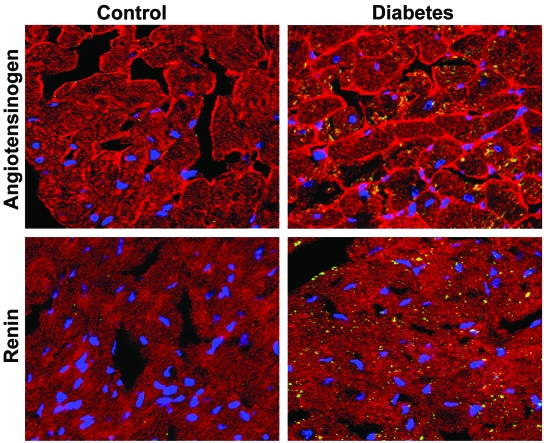
Intracellular synthesis of Ang II would require the presence of the precursor molecule AGT and processing enzyme, renin, intracellularly. To demonstrate this possibility, immunohistochemistry was performed on heart sections using anti-AGT and anti-renin antibodies, along with counterstaining for anti-sarcomeric actin, as described previously (24). Elevated levels of AGT and renin were apparent in cardiac myocytes of diabetic hearts (green/yellow staining) compared with control, confirming activation of the intracellular RAS in hyperglycemic conditions (Fig. 2).
FIG. 2.
Representative confocal immunofluorescence images of AGT and renin staining in hearts from control and diabetic rats. Pictures shown are merged images of staining for anti-sarcomeric actin (red), laminin (peripheral red staining in top), AGT (top) or renin (bottom) (green and yellow staining), and nuclei (blue). Magnification ×900. (Please see http://dx.doi.org/10.2337/db08-0805 for a high-quality digital representation of this figure.)
iAng II is correlated with hyperglycemia-induced oxidative stress.
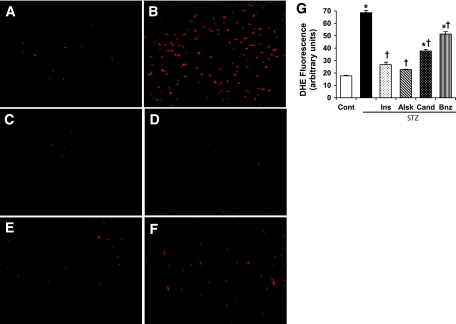
Hyperglycemia is known to induce myocardial oxidative stress, which may be related to glucose metabolism or activation of cytokines and other hormones. To determine whether there was a role of iAng II, superoxide production was detected by DHE staining in frozen heart sections of diabetic rats treated with different RAS inhibitors. As shown in Fig. 3, diabetic hearts showed enhanced superoxide production, which was prevented in insulin-treated animals. Treatment of diabetic rats with candesartan or benazepril significantly, but not completely, reduced oxidative stress, whereas aliskiren blocked completely. Our previous studies had indicated that ARBs and ACE inhibitors were ineffective in blocking the intracellular RAS, unlike a renin inhibitor that blocks both the intracellular and extracellular systems (14,19,22). The observed partial efficacy of candesartan and benazepril strongly suggested that iAng II contributed to hyperglycemia-induced oxidative stress in the myocardium.
FIG. 3.
Measurement of oxidative stress in heart sections by DHE staining. These are representative images of DHE-stained heart sections from control rats (A); diabetic rats (B); and diabetic rats treated with insulin (C), aliskiren (D), candesartan (E), or benazepril (F). Magnification ×60. G: DHE fluorescence intensity was calculated from five images per heart and three hearts per group. Values are expressed as means ± SE (n = 15). *P < 0.05 vs. control, †P < 0.05 vs. diabetic rats without any treatment. (Please see http://dx.doi.org/10.2337/db08-0805 for a high-quality digital representation of this figure.)
iAng II is correlated with hyperglycemia-induced cardiac myocyte apoptosis.
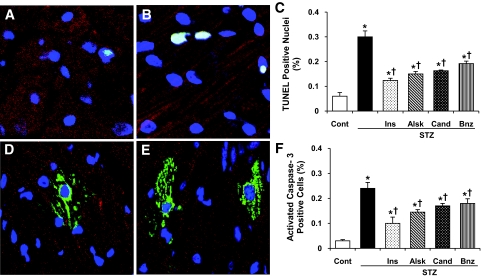
Cardiac myocyte apoptosis was determined by TUNEL assay and activated caspase-3 immunostaining. Figure 4 shows an increased number of apoptotic cells in the heart sections. Quantification of apoptotic cells (Fig. 4C and F) showed a five- to eightfold increase in diabetic hearts compared with control by both TUNEL assay and caspase-3 staining. Normalization of blood glucose by insulin or blockade of the RAS with the three different inhibitors significantly reduced the number of apoptotic cells but did not prevent apoptosis completely. There was a significant difference between aliskiren- and benazepril-treated animals, with aliskiren being more protective.
FIG. 4.
Detection of apoptosis in cardiac myocytes by TUNEL assay and cleaved caspase-3 staining. A and B: TUNEL assay on heart sections from control (A) and diabetic (B) rats. D and E: Staining for cleaved caspase-3 in the hearts of control (D) and diabetic (E) rats. C and F: Quantification of TUNEL+ (C) and cleaved caspase-3+ (F) cells (∼25,000 cells were counted in each case). Values are expressed as means ± SE. *P < 0.05 vs. control, †P < 0.05 vs. diabetic rats without any treatment. (Please see http://dx.doi.org/10.2337/db08-0805 for a high-quality digital representation of this figure.)
iAng II is correlated with hyperglycemia-induced cardiac fibrosis.
Cardiac fibrosis is an important pathogenic factor in diabetes-induced diastolic dysfunction. Paraffin-embedded heart sections were stained with Masson's Trichrome, and the degree of blue staining was evaluated on a scale of 0–4, as described in research design and methods. Even after only 1 week of diabetes, the overall staining for fibrosis was enhanced in hearts from diabetic rats (grade 1.5) compared with control animals (grade 0) (Fig. 5). Insulin treatment completely prevented the increase in fibrosis (grade 0.04). Candesartan and benazepril reduced the degree of fibrosis (grade 0.43 and 0.88, respectively), whereas aliskiren had a more pronounced reduction of fibrosis (grade 0.25) in diabetic rat hearts (Fig. 5).
FIG. 5.
Detection of cardiac fibrosis by Masson's Trichrome staining in heart sections from control rats (A); diabetic rats (B); and diabetic rats treated with insulin (C), aliskiren (D), candesartan (E), or benazepril (F). Representative images of five sections per heart and three hearts per group were observed with a ×40 objective. (Please see http://dx.doi.org/10.2337/db08-0805 for a high-quality digital representation of this figure.)
DISCUSSION
In this study, we observe a dramatic activation of the intracellular RAS, a novel aspect of the tissue RAS, in diabetic rat hearts. We also demonstrate that iAng II is correlated with the development of pathological conditions associated with diabetes. Significantly, we observed that blockade of the RAS by a renin inhibitor in diabetic rats provided greater protection from oxidative stress and cardiac fibrosis compared with inhibition with an AT1 antagonist or ACE inhibitor.
We first described a physiologically relevant intracellular RAS in NRVMs (14), defined as the presence of the precursor protein and enzymes and synthesis of Ang II inside the cell and which was coupled to a biological action (11). The regulation and separation of the intracellular from the extracellular RAS becomes very obvious in hyperglycemic conditions. High glucose promoted accumulation of AGT, renin, and Ang II intracellularly, resulting in a dramatic rise in iAng II concentrations without affecting extracellular Ang II levels (14). Similar to NRVMs, the intracellular accumulation of RAS components and iAng II synthesis have also been described in VSMCs and renal mesangial cells in high-glucose culture conditions (15,17,29). To extend the in vitro observations, we determined whether a similar activation of the intracellular RAS occurs in adult diabetic animals. We observed a significant increase in intracellular levels of AGT and renin and iAng II synthesis in diabetic rat hearts, as determined by ELISA and confocal immunocytochemistry. In a previous human study, a threefold increase in Ang II staining was described in hearts from diabetic compared with nondiabetic patients, which was enhanced an additional 2.5-fold in diabetic hypertensive patients (30). However, in that study, it was not clear whether the increased Ang II staining represented activation of an intracellular RAS or was due to internalization of extracellularly synthesized Ang II. We observed an increase in iAng II levels even in the presence of AT1 blockade with candesartan, which strongly supported activation of the intracellular RAS. The latter conclusion was further strengthened by an observed increase in intracellular staining for renin and AGT.
The observation of elevated Ang II levels after removal of the high-glucose stimulus, suggested that iAng II was highly stable. The reported half-life of Ang II in the heart is 15 min in vivo and 30 min ex vivo (31). In this regard, it is important to make a distinction between Ang II that was internalized versus that which was synthesized intracellularly, because the intracellular location is likely to be different, which could substantially affect the half-life. The half-life reported in the literature was for Ang II that was internalized through AT1 receptor (31), a major part of which was likely targeted to lysosomes for degradation (32). We measured Ang II that was synthesized intracellularly, which is most likely to occur in organelles or at sites that are not associated with protein degradation.
An interesting and therapeutically significant aspect of the intracellular RAS is that high glucose–induced iAng II synthesis in cardiac myocytes is catalyzed by chymase, not ACE. Chymase levels are significantly elevated in NRVMs after exposure to high glucose, whereas ACE levels remain unchanged (14). Similarly, no change in gene expression of ACE was observed in diabetic rat hearts (33). In the above-referenced human study (30), diabetic patients who had elevated iAng II levels were on ACE-inhibitor therapy. In the latter case, a rise in Ang II levels could be attributed to an “ACE-escape” phenomenon, which is believed to occur after prolonged treatment with ACE inhibitors (34). In the present study, the lack of reduction in iAng II levels after only 1 week of ACE-inhibitor treatment strongly suggests an ACE-independent mechanism of iAng II synthesis, corroborating in vitro observations. Upregulation of vascular chymase in diabetic patients and chymase-mediated Ang II generation in human and rat VSMCs and human mesangial cells has been previously reported (15,17,18,35). Involvement of chymase further strengthens the concept of an intracellular RAS in diabetes.
Renin inhibition by aliskiren completely prevented hyperglycemia-induced iAng II synthesis. The source of renin in the heart has been an issue of debate (36). Circulating prorenin levels are elevated severalfold in diabetes, which might also contribute to cardiac levels of pro(renin) (37). Cardiac myocytes have been shown to internalize and activate prorenin, which could contribute to iAng II synthesis (38,39). In addition, several reports have described expression of renin by cardiac myocytes, fibroblasts, and cardiac mast cells (13,40–42). Adult rat cardiac myocytes have been shown to express an intracellular form of prorenin, which lacked a portion of the preprofragment, eliminating the need for proteolytic activation (43). We have previously described a significant increase in intracellular renin levels in NRVMs exposed to high-glucose conditions (14). In the current study, we observed enhanced staining for renin in the heart of diabetic rats. Thus, inhibition of cardiac iAng II synthesis by aliskiren was consistent with these observations.
Although aliskiren is the most potent inhibitor of human renin (half-maximal inhibitory concentration [IC50] 0.6 nmol/l), it inhibits rat renin at higher concentrations (IC50 80 nmol/l) (44). We chose an aliskiren dose of 30 mg · kg−1 · day−1 based on significant blood pressure–lowering effects of this dose in spontaneously hypertensive rats (45). Pharmacokinetic studies of aliskiren in Sprague-Dawley rats (species used in this study) demonstrated that an oral dose of 30 mg/kg resulted in an area under the curve of 3.06 ± 1.8 μmol · l−1 · h−1, indicating sufficient drug in the bloodstream (45). Several additional characteristics of aliskiren could explain the observed effects in diabetic rats. These include cellular uptake, accumulation on multiple dosing, longer half-life, and rapid binding to renin with slow dissociation (46). We observed that neonatal rat cardiac myocytes internalized aliskiren in a concentration-dependent manner, levels of which were measured at 1, 24, and 48 h after addition to the culture medium. Maximum intracellular levels of aliskiren were observed at 24 h, which were not reduced even after 48 h (data not shown). Aliskiren levels were measured by liquid chromatography tandem mass spectrometry, as previously described (47). Similarly, rats that were treated with aliskiren at a dose of 10 mg · kg−1 · day−1 for 2 weeks, showed a kidney-to-plasma ratio of aliskiren in the range of 45 to 64 (47). The latter indicated extensive partitioning of aliskiren to the kidneys, which localized to glomeruli and the walls of small cortical arteries. Persistent renal protective effects of aliskiren after discontinuation of treatment also suggest slow clearance and accumulation in tissues (48). The above findings suggest similar partitioning of aliskiren in the heart, which might have resulted in sufficiently high intracellular levels of aliskiren to inhibit rat renin in our studies. Furthermore, tissue accumulation of aliskiren may result in effects on target organs, even at doses that do not affect blood pressure. This was evident from a recent study wherein 2.5 mg/kg aliskiren did not produce a statistically significant sustained reduction in blood-pressure but reduced atherosclerotic lesion size significantly in hypercholesterolemic Ldlr−/− mice (49). Higher doses of aliskiren (up to 50 mg/kg) produced similar results. In the double human renin-AGT transgenic rat model, a dose (of aliskiren) of 0.03 mg/kg, which did not decrease blood pressure, reduced albuminuria and cardiac hypertrophy (50).
iAng II has been shown to produce multiple biological actions, including cardiac hypertrophy (11,19). Many of the reported iAng II effects are not prevented by ARBs, either due to limited cell permeability of these drugs or to an AT1-independent mechanism of iAng II–mediated effects. We previously demonstrated that iAng II–induced NRVM cell growth and cardiac hypertrophy was not inhibited by ARBs (19). Proliferation of Chinese hamster ovary cells, which are deficient in AT1 receptor, demonstrated that some of the effects of iAng II do not require AT1 receptor (22). In addition, enhanced transforming growth factor-β/smad signaling was reported in kidneys from diabetic AT1-knockout mice (51). These findings, together with the observation that iAng II synthesis is chymase dependent, suggest that ARBs and ACE inhibitors do not block the intracellular RAS, which is activated in diabetes. A renin inhibitor prevents both intracellular and extracellular Ang II synthesis (14) and thus may prove more beneficial in diabetic conditions. Consistent with the latter hypothesis, we observed that aliskiren was more effective in preventing oxidative stress and cardiac fibrosis compared with candesartan or benazepril. Effects of aliskiren were unlikely to be mediated through Ang II–independent mechanisms because aliskiren does not inhibit renin binding to the (pro)renin receptor and extracellular signal–related kinase activation (8,47). The effect of these drugs on cardiac myocyte apoptosis demonstrated that aliskiren was significantly more beneficial than benazepril.
Reversal of cardiac effects in diabetic animals by insulin treatment indicates that the observed effects were due to hyperglycemia. Although STZ-induced diabetes is representative of type 1 diabetes, we also predict activation of the intracellular RAS in type 2 diabetes. This is based on our unpublished observations of no effect of insulin treatment on high glucose–induced iAng II synthesis in NRVMs and patients with type 2 diabetes, who showed enhanced iAng II staining in the heart (30).
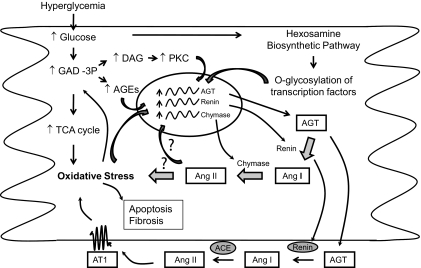
As depicted in Fig. 6, activation of the RAS appears to be a major event in hyperglycemia as a result of increased oxidative stress, increased protein kinase C (PKC) levels, and/or increased activity of the hexosamine biosynthesis pathway. iAng II could directly produce oxidative stress and cellular apoptosis through unidentified mechanisms and/or could enhance expression of RAS components through a positive feedback mechanism (52), resulting in enhanced extracellular Ang II levels as well, particularly via cardiac fibroblasts (13). Extracellular Ang II in turn causes oxidative stress, cardiac myocyte apoptosis, and cardiac fibrosis through the AT1 receptor. Interrupting this cycle by blocking Ang II synthesis protects from hyperglycemia-induced pathological events. ACE inhibitors or ARBs would block only the extracellular synthesis or actions, respectively, of Ang II; whereas a renin inhibitor would block both intra- and extracellular Ang II synthesis, the latter providing an explanation for the more pronounced effects of aliskiren observed in this study. In diabetic heart, the source and target of Ang II could be represented by multiple cell types. In addition to NRVMs, we previously demonstrated that cardiac fibroblasts respond to high glucose with enhanced activity of the RAS and extracellular matrix production (13). Cardiac fibroblasts increase both intracellular and extracellular Ang II, in contrast to cardiac myocytes, which demonstrate only increased iAng II in high-glucose conditions. Additionally, Ang II synthesis by cardiac fibroblasts, extracellular as well as intracellular, is catalyzed by ACE (13). Thus, ACE inhibitors would block Ang II synthesis by cardiac fibroblasts, and ARBs would block autocrine/paracrine effects of extracellular Ang II. This explains the partial effects of these agents in diabetic rats. However, these agents would not block high glucose–stimulated iAng II synthesis or intracellular actions in cardiac myocytes. In addition to a direct effect on cardiac myocytes, iAng II synthesized in cardiac myocytes would likely have indirect functional effects on other cells by stimulating synthesis and release of growth factors and cytokines from myocytes (53). Thus, the intracellular RAS possibly explains progression from microalbuminuria to proteinuria in diabetic patients on ACE inhibitor therapy, the mechanism of resistance to antihypertensive therapy in type 2 diabetes and higher cardiovascular morbidity and mortality in hypertensive patients with diabetes (10,54–56). Although ARBs and ACE inhibitors would provide some protection to the cardiovascular system through positive hemodynamic effects, partial inhibition of the local RAS, or other non-RAS–related mechanisms, such as an effect on peroxisome proliferator–activated receptor-γ and the kallikrein-kinin system (57–59); additional blockade of the intracellular RAS using agents such as a renin inhibitor might prove more beneficial in diabetes. Long-term studies that include cardiac functional analysis will be necessary to validate the above hypothesis.
FIG. 6.
Schematic representation of the relationship between hyperglycemia, iAng II, and pathological effects. In hyperglycemia, there is an increase in glucose oxidation through the tricarboxylic acid cycle in mitochondria, which results in enhanced generation of reactive oxygen species. Overproduction of superoxide inhibits glyceraldehyde-3-phosphate dehydrogenase activity, resulting in an accumulation of upstream metabolites of the glycolytic pathway. Increased levels of glyceraldehyde-3-phosphate (GAD-3P) cause activation of PKC isoforms through diacylglycerol (DAG) production and synthesis of advanced glycation end products (AGEs). There is increased shuttling of glucose through the hexosamine biosynthesis pathway, resulting in the modification of transcription factors through o-glycosylation. All of these products of hyperglycemia, i.e., oxidative stress, AGEs, PKC, and o-glycosylation of transcription factors, activate expression of RAS components. Cardiac myocytes synthesize and retain Ang II intracellularly in hyperglycemia, whereas cardiac fibroblasts increase both intra- and extracellular Ang II. iAng II could directly increase oxidative stress and cellular apoptosis through unidentified mechanisms and/or could enhance expression of RAS components through a positive feedback mechanism, resulting in enhanced extracellular Ang II levels as well, particularly via cardiac fibroblasts. Extracellular Ang II in turn causes oxidative stress, cardiac myocyte apoptosis, and cardiac fibrosis through the AT1 receptor. Interrupting this cycle by blocking Ang II synthesis provides protection from hyperglycemia-induced pathological events. ACE inhibitors or ARBs would block only the extracellular synthesis or actions, respectively, of Ang II; whereas a renin inhibitor would block both intra- and extracellular Ang II synthesis, the latter providing an explanation for the more pronounced effects of aliskiren observed in this study.
Supplementary Material
Acknowledgments
We thank the Texas A&M Health Science Center Microscopy Imaging Center for providing confocal microscopy services. R.K. has received an American Heart Association, Texas Affiliate, Beginning Grant-In-Aid.
Published ahead of print at http://diabetes.diabetesjournals.org on 1 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Paul M, Poyan Mehr A, Kreutz R: Physiology of local renin-angiotensin systems. Physiol Rev 86 :747 –803,2006 [DOI] [PubMed] [Google Scholar]
- 2.Connelly KA, Boyle AJ, Kelly DJ: Angiotensin II and the cardiac complications of diabetes mellitus. Curr Pharm Des 13 :2721 –2729,2007 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD: Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int 50 :1897 –1903,1996 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109 :1417 –1427,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M: Increased plasma inactive renin in diabetes mellitus: a marker of microvascular complications. N Engl J Med 312 :1412 –1417,1985 [DOI] [PubMed] [Google Scholar]
- 6.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J: The collecting duct is the major source of prorenin in diabetes. Hypertension 51 :1597 –1604,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saris JJ, ‘t Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH: Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48 :564 –571,2006 [DOI] [PubMed] [Google Scholar]
- 8.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN: Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51 :682 –688,2008 [DOI] [PubMed] [Google Scholar]
- 9.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, Woodward M, MacMahon S: Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 165 :1410 –1419,2005 [DOI] [PubMed] [Google Scholar]
- 10.Ficociello LH, Perkins BA, Silva KH, Finkelstein DM, Ignatowska-Switalska H, Gaciong Z, Cupples LA, Aschengrau A, Warram JH, Krolewski AS: Determinants of progression from microalbuminuria to proteinuria in patients who have type 1 diabetes and are treated with angiotensin-converting enzyme inhibitors. Clin J Am Soc Nephrol 2 :461 –469,2007 [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Singh VP, Baker KM: The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 18 :208 –214,2007 [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Singh VP, Baker KM: The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens 17 :168 –173,2008 [DOI] [PubMed] [Google Scholar]
- 13.Singh VP, Baker KM, Kumar R: Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol 294 :H1675 –H1684,2008 [DOI] [PubMed] [Google Scholar]
- 14.Singh VP, Le B, Bhat VB, Baker KM, Kumar R: High glucose induced regulation of intracellular angiotensin II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol 293 :H939 –H948,2007 [DOI] [PubMed] [Google Scholar]
- 15.Lavrentyev EN, Estes AM, Malik KU: Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res 101 :455 –464,2007 [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Leehey DJ: Effect of ACE inhibitors on angiotensin II in rat mesangial cells cultured in high glucose. Biochem Biophys Res Commun 357 :1040 –1045,2007 [DOI] [PubMed] [Google Scholar]
- 17.Cristovam PC, Arnoni CP, de Andrade MC, Casarini DE, Pereira LG, Schor N, Boim MA: ACE- and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med 233 :1035 –1043,2008 [DOI] [PubMed] [Google Scholar]
- 18.Koka V, Wang W, Huang XR, Kim-Mitsuyama S, Truong LD, Lan HY: Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation 113 :1353 –1360,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R: Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept 120 :5 –13,2004 [DOI] [PubMed] [Google Scholar]
- 20.Cook JL, Zhang Z, Re RN: In vitro evidence for an intracellular site of angiotensin action. Circ Res 89 :1138 –1146,2001 [DOI] [PubMed] [Google Scholar]
- 21.Filipeanu CM, Brailoiu E, Kok JW, Henning RH, De Zeeuw D, Nelemans SA: Intracellular angiotensin II elicits Ca2+ increases in A7r5 vascular smooth muscle cells. Eur J Pharmacol 420 :9 –18,2001 [DOI] [PubMed] [Google Scholar]
- 22.Baker KM, Kumar R: Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol 291 :C995 –C1001,2006 [DOI] [PubMed] [Google Scholar]
- 23.Weber MA, Giles TD: Inhibiting the renin-angiotensin system to prevent cardiovascular diseases: do we need a more comprehensive strategy? Rev Cardiovasc Med 7 :45 –54,2006 [PubMed] [Google Scholar]
- 24.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P: IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50 :1414 –1424,2001 [DOI] [PubMed] [Google Scholar]
- 25.Raimondi L, De Paoli P, Mannucci E, Lonardo G, Sartiani L, Banchelli G, Pirisino R, Mugelli A, Cerbai E: Restoration of cardiomyocyte functional properties by angiotensin II receptor blockade in diabetic rats. Diabetes 53 :1927 –1933,2004 [DOI] [PubMed] [Google Scholar]
- 26.Nussberger J, Wuerzner G, Jensen C, Brunner HR: Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension 39 :E1 –E8,2002 [DOI] [PubMed] [Google Scholar]
- 27.Delacretaz E, Nussberger J, Biollaz J, Waeber B, Brunner HR: Characterization of the angiotensin II receptor antagonist TCV-116 in healthy volunteers. Hypertension 25 :14 –21,1995 [DOI] [PubMed] [Google Scholar]
- 28.Juillerat L, Nussberger J, Menard J, Mooser V, Christen Y, Waeber B, Graf P, Brunner HR: Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension 16 :564 –572,1990 [DOI] [PubMed] [Google Scholar]
- 29.Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA: High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286 :F1039 –F1045,2004 [DOI] [PubMed] [Google Scholar]
- 30.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P: Myocardial cell death in human diabetes. Circ Res 87 :1123 –1132,2000 [DOI] [PubMed] [Google Scholar]
- 31.van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA: Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension 30 :42 –49,1997 [DOI] [PubMed] [Google Scholar]
- 32.Bianchi C, Gutkowska J, De Lean A, Ballak M, Anand-Srivastava MB, Genest J, Cantin M: Fate of [125I]angiotensin II in adrenal zona glomerulosa cells. Endocrinology 118 :2605 –2607,1986 [DOI] [PubMed] [Google Scholar]
- 33.Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J: Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II- dependent. Lab Invest 80 :513 –527,2000 [DOI] [PubMed] [Google Scholar]
- 34.van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, van Gilst WH, Voors AA: Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol 106 :367 –372,2006 [DOI] [PubMed] [Google Scholar]
- 35.Li M, Liu K, Michalicek J, Angus JA, Hunt JE, Dell’Italia LJ, Feneley MP, Graham RM, Husain A: Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest 114 :112 –120,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krop M, Danser AH: Circulating versus tissue renin-angiotensin system: on the origin of (pro)renin. Curr Hypertens Rep 10 :112 –118,2008 [DOI] [PubMed] [Google Scholar]
- 37.Franken AA, Derkx FH, Man in’t Veld AJ, Hop WC, van Rens GH, Peperkamp E, de Jong PT, Schalekamp MA: High plasma prorenin in diabetes mellitus and its correlation with some complications. J Clin Endocrinol Metab 71 :1008 –1015,1990 [DOI] [PubMed] [Google Scholar]
- 38.van Kesteren CA, Danser AH, Derkx FH, Dekkers DH, Lamers JM, Saxena PR, Schalekamp MA: Mannose 6-phosphate receptor-mediated internalization and activation of prorenin by cardiac cells. Hypertension 30 :1389 –1396,1997 [DOI] [PubMed] [Google Scholar]
- 39.Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MGF, Mullins JJ: Functional significance of prorenin internalization in the rat heart. Circ Res 90 :1135 –1141,2002 [DOI] [PubMed] [Google Scholar]
- 40.Peters J: Secretory and cytosolic (pro)renin in kidney, heart, and adrenal gland. J Mol Med 86 :711 –714,2008 [DOI] [PubMed] [Google Scholar]
- 41.Sanghi S, Kumar R, Smith M, Baker KM, Dostal DE: Activation of protein kinase A by atrial natriuretic peptide in neonatal rat cardiac fibroblasts: role in regulation of the local renin-angiotensin system. Regul Pept 132 :1 –8,2005 [DOI] [PubMed] [Google Scholar]
- 42.Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, Silver RB, Levi R: Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 116 :1063 –1070,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters J, Clausmeyer S: Intracellular sorting of renin: cell type specific differences and their consequences. J Mol Cell Cardiol 34 :1561 –1568,2002 [DOI] [PubMed] [Google Scholar]
- 44.Wood JM, Maibaum J, Rahuel J, Grutter MG, Cohen NC, Rasetti V, Ruger H, Goschke R, Stutz S, Fuhrer W, Schilling W, Rigollier P, Yamaguchi Y, Cumin F, Baum HP, Schnell CR, Herold P, Mah R, Jensen C, O’Brien E, Stanton A, Bedigian MP: Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun 308 :698 –705,2003 [DOI] [PubMed] [Google Scholar]
- 45.Wood JM, Schnell CR, Cumin F, Menard J, Webb RL: Aliskiren, a novel, orally effective renin inhibitor, lowers blood pressure in marmosets and spontaneously hypertensive rats. J Hypertens 23 :417 –426,2005 [DOI] [PubMed] [Google Scholar]
- 46.Hong Y, Dingemanse J, Mager DE: Pharmacokinetic/pharmacodynamic modeling of renin biomarkers in subjects treated with the renin inhibitor aliskiren. Clin Pharmacol Ther 84 :136 –143,2008 [DOI] [PubMed] [Google Scholar]
- 47.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G: Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 52 :130 –136,2008 [DOI] [PubMed] [Google Scholar]
- 48.Feldman DL, Jin L, Miserindino-Moltini R, Xuan H, Luft FC, Muller DN: P-613: Aliskiren, a human renin inhibitor induces persistent renoprotection comparable to ACE inhibition in double transgenic rats (dTGR). Am J Hypertens 18 :230A ,2005 [Google Scholar]
- 49.Lu H, Rateri DL, Feldman DL, Jr RJ, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A: Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest 118 :984 –993,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dechend R, Shagdarsuren E, Gratze P, Fiebeler A, Pilz B, Meiners S, Derer W, Feldman DL, Webb R, Muller DN: Low-dose renin inhibitor and low-dose AT(1)-receptor blocker therapy ameliorate target-organ damage in rats harbouring human renin and angiotensinogen genes. J Renin Angiotensin Aldosterone Syst 8 :81 –84,2007 [DOI] [PubMed] [Google Scholar]
- 51.Okazaki Y, Yamasaki Y, Uchida HA, Okamoto K, Satoh M, Maruyama K, Maeshima Y, Sugiyama H, Sugaya T, Kashihara N, Makino H: Enhanced TGF-beta/Smad signaling in the early stage of diabetic nephropathy is independent of the AT1a receptor. Clin Exp Nephrol 11 :77 –87,2007 [DOI] [PubMed] [Google Scholar]
- 52.Singh VP, Baker KM, Kumar R: Intracellular angiotensin II is a positive regulator of the cardiac renin-angiotensin system (Abstract). Hypertension 52 :e91 ,2008 [Google Scholar]
- 53.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C,Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschope C: Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia 49 :2507 –2513,2006 [DOI] [PubMed] [Google Scholar]
- 54.Eguchi K, Pickering TG, Kario K: Why is blood pressure so hard to control in patients with type 2 diabetes? J Cardiometab Syndr 2 :114 –118,2007 [DOI] [PubMed] [Google Scholar]
- 55.Aksnes TA, Kjeldsen SE, Rostrup M, Omvik P, Hua TA, Julius S: Impact of new-onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial population. Hypertension 50 :467 –473,2007 [DOI] [PubMed] [Google Scholar]
- 56.Okin PM, Devereux RB, Gerdts E, Snapinn SM, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Lindholm LH, Dahlof B: Impact of diabetes mellitus on regression of electrocardiographic left ventricular hypertrophy and the prediction of outcome during antihypertensive therapy: the Losartan Intervention For Endpoint (LIFE) Reduction in Hypertension Study. Circulation 113 :1588 –1596,2006 [DOI] [PubMed] [Google Scholar]
- 57.Schupp M, Janke J, Clasen R, Unger T, Kintscher U: Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation 109 :2054 –2057,2004 [DOI] [PubMed] [Google Scholar]
- 58.Watanabe T, Suzuki J, Yamawaki H, Sharma VK, Sheu SS, Berk BC: Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: angiotensin II type 1 receptor-independent effects of EXP3179. Circulation 112 :1798 –1805,2005 [DOI] [PubMed] [Google Scholar]
- 59.Yoshiyama M, Nakamura Y, Omura T, Izumi Y, Matsumoto R, Oda S, Takeuchi K, Kim S, Iwao H, Yoshikawa J: Angiotensin converting enzyme inhibitor prevents left ventricular remodelling after myocardial infarction in angiotensin II type 1 receptor knockout mice. Heart 91 :1080 –1085,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.