Abstract
OBJECTIVE—Previous work has demonstrated that chronic administration of the serotonin reuptake inhibitor (SSRI) fluoxetine augments counterregulatory responses to hypoglycemia in healthy humans. However, virtually no information exists regarding the effects of fluoxetine on integrated physiological counterregulatory responses during hypoglycemia in type 1 diabetes. Therefore, the specific aim of this study was to test the hypothesis that 6-week use of the SSRI fluoxetine would amplify autonomic nervous system (ANS) counterregulatory responses to hypoglycemia in individuals with type 1 diabetes.
RESEARCH DESIGN AND METHODS—Eighteen type 1 diabetic patients (14 men/4 women aged 19–48 years with BMI 25 ± 3 kg/m2 and A1C 7.0 ± 0.4%) participated in randomized, double-blind 2-h hyperinsulinemic (9 pmol · kg−1 · min−1)-hypoglycemic clamp studies before and after 6 weeks of fluoxetine administration (n = 8) or identical placebo (n = 10). Glucose kinetics was determined by 3-tritiated glucose. Muscle sympathetic nerve activity (MSNA) was determined by microneurography.
RESULTS—Hypoglycemia (2.8 ± 0.1 mmol/l) and insulinemia (646 ± 52 pmol/l) were similar during all clamp studies. ANS, neuroendocrine, and metabolic counterregulatory responses remained unchanged in the placebo group. However, fluoxetine administration significantly (P < 0.05) increased key ANS (epinephrine, norepinephrine, and MSNA), metabolic (endogenous glucose production and lipolysis), and cardiovascular (systolic blood pressure) counterregulatory responses during hypoglycemia.
CONCLUSIONS—This study has demonstrated that 6-week administration of the SSRI fluoxetine can amplify ANS and metabolic counterregulatory mechanisms during moderate hypoglycemia in patients with type 1 diabetes. These data also suggest that the use of fluoxetine may be useful in increasing epinephrine responses during hypoglycemia in clinical practice.
Selective serotonin reuptake inhibitors (SSRIs) are effective drugs for the treatment of depressive disorders associated with reduced serotonergic function. Serotonergic neurons play an important role in the regulation of neuroendocrine function carried out via both sympathoadrenal and hypothalamic-pituitary-adrenal (HPA) pathways.
Two studies have reported increased hypoglycemia and loss of awareness to hypoglycemia related to the use of SSRIs in depressed patients with type 1 diabetes (1,2). Although SSRIs are potent inhibitors of neuronal serotonin uptake, they also have the ability to block norepinephrine transport (3,4). This would be predicted to increase sympathetic outflow activity (4–7). Supporting this, previous studies by a number of investigators have demonstrated that SSRIs can modulate sympathetic nervous system activity and increase counterregulation in rats (8).
Two recent studies in healthy humans (9) and conscious rats (10) have provided further insight into the effects of SSRIs on counterregulatory physiology during hypoglycemia. Briscoe et al. (9) investigated the effects of 6 weeks of high-dose fluoxetine administration on physiological responses to hypoglycemia in a group of healthy, nondepressed humans. Key sympathetic nervous system (epinephrine, norepinephrine, and muscle sympathetic nerve activity [MSNA]) and metabolic (glucose production and lipolysis) counterregulatory mechanisms were significantly amplified by the SSRI. Sanders et al. (10) elegantly studied the chronic effects of another SSRI (sertraline) in a conscious rat model. After 20 days’ administration of the SSRI, epinephrine and glucagon responses were significantly increased during hypoglycemia. Additionally, sertraline preserved levels of epinephrine during repeated hypoglycemia, thereby preventing the blunting effects of antecedent hypoglycemia on subsequent autonomic nervous system (ANS) counterregulatory responses. Taken together, the above data suggest that seroteonergic transmission may be an important mechanism in upregulating sympathetic nervous system drive during hypoglycemia in both rats and healthy humans.
However, the effects of SSRIs on ANS, neuroendocrine, and metabolic counterregulatory mechanisms during hypoglycemia in type 1 diabetes do not appear to have been studied. To address this question, the specific aim of this study was to test the hypothesis that chronic administration of the commonly used SSRI fluoxetine would result in an amplification of metabolic and ANS counterregulatory mechanisms during hypoglycemia in nondepressed individuals with type 1 diabetes. The glucose clamp technique was used so that insulin and glucose levels could be controlled in all studies.
RESEARCH DESIGN AND METHODS
Twenty type 1 diabetic patients (15 men/5 women aged 19–48 years with BMI 25 ± 3 kg/m2, diabetes duration 18 ± 9 years, and A1C 7.0 ± 0.4% [normal range 4–6.5%]) were studied. The Zung Self-Rating Depression Scale (11,12) was completed by each subject to rule out symptoms of clinical depression. None had a history of epilepsy or any major psychiatric illness. None were taking any psychotropic medication. Each subject had a normal blood count, plasma electrolytes, and liver and renal function. All gave written informed consent. Studies were approved by the Vanderbilt University Human Subjects Institutional Review Board.
All study patients were asked to avoid any exercise and consume their usual weight-maintaining diet for 3 days before each experiment. All patients performed intensive home blood glucose monitoring (i.e., at least four glucose tests per day) and were asked to avoid hypoglycemia for at least 5 days before a study. On the day before a study, intermediate or long-acting insulin was discontinued and replaced by injections of regular insulin before breakfast and lunch. Each subject was admitted to the Vanderbilt General Clinical Research Center (GCRC) at 5:00 p.m. on the evening before an experiment. At this time, two intravenous cannulae were inserted under 1% lidocaine local anesthesia. One cannula was placed in a retrograde fashion into a vein on the back of the hand. This hand would be placed in a heated box (55–60°C) during the study so that arterialized blood could be obtained (13). The other cannula was placed in the contralateral arm for infusions. Patients then received a standardized evening meal, and a continuous low-dose infusion of insulin was started to normalize plasma glucose. The insulin infusion was adjusted overnight to maintain blood glucose between 4.4 and 7.2 mmol/l.
Hypoglycemia experiments.
After an overnight 10-h fast at 0 min, a primed (18-μCi) continuous infusion (0.18 μCi/min) of high-performance liquid chromatography (HPLC)–purified [3-3H]glucose (11.5 mCi · mmol−1 · l−1; Perkin Elmer Life Sciences, Boston, MA) was administered via a precalibrated infusion pump (Harvard Apparatus, South Natick, MA). A period of 90 min was allowed to elapse followed by a 30-min basal control period and a 120-min hyperinsulinemic-hypoglycemic experimental period. An insulin infusion solution was prepared with normal saline containing 3% (vol/vol) of the subject's own plasma. At time 120 min, a primed constant (9.0 pmol · kg−1 · min−1) infusion of insulin (Human Regular Insulin; Eli Lilly, Indianapolis, IN) was started and continued until 240 min. The rate of fall of glucose was controlled (0.06 mmol/min) and the glucose nadir (2.8 mmol/l) was achieved using a modification of the glucose clamp technique (14). During the clamp period, plasma glucose was measured every 5 min, and a 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 2.8 ± 0.1 mmol/l (15). Potassium chloride (20 mmol/l) was infused during the clamp to reduce insulin-induced hypokalemia. After completion of the 2-h test period, the plasma glucose was rapidly restored to euglycemia with 20% dextrose. A second identical hyperinsulinemic-hypoglycemic clamp was performed after 6 weeks of the study medication.
Study medication.
After the initial 1-day clamp study, volunteers were given a 1-week medication supply of either fluoxetine or placebo for 6 weeks. The fluoxetine dose was as follows: 20 mg/day during week 1, 40 mg/day during week 2, 60 mg/day during week 3, and 80 mg/day during weeks 4–6. The study was carried out in a double-blind fashion with volunteers and investigators blinded to the treatment group assigned. Stratified blocked randomization was performed by the Vanderbilt Investigational Drug Pharmacy. The subjects were stratified according to sex because it is known to affect the counterregulatory response (16). Randomization was performed within each sex, and blocks of two were used to ensure an equal number of men and women in the placebo and fluoxetine treatment groups.
During the 6-week treatment period, volunteers came to the GCRC once a week for monitoring of compliance and adverse events. Compliance was determined via a pill count and a blood draw to measure serum fluoxetine levels. Two subjects (1 man/1 woman) in the fluoxetine group withdrew from the study because of side effects. The withdrawals occurred early at the 20-mg dose and were described as feelings of tiredness and nonspecific malaise. In the remaining subjects, fluoxetine was very well tolerated, with no reports of side effects. After taking either placebo or fluoxetine for 6 weeks, subjects underwent another single-day hypoglycemic clamp study as previously described. Thus, 18 subjects (8 fluoxetine/10 placebo) completed both hypoglycemia clamp studies. On completion of this second 1-day study, subjects were tapered off the study medication (placebo or fluoxetine). Those receiving fluoxetine were given it for 1 week at 40 mg/day and 1 week at 20 mg/day. Once subjects finished the medication, they were unblinded as to the medication they had taken.
Direct measurement of MSNA.
MSNA was recorded from the peroneal nerve at the level of the fibular head and popliteal fossa (17,18). Nerve activity was recorded on a PC-based Windaq data acquisition system at 1,000 H2Z channel-1 (DATAQ Instruments, Akron, OH). Five-minute Windaq files were analyzed with a MatLab GUIDE interface (to adjust for an individual's 1.3-s nerve burst delay from a once-removed R-R interval, automatically detected by pulse synchronicity, a 2:1 signal:noise ratio, and wave-form shape). Further criteria for acceptable MSNA recordings were that 1) electrical stimulation produced muscle twitches but not paresthesia, 2) nerve activity increased during phase II of the Valsalva maneuver (hypotensive phase) and was suppressed during phase IV (blood pressure overshoot), and 3) nerve activity increased in response to held expiration.
Tracer calculations.
Rates of glucose appearance (Ra), endogenous glucose production (EGP), and glucose utilization were calculated according to the methods of Wall et al. (19). EGP was calculated by determining the total Ra (this comprises EGP and any exogenous glucose infused to maintain the desired hypoglycemia) and subtracting it from the amount of exogenous glucose infused. It is now recognized that this approach is not fully quantitative because underestimates of total Ra and rate of glucose disposal (Rd) can be obtained. The use of a highly purified tracer and taking measurements under steady-state conditions (i.e., constant specific activity) in the presence of low glucose flux eliminates most, if not all, of the problems. In addition, to maintain a constant specific activity, isotope delivery was increased commensurate with increases in exogenous glucose infusion. During these studies, only glucose flux results from the steady-state basal and the final 30-min periods of the hypoglycemic clamps are reported.
Analytical methods.
Plasma glucose concentrations were measured in triplicate using the glucose oxidase method with a glucose analyzer (Beckman, Fullerton, CA). Glucagon was measured by radioimmunoassay (RIA) with an interassay coefficient of variation (CV) of 12% (20). Insulin was measured as previously described (21) with an interassay CV of 9%. Catecholamines were determined by HPLC (22) with an interassay of 12% for epinephrine and 8% for norepinephrine. Cortisol was assayed using the Clinical Assays Gamma Coat RIA kit with an interassay CV of 6%. Growth hormone was determined by RIA (23) with a CV of 8.6%. Pancreatic polypeptide was measured by RIA using the method of Hagopian et al. (24) with an interassay CV of 8%. Lactate, glycerol, alanine, and β-hydroxybutyrate were measured in deproteinized whole blood using the method of Lloyd et al. (25). Nonesterified fatty acids (NEFAs) were measured using the WAKO kit adopted for use on a centrifugal analyzer (26). Fluoxetine and norfluoxetine were determined by gas chromatography with electron capture detection based on a modification described by Torok-Both et al. (27).
Blood for hormones and intermediary metabolites was drawn twice during the control period and every 15 min during the experimental period. Cardiovascular parameters (pulse, systolic, diastolic, and mean arterial pressure) were measured noninvasively by a Dinamap (Critikon, Tampa, FL) every 10 min throughout each study starting at 80 min.
Hypoglycemic symptoms were quantified using a previously validated semiquantitative questionnaire (28). Each individual was asked to rate his/her experience of the symptoms twice during the control period and every 15 min during experimental periods. Symptoms measured included sweat, tremor/shaking, feeling hot, thirsty/dry mouth, agitation/irritability, palpitations, feeling tired/fatigued, confusion, dizziness, difficulty thinking, blurriness of vision, and sleepiness. The ratings of the first six symptoms were summed to get the autonomic score, whereas the ratings from the last six symptoms provide a neuroglycopenic symptom score.
Statistical analysis.
Data are expressed as means ± SE and were analyzed using standard, parametric, and one- and two-way ANOVA and with repeated measures where appropriate (SigmaStat; SPSS Science, Chicago, IL). Tukey's post hoc analysis was used to delineate statistical significance across time within each group and for each group compared with the control group. A P value of <0.05 was accepted as statistically significant. The baseline and final 30 min of hypoglycemia were compared for most parameters because steady-state glucose levels, insulin levels, and glucose infusion rates were achieved by this time.
RESULTS
Glucose, insulin, and fluoxetine levels.
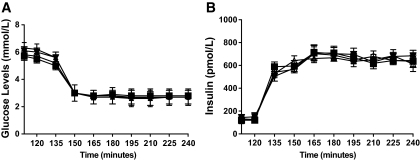
Basal plasma glucose levels were 6.3 ± 0.3 and 5.6 ± 0.2 mmol/l in the clamps before fluoxetine and before placebo. Basal glucose levels were similar (6.1 ± 0.3 and 5.5 ± 0.3 mmol/l) after 6 weeks of fluoxetine or placebo. Plasma glucose levels reached steady state by 30 min, and equivalent hypoglycemia (2.8 ± 0.1 mmol/l) was maintained during all clamp procedures (Fig. 1). Basal and steady-state insulin levels for both fluoxetine and placebo groups were similar during studies before the clamp (fluoxetine, 120 ± 18 and 660 ± 24 pmol/l, and placebo, 126 ± 30 and 648 ± 54 pmol/l) and after clamp (fluoxetine 120 ± 18 and 630 ± 42 pmol/l, and placebo, 144 ± 30 and 667 ± 36 pmol/l) (Fig. 2). A1C levels were unchanged during fluoxetine (7.0 ± 0.3 to 6.8 ± 0.3%) and placebo (6.9 ± 0.3 to 6.9 ± 0.3%) administration. Similarly, weight was constant during both fluoxetine (74.2 ± 13 to 73 ± 7±14.3 kg) and placebo (77.5 ± 9 to 77.4 ± 9 kg) administration. Mean fluoxetine and norfluoxetine levels at the end of the study were 274.13 ± 45 and 188.38 ± 34 ng/ml, respectively, in the SSRI group and were undetectable in the placebo group.
FIG. 1.
Plasma glucose (A) and insulin (B) concentrations (means ± SE) during hypoglycemic clamp studies in 18 individuals (14 men/4 women) with type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration. pre-SSRI, ○; post-SSRI, ▪; pre-placebo, ▵; post-placebo, ▾.
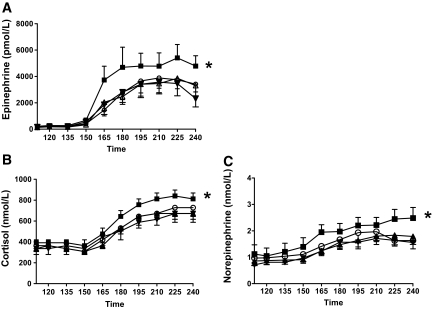
FIG. 2.
Mean plasma epinephrine (A), cortisol (B), and norepinephrine (C) levels (means ± SE) during the basal period and the final 30 min of hypoglycemic clamp studies in 18 individuals (14 men/four women) with type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration. *Plasma epinephrine, norepinephrine, and cortisol levels are significantly increased (P < 0.05) following fluoxetine. pre-SSRI, ○; post-SSRI, ▪; pre-placebo, ▵; post-placebo, ▾.
Neuroendocrine counterregulatory hormones.
Epinephrine responses were significantly higher (P < 0.05) during the final 30 min of hypoglycemia after fluoxetine (5,436 ± 808 pmol/l) compared with before treatment (3,815 ± 841 pmol/l) and after placebo (3,198 ± 791 pmol/l) hypoglycemic clamp. Epinephrine responses were similar during the final 30 min of the hypoglycemic clamps before and after placebo (Fig. 2).
Norepinephrine responses were significantly higher (P < 0.05) during the final 30 min of after fluoxetine (2.3 ± 0.3 nmol/l) compared with before treatment (1.7 ± 0.2 nmol/l) and after placebo (1.6 ± 0.2 nmol/l). Norepinephrine responses were similar during the final 30 min of hypoglycemia during the studies before and after placebo (Fig. 2).
Cortisol levels were increased (P < 0.05) in the after- fluoxetine versus the after-placebo groups (800 ± 55 vs. 635 ± 83 nmol/l). Cortisol levels remained unchanged during placebo administration.
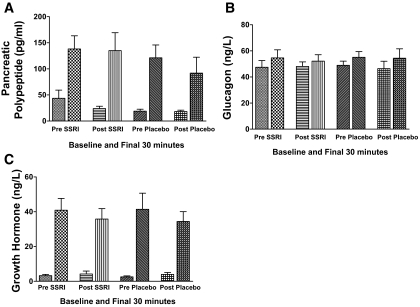
Peak pancreatic polypeptide levels during hypoglycemia were practically unchanged (135 ± 37 vs. 138 ± 27 nmol/l) after fluoxetine administration compared with values before treatment, respectively. Pancreatic polypeptide levels were also similar for the control group after placebo (115 ± 35 nmol/l) versus before placebo (121 ± 26 nmol/l) during the final 30 min of hypoglycemia (Fig. 3).
FIG. 3.
Mean plasma pancreatic polypeptide (A), glucagon (B), and growth hormone (C) levels (means ± SE) during the basal period and the final 30 min of hypoglycemic clamp studies in 18 individuals (14 men/four women) with type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration.
Glucagon responses were similar during hypoglycemia in all groups (before/after fluoxetine and before/after placebo) (Fig. 3). Fluoxetine and placebo had similar effects on growth hormone responses during hypoglycemia. Growth hormone levels during the studies before and after fluoxetine were 41 ± 7 vs. 36 ± 6 ng/l, respectively, and were 41 ± 10 vs. 34 ± 7 ng/l for the before- and after- placebo groups, respectively (Fig. 3).
MSNA.
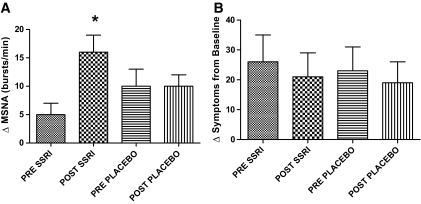
MSNA increased by a significantly greater amount (P < 0.05) during hypoglycemia after fluoxetine (16 ± 3 bursts/min) versus both after placebo (10 ± 2 bursts/min) and before fluoxetine (5 ± 2 bursts/min). There were no differences in the after-placebo versus before-placebo responses (Fig. 4).
FIG. 4.
ΔMSNA (A) and total symptom (B) responses during the final 30 min of hypoglycemic clamp studies in 18 individuals (14 men/4 women) with type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration. Data are means ± SE. *MSNA responses are significantly increased (P < 0.05) following fluoxetine.
Glucose kinetics.
Glucose specific activity (disintegrations per minute per milligram) was in a steady state during the basal period and the final 30 min of all hyperinsulinemic-hypoglycemic clamps (Table 1).
TABLE 1.
Glucose specific activity during the basal period and the final 30 min of hyperinsulinemic-hypoglycemic clamps in subjects with type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration
| Glucose specific activity (dpm/mmol) |
||||||
|---|---|---|---|---|---|---|
| −20 min | −10 min | 0 min | 90 min | 105 min | 120 min | |
| Before fluoxetine | 364 ± 22 | 341 ± 25 | 367 ± 18 | 273 ± 22 | 289 ± 13 | 276 ± 12 |
| After fluoxetine | 334 ± 42 | 353 ± 33 | 359 ± 33 | 262 ± 22 | 260 ± 20 | 247 ± 20 |
| Before placebo | 326 ± 18 | 334 ± 24 | 338 ± 19 | 271 ± 22 | 263 ± 17 | 259 ± 19 |
| After placebo | 365 ± 24 | 369 ± 22 | 376 ± 24 | 300 ± 20 | 289 ± 18 | 272 ± 18 |
Data are means ± SE.
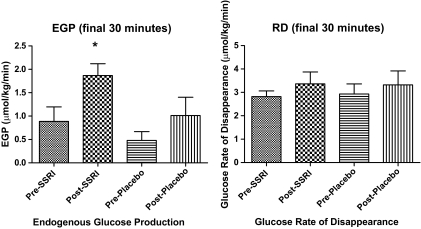
EGP after fluoxetine (11 ± 1.1 μmol · kg−1 · min−1) was significantly increased (P < 0.05) during the final 30 min of hypoglycemia compared with before fluoxetine (5.1 ± 1.1 μmol · kg−1 · min−1), before placebo (2.2 ± 1.6 μmol · kg−1 · min−1), and after placebo (5.0 ± 2.2 μmol · kg−1 · min−1). Glucose infusion rates were 14.3 ± 6.1and 8.9 ± 3.9 μmol · kg−1 · min−1 after placebo and after fluoxetine, respectively. Glucose Rds during the final 30 min of hypoglycemia were 19 ± 2.8 and 19.8 ± 3.3 after placebo and after fluoxetine, respectively (Fig. 5).
FIG. 5.
Glucose kinetics during the basal period and the final 30 min of hypoglycemic clamp studies in 18 individuals (14 men/4 women) with type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration. Data are means ± SE. *EGP is significantly increased (P < 0.05) following fluoxetine administration.
Intermediary metabolism.
Baseline glycerol, lactate, β-hydroxybutyrate, NEFA, and alanine levels were similar among groups (Table 2). Glycerol levels were significantly greater (P < 0.05) after fluoxetine (115 ± 18 μmol/l) compared with after placebo (83 ± 16 μmol/l) or before fluoxetine (73 ± 5 μmol/l). There was no difference in the increase of glycerol in the studies before and after placebo (Table 2).
TABLE 2.
Plasma glycerol, lactate, β-hydroxybutyrate, NEFA, and alanine levels during the basal period and the final 30 min of hyperinsulinemic-hypoglycemic clamp studies in type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration
| Basal period | Final 30 min | |
|---|---|---|
| Glycerol (μmol/l) | ||
| Before treatment | 58 ± 7 | 65 ± 8 |
| After fluoxetine | 95 ± 25 | 110 ± 21* |
| Before treatment | 67 ± 15 | 74 ± 15 |
| After placebo | 73 ± 11 | 80 ± 17 |
| Lactate (mmol/l) | ||
| Before treatment | 0.4 ± 0.04 | 0.75 ± 0.09 |
| After fluoxetine | 0.3 ± 0.02 | 0.83 ± 0.15 |
| Before treatment | 0.4 ± 0.04 | 0.75 ± 0.09 |
| After placebo | 0.4 ± 0.05 | 0.84 ± 0.00 |
| β-Hydroxybutyrate (μmol/l) | ||
| Before treatment | 0.099 ± 0.089 | 0.05 ± 0.03 |
| After fluoxetine | 0.046 ± 0.026 | 0.02 ± 0.01 |
| Before treatment | 0.07 ± 0.004 | 0.019 ± 0.002 |
| After placebo | 0.07 ± 0.005 | 0.02 ± 0.01 |
| NEFA (μmol/l) | ||
| Before treatment | 149 ± 38 | 93 ± 24 |
| After fluoxetine | 165 ± 41 | 182 ± 64* |
| Before treatment | 131 ± 34 | 86 ± 22 |
| After placebo | 212 ± 37 | 91 ± 24 |
| Alanine (μmol/l) | ||
| Before treatment | 0.23 ± 0.02 | 0.22 ± 0.02 |
| After fluoxetine | 0.21 ± 0.02 | 0.21 ± 0.03 |
| Before treatment | 0.21 ± 0.02 | 0.20 ± 0.02 |
| After placebo | 0.24 ± 0.03 | 0.22 ± 0.02 |
Data are means ± SE. *Significantly increased responses during final 30 min of hypoglycemia after 6 weeks of fluoxetine administration (P < 0.05).
There was an increase in NEFA levels during hypoglycemia after fluoxetine (182 ± 64 μmol/l) compared with after placebo (91 ± 25 μmol/l) and before fluoxetine (93 ± 24 μmol/l) (P < 0.05) studies. There was no difference in the increase of NEFA levels during hypoglycemia in the studies before and after placebo. Blood lactate, alanine, and β-hydroxybutyrate levels were similar during the four series of hypoglycemic clamps and were unaffected by fluoxetine or placebo administration (Table 2).
Cardiovascular parameters.
Basal heart rate and blood pressure were not different after a 6-week administration of fluoxetine. Heart rate was significantly higher (P < 0.05) during the final 30 min of groups after fluoxetine compared with before fluoxetine and after placebo (80 ± 4 vs. 74 ± 5 and 69 ± 5 bpm, respectively). Heart rate was similar in the control group (after placebo 69 ± 5 bpm vs. before placebo, 67 ± 8 bpm). Systolic blood pressure was significantly increased during hypoglycemia in the group after fluoxetine versus the before-fluoxetine and after- placebo groups (127 ± 4 vs. 113 ± 3 and 115 ± 6 mmHg, respectively; P < 0.05). There were no differences in systolic blood pressure in the placebo control group (after placebo 115 ± 6 mmHg vs. before placebo 119 ± 5 mmHg). Diastolic blood pressure was also increased after fluoxetine (P < 0.05) compared with after placebo and before fluoxetine (67 ± 2 vs. 61 ± 3 and 61 ± 1 mmHg, respectively; Table 3).
TABLE 3.
Cardiovascular responses during hyperinsulinemic-hypoglycemic clamp studies in type 1 diabetes before and after 6 weeks of fluoxetine or placebo administration
| Basal period | Final 30 min | |
|---|---|---|
| Heart rate (beats/min) | ||
| Before fluoxetine | 69 ± 4 | 74 ± 5 |
| After fluoxetine | 69 ± 4 | 80 ± 4* |
| Before placebo | 67 ± 5 | 67 ± 8 |
| After placebo | 65 ± 5 | 69 ± 5 |
| Systolic blood pressure (mmHg) | ||
| Before fluoxetine | 107 ± 3 | 113 ± 3 |
| After fluoxetine | 115 ± 7 | 127 ± 4* |
| Before placebo | 110 ± 3 | 119 ± 5 |
| After placebo | 110 ± 4 | 115 ± 6 |
| Diastolic blood pressure (mmHg) | ||
| Before fluoxetine | 66 ± 1 | 61 ± 1 |
| After fluoxetine | 68 ± 3 | 67 ± 2* |
| Before placebo | 67 ± 3 | 63 ± 3 |
| After placebo | 65 ± 3 | 61 ± 3 |
Data are means ± SE.
Significantly increased responses during final 30 min of hypoglycemia after 6 weeks of fluoxetine administration (P < 0.05).
Symptom response.
There were no differences in total symptom scores in the experimental group when compared with groups before fluoxetine or after placebo. Similarly, no significant differences occurred in the control group after placebo compared with the clamp study before placebo (Fig. 4). Both autonomic and neuroglycopenic symptoms scores were similar during hypoglycemia before and after fluoxetine administration (autonomic symptoms before fluoxetine were 23 ± 4 vs. 19 ± 4 after fluoxetine; neuroglycopenic scores were 19 ± 4 before fluoxetine and 20 ± 4 after fluoxetine).
DISCUSSION
This study has determined the effects of 6-week administration of the SSRI fluoxetine on counterregulatory responses to hypoglycemia in nondepressed patients with type 1 diabetes. Our results demonstrate that a period of 6 weeks of high-dose fluoxetine significantly increases sympathetic nervous system, HPA, and metabolic (EGP and lipolysis) counterregulatory responses in patients with long-duration type 1 diabetes.
Hypoglycemia remains the major barrier to even near normalization of glucose in patients with type 1 diabetes. We have recently reported the beneficial effects of 6 weeks’ fluoxetine administration on amplifying counterregulatory mechanisms during hypoglycemia in nondiabetic individuals (9). In this present study, we have tested the hypothesis that 6 weeks’ administration of fluoxetine will enhance counterregulatory responses during hypoglycemia in type 1 diabetes. Similar to studies in nondiabetic humans, this study has determined that fluoxetine can have marked effects in amplifying catecholamine responses and muscle sympathetic nervous activity during hypoglycemia. The increased sympathetic nervous system (SNS) activity also had significant effects on enhancing the key counterregulatory metabolic mechanisms of EGP and lipolysis.
In longer duration type 1 diabetes patients, epinephrine becomes the critical counterregulatory hormone in the defense against acute hypoglycemia. This is due to the fact that with increasing disease duration, the glucagon response to hypoglycemia is lost in type 1 diabetes (29). Unfortunately, in type 1 diabetic patients with repeated episodes of hypoglycemia and intensive glucose control, the epinephrine response to hypoglycemia is significantly reduced (30). The combination of absent glucagon and severely blunted epinephrine responses consequently results in a significant magnification of the risk for severe hypoglycemia (30). Perhaps the most notable finding from the present study was the striking increase in epinephrine responses during hypoglycemia following fluoxetine. Despite equivalent insulin and glucose levels during the hypoglycemic clamps, fluoxetine resulted in a 90% increase in epinephrine levels. In fact, fluoxetine increased the response of epinephrine to levels even higher than previously observed in nondiabetic individuals during similar conditions of hypoglycemia (31). The increased SNS response following fluoxetine also resulted in a marked increase in EGP and lipolysis. As can be observed from the placebo studies, EGP is typically suppressed during hypoglycemia in type 1 diabetes. Thus, the amplified (doubled) EGP response following fluoxetine represents a potentially important defense against hypoglycemia in type 1 diabetes. The increased lipolytic response following fluoxetine would also be expected to contribute to the elevated rates of EGP. Both glycerol, an important gluconeogenic precursor during hypoglycemia (32), and NEFA, a significant provider of energy for gluconeogenesis (32), were significantly increased. Rates of glucose uptake were unchanged during the series of hypoglycemic clamps. Previous studies have demonstrated that the dose-response effects of epinephrine to inhibit glucose uptake are relatively flat (33,34) in association with increasing the concentrations of the catecholamines from 600 to 1,000 pg/ml, producing limited actions. Similar to the findings in healthy humans, fluoxetine resulted in an increased response of cortisol during hypoglycemia. This also demonstrates that the SSRI was having effects to activate multiple neural pathways within the central nervous system (7).
Accompanying the amplified SNS responses were significant increases in blood pressure and heart rate during hypoglycemia following fluoxetine. Somewhat surprisingly, there was not an increase in symptom scores despite the significant increases in central ANS drive and the respective target organ responses (i.e., liver, adipose tissue, and heart) following fluoxetine. This apparent dissociation between neutral symptom responses and increases in other components of the sympathetic nervous system following fluoxetine was also clearly demonstrated in the previous study with nondiabetic individuals (9). These data further suggest that serotonergic pathways are involved in the generation of symptom responses during hypoglycemia. Recent work in humans has demonstrated that hippocampal and thalamic regions of the brain are activated during hypoglycemia (35). These areas are involved in mood and function known to be affected by SSRIs (36). We therefore hypothesize that fluoxetine exerted a relatively restraining effect on symptom generation while stimulating neurohumoral and cardiovascular sympathetic tone during hypoglycemia. Similar to our findings in nondiabetic individuals, despite the increased sympathetic nervous system drive and elevated epinephrine levels, fluoxetine had no effects on amplifying glucagon responses during hypoglycemia (9). This would add support to the hypothesis that there is an inherent B-cell defect restricting glucagon release during hypoglycemia in type 1 diabetes rather than simply just a loss of ANS input into the α-cells (37).
Another point that should be noted is that fluoxetine only amplified counterregulatory responses during hypoglycemia and did not increase basal homeostatic mechanisms. Thus, there were no differences in baseline cardiovascular, metabolic, and neuroendocrine parameters following fluoxetine or placebo. The lack of chronic effects of an SSRI to increase basal neuroendocrine activity before hypoglycemia was also reported by Sanders et al. (10) in their recent study using sertraline in nondiabetic conscious rats. We also found that chronic administration of the SSRI fluoxetine in nondiabetic individuals had no effects on increasing basal cardiovascular, ANS, and metabolic function. However, basal plasma cortisol (but not glucagon and growth hormone) was increased by fluoxetine in nondiabetic individuals.
Data are accumulating regarding possible mechanisms for SSRI enhancement of ANS and HPA axis responses during hypoglycemia. Activation of a number of serotonergic receptors (5HT1A, 5HT1C, 5HT2, and 5HT3) has been demonstrated to increase sympathetic nervous system outflow (5,6,38,39). Additionally, both systemic and central administration of SSRIs have specifically increased adrenal catecholamine and epinephrine release (7,8). Two recent studies have also demonstrated that chronic administration of different SSRIs can amplify counterregulatory responses during hypoglycemia in both healthy humans and conscious rats (9,10). The findings of increased cortisol and catecholamines cannot determine whether the SSRI was being sensed at central (i.e., brain), peripheral (i.e., adrenal gland), or even both sites to augment counterregulatory responses. However, the finding that MSNA was increased in the present study certainly indicates that central sensing and action of the SSRI were occurring. The effects of SSRIs on HPA responses during stress appear to be more complex. We have found that fluoxetine can increase plasma cortisol responses during hypoglycemia in both healthy and type 1 diabetes humans. Durand et al. (40) have also demonstrated that fluoxetine can amplify corticosteroid responses to stress in conscious rats. However, Sanders et al. (10), studying a different SSRI (sertraline) and species of rat (Spraque-Dawley), found no amplification of HPA axis responses during hypoglycemia. Thus, the physiological effects of SSRI may be different depending on the specific agent and experimental model under investigation.
The present study has provided an evaluation of the effects of fluoxetine on physiological responses during hypoglycemia in a group of metabolically well-controlled type 1 diabetic patients. These individuals typically have the highest prevalence of hypoglycemia and might be expected to benefit most from strategies aimed at improving ANS responses. Six-week administration of high-dose fluoxetine had marked effects in amplifying epinephrine and metabolic counterregulatory responses. However, the dose of fluoxetine used in this study is higher than the average dose of the drug used in clinical practice, ∼33 mg/day (41). Fluoxetine was increased to the highest clinically approved dose of the drug because we wanted to ensure that the largest experimental signal was generated in these initial studies. Additionally, the subjects enrolled in our study were not depressed and did not have any major psychopathology. Thus, we cannot determine whether similar results would be obtained in depressed individuals. In addition, we did not formally assess whether fluoxetine would have any effects on cognitive function during hypoglycemia. This is an important clinical consideration and deserves further study. Overall, fluoxetine was well tolerated. Body weight and A1C were no different in the placebo or fluoxetine groups. Rates of hypoglycemia (albeit anecdotally reported by the patients) appeared to be unchanged (n = 3), improved (n = 3), or relatively improved (n = 2) (i.e., unchanged frequency of hypoglycemia with improvement in A1C of ≥0.8%). Therefore, we cannot determine whether fluoxetine increased sympathoadrenal responses by reducing episodes of antecedent hypoglycemia and/or by directly stimulating ANS responses during any given episode of hypoglycemia. Fluoxetine administration was started at 20 mg and increased in a stepwise fashion to 80 mg in an attempt to limit side effects of the drug. However, two subjects receiving fluoxetine withdrew early from the study. Both of these subjects were only receiving 20 mg of the drug and reported nonspecific side effects. Interestingly, the higher doses of the drug (60–80 mg/day) were very well tolerated, with no reports of unpleasant side effects. Thus, in the present study (and our previous study in nondiabetic individuals), the side effects with fluoxetine appeared early, dissipated after continued use, and were not dose related.
In summary, this study has demonstrated that 6 weeks’ administration of fluoxetine can markedly increase key sympathetic nervous system (epinephrine, norepinephrine, and muscle sympathetic nervous system), metabolic (EGP and lipolysis), and cardiovascular counterregulatory responses during clamped moderate (2.8 mmol/l) hypoglycemia in type 1 diabetes. The study also demonstrates that serotonergic mechanisms can play a significant role in regulating ANS and HPA physiological responses during hypoglycemia in type 1 diabetic individuals. In conclusion, the present results have provided novel findings demonstrating that under the conditions of the present study, SSRIs (specifically fluoxetine) may provide beneficial adjunct effects in amplifying epinephrine levels during hypoglycemia in type 1 diabetic individuals.
Acknowledgments
This study has received National Institutes of Health grants R01-DK-069803, MO1-RR-000095, P01-HL-056693, and P60-DK-020593.
We thank the expert technical assistance of Eric Allen, Susan Hajizadeh, Nathan Jones, and Mary Garmon. We also acknowledge the superb care provided by the staff of the Vanderbilt General Clinical Research Center.
Published ahead of print at http://diabetes.diabetesjournals.org on 3 October 2008. Clinical trial reg. no. NCT00592670, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sawka AM, Burgart V, Zimmerman D: Loss of hypoglycemia awareness in an adolescent with type 1 diabetes mellitus during treatment with fluoxetine hydrochloride. J Pediatr 136 :394 –396,2000 [DOI] [PubMed] [Google Scholar]
- 2.Sawka AM, Burgart V, Zimmerman D: Loss of awareness of hypoglycemia temporally associated with selective serotonin reuptake inhibitors. Diabetes Care 24 :1845 –1846,2001 [DOI] [PubMed] [Google Scholar]
- 3.Perry KW, Fuller RW: Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci 50 :1683 –1690,1992 [DOI] [PubMed] [Google Scholar]
- 4.Perry KW, Fuller RW: Fluoxetine increases norepinephrine release in rat hypothalamus as measured by tissue levels of MHPG-SO4 and microdialysis in conscious rats. J Neural Transm 104 :953 –966,1997 [DOI] [PubMed] [Google Scholar]
- 5.Chaouloff F, Gunn SH, Young JB: Influence of 5-HT1 and 5 HT2 receptor antagonists on insulin-induced adrenomedullary catecholamine release. Neuroendocrinology 54 :639 –645,1991 [DOI] [PubMed] [Google Scholar]
- 6.Baudie V, Chaouloff F: Mechanisms involved in the hyperglycemic effect of the 5-HT1c/5 HT2 receptor agonist, DOI. Eur J Pharmacol 213 :41 –46,1992 [DOI] [PubMed] [Google Scholar]
- 7.Blardi P, de Lalla A, Auteri A, Iapichino S, Dell’Erba A, Castrogiovanni P: Plasma catecholamine levels after fluox treatment in depressive patients. Neuropsychobiology 51 :72 –76,2005 [DOI] [PubMed] [Google Scholar]
- 8.Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW: Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology 160 :353 –361,2002 [DOI] [PubMed] [Google Scholar]
- 9.Briscoe VJ, Ertl AC, Tate DB, Dawling S, Davis SN: Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy man. Diabetes 57 :2453 –2460,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders NM, Wilkinson CW, Taborsky GJ, Salwa A, Daumen W, Zavosh A, Figlewicz DP: The selective serotonin reuptake inhibitor sertraline enhances counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab 294 :E853 –E860,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zung WW, Richards CB, Short MJ: Self-rating depression scale in an outpatient clinic: further validation of the SDS.A self-rating depression scale. Arch Gen Psychiatry 13 :508 –515,1965 [DOI] [PubMed] [Google Scholar]
- 12.Carroll BJ, Fielding JM, Blashki TG: Depression rating scales: A critical review. Arch Gen Psychiatry 28 :361 –366,1973 [DOI] [PubMed] [Google Scholar]
- 13.Abumrad NN, Rabin D, Diamond MC, Lacy WW: Use of a heated superficial hand vein as an alternative site for measurement of amino acid concentration and for the study of glucose and alanine kinetics in man. Metabolism 30 :936 –940,1981 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin K, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237 :E216 –E223,1979 [DOI] [PubMed] [Google Scholar]
- 15.Amiel SA, Tambolane WV, Simonson DC, Sherwin R: Defective glucose counterregulation after strict control of insulin-dependent diabetes mellitus. N Engl J Med 316 :1376 –1383,1987 [DOI] [PubMed] [Google Scholar]
- 16.Davis SN, Shavers C, Costa F: Differential gender responses to hypoglycemia are due to alterations in CNS drive and not glycemic thresholds. Am J Physiol Endocrinol Metab 279 :E1054 –E1063,2000 [DOI] [PubMed] [Google Scholar]
- 17.Wallin B, Sundiot G, Eriksson B, Domniak P, Grobecker H, Lindblad L: Plasma noradrenaline correlates by sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand 11 :69 –73,1981 [DOI] [PubMed] [Google Scholar]
- 18.Sandoval D, Ertl AC, Richardson A, Tate D, Davis SN: Estrogen blunts neuroendocrine and metabolic responses to hypoglycemia. Diabetes 52 :1749 –1755,2003 [DOI] [PubMed] [Google Scholar]
- 19.Wall JS, Steele R, Debodo RD, Altszuler N: Effect of insulin on utilization and production of circulating glucose. Am J Physiol 189 :43 –50,1957 [DOI] [PubMed] [Google Scholar]
- 20.Aguilar-Parada E, Eisentraut AM, Unger RH: Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci 257 :415 –419,1969 [DOI] [PubMed] [Google Scholar]
- 21.Wide L, Porath J: Radioimmunoassay of proteins with the uses of sephadex-coupled antibodies. Biochim Biophys Acta 130 :257 –260,1966 [Google Scholar]
- 22.Causon R, Caruthers M, Rodnight R: Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem 116 :223 –226,1982 [DOI] [PubMed] [Google Scholar]
- 23.Hunter W, Greenwood F: Preparation of [131I]-labeled human growth hormone of high specific activity. Nature 194 :495 –496,1962 [DOI] [PubMed] [Google Scholar]
- 24.Hagopian W, Lever EG, Cohen D, Emmanouel D, Polonsky KS, Pugh W, Moossa A, Jaspan JB: Predominance of renal and absence of hepatic metabolism of pancreatic polypeptide in the dog. Am J Physiol 245 :171 –177,1983 [DOI] [PubMed] [Google Scholar]
- 25.Lloyd B, Burrin J, Smythe P, Alberti KG: Enzymatic fluorometric continuous flow assays for blood glucose, lactate, pyruvate, alanine, glycerol and 2-hydroxybutyrate. Clin Chem 24 :1724 –1729,1978 [PubMed] [Google Scholar]
- 26.Ho RJ: Radiochemical assay of long chain fatty acids using 63NI as tracer. Anal Biochem 26 :105 –113,1970 [DOI] [PubMed] [Google Scholar]
- 27.Torok-Both GA, Baker GB, Coutts RT, McKenna KF, Aspeslet LJ: Simultaneous determination of fluoxetine and norfluoxetine enantiomers in biological samples by gas chromatography with electron capture detection. J Chromatogr Biomed Appl 579 :99 –106,1992 [DOI] [PubMed] [Google Scholar]
- 28.Deary L, Hepburn D, Macleod K, Frier BM: Partitioning the symptoms of hypoglycemia using multi-sample confirmatory factor analysis. Diabetologia 36 :771 –770,1993 [DOI] [PubMed] [Google Scholar]
- 29.Gerich J, Noacco C, Karam J, Forsham P: Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha-cell defect. Science 182 :171 ,1973 [DOI] [PubMed] [Google Scholar]
- 30.Cryer PE: Hypoglycemia: the limiting factor in the management of IDDM. Diabetes 43 :1378 –1389,1994 [DOI] [PubMed] [Google Scholar]
- 31.Davis SN, Shavers C, Davis B, Costa F: Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. J Clin Invest 100 :429 –438,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanelli C, DeFeo P, Porcellati F, Perriello G, Torlone E, Santeusanio F, Brunetti P, Bolli GB: Adrenergic mechanisms contribute to the late phase of hypoglycemic glucose counterregulation in humans by stimulating lipolysis. J Clin Invest 89 :2005 –2013,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizza R, Haymon M, Cryer P, Gerich J: Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol Endocrinol Metab 237 :E356 –E362,1979 [DOI] [PubMed] [Google Scholar]
- 34.Guy DA, Sandoval D, Richardson MA, Tate D, Flakoll PJ, Davis SN: Differing physiological effects of epinephrine in type 1 diabetes and nondiabetic humans. Am J Physiol Endocrinol Metab 288 :E178 –E186,2005 [DOI] [PubMed] [Google Scholar]
- 35.Teves D, Videen TO, Cryer PE, Powers WJ: Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 101 :6217 –6221,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayberg H: Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48 :830 –843,2003 [DOI] [PubMed] [Google Scholar]
- 37.Taborsky GJ, Ahren B, Mundinger TO, Mei Q, Havel PJ: Autonomic mechanism and defects in the glucagon response to insulin induced hypoglycemia. Diabetes Nutr Metab 15 :318 –322,2000 [PubMed] [Google Scholar]
- 38.Carvalho F, Barros D, Silva J, Rezende E, Soares M, Fregoneze J, De Castro e Silva E: Hyperglycemia induced by acute central fluoxetine administration: role of the central CRH system and 5-HT3 receptors. Neuropeptides 38 :98 –105,2004 [DOI] [PubMed] [Google Scholar]
- 39.Chaouloff F, Gunn SH, Young JB: Central 5-hydroxytryptamine2 receptors are involved in the adrenal catecholamine-releasing and hyperglycemic effects of the 5-hydroxytryptamine indirect agonist D-fenfluramine in the conscious rat. J Pharmacol Exp Ther 260 :1008 –1016,1992 [PubMed] [Google Scholar]
- 40.Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormede P, Chaouloff F: Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis in SHR and WKY rats. Neuropharmacology 38 :893 –907,1999 [DOI] [PubMed] [Google Scholar]
- 41.Schleis T: Realities of the fluoxetine-to-sertraline switch. Am J Health Syst Pharm 52 :423 ,1995 [DOI] [PubMed] [Google Scholar]