Abstract
OBJECTIVE— It has been suggested that retinol-binding protein 4 (RBP4) links adiposity, insulin resistance, and type 2 diabetes. However, circulating RBP4 levels are also affected by kidney function. Therefore, the aim of this study was to test whether RBP4 serum levels are primarily associated with kidney function or type 2 diabetes.
RESEARCH DESIGN AND METHODS— RBP4 serum concentration was determined by enzyme-linked immunosorbent assay in 126 nondiabetic and 104 type 2 diabetic subjects. The study population was divided according to estimated glomerular filtration rate (eGFR) into the following groups: eGFR >90 ml/min per 1.73 m2 (n = 53), 60–90 ml/min per 1.73 m2 (n = 90), 30–60 ml/min per 1.73 m2 (n = 38), and <30 ml/min per 1.73 m2 (n = 49). Each group was subdivided into nondiabetic and type 2 diabetic subjects.
RESULTS— RBP4 serum concentration was elevated (2.65 vs. 2.01 μmol/l; P < 0.001) and eGFR was reduced (56 vs. 74 ml/min per 1.73 m2; P < 0.001) in type 2 diabetic vs. nondiabetic subjects, respectively. By stratifying for eGFR, no more differences in RBP4 serum concentration were detectable between type 2 diabetic and nondiabetic subjects. A linear regression analysis revealed an influence of eGFR (r = −0.477; P < 0.001) but not A1C (r = 0.093; P = 0.185) on RBP4 serum concentration.
CONCLUSIONS— Existing human data showing elevated RBP4 levels in type 2 diabetic patients may be the result of moderate renal insufficiency rather than support for the suggestion that RBP4 links obesity to type 2 diabetes.
Retinol-binding protein 4 (RBP4) is a small visceral protein (∼21 kDa), mainly synthesized in the liver and catabolized in the kidneys after glomerular filtration (1). To prevent the renal loss of RBP4 before delivering its ligand retinol to the target tissues, RBP4 is complexed by transthyretin, a homotetrameric protein with a molecular weight of ∼55 kDa, formerly known as prealbumin (2). RBP4 was recently discussed as a new adipokine that is possibly linked to insulin resistance and type 2 diabetes (3–5). Although it is known that RBP4 serum levels are elevated in states of impaired kidney function (1,6–8) (which is a common feature of diabetes, metabolic syndrome, and obesity [9,10]), parameters of kidney function have not been reported in most of the studies concerning RBP4 and insulin resistance and/or diabetes (3–5). Therefore, the aim of this study was to determine whether RBP4 serum concentration is associated with kidney function rather than type 2 diabetes.
RESEARCH DESIGN AND METHODS
Serum samples of 230 age-matched subjects (131 male, including 59 with type 2 diabetes, and 99 female, including 45 with type 2 diabetes) were collected by the Department of Endocrinology, Diabetes and Nutrition and the Department of Nephrology—both of Charité-Universitätsmedizin Berlin, Campus Benjamin Franklin, Berlin, Germany—and by the Department of Clinical Nutrition of the German Institute of Human Nutrition, Potsdam-Rehbruecke, Germany. The study protocol was approved by the ethics committees of the hospitals and the University of Potsdam. Written informed consent was obtained from each subject. Blood was taken from antecubital veins after an overnight fast and centrifuged, and serum was immediately frozen at −80°C until processing. The renal function of all subjects was quantified by the estimated glomerular filtration rate (eGFR), which was calculated using the simplified Modification of Diet in Renal Disease (MDRD) formula including serum creatinine concentration, age, and sex (11). The study population was subdivided into nondiabetic and diabetic subjects, and each subpopulation was assigned according to eGFR to the following groups: eGFR >90, 60–90, 30–60, and <30 ml/min per 1.73 m2.
Measurement of laboratory parameters.
Anthropometry was performed by the attending physician as previously described (12). Serum samples were analyzed for glucose, total cholesterol, LDL and HDL cholesterol, triglycerides, and creatinine with a Cobas Mira Analyzer (Roche, Mannheim, Germany). The intra-assay coefficients of variation (CVs) were as follows: glucose, 5.5%; total cholesterol, 5.1%; HDL cholesterol, 5.4%; and triglycerides, 5.1%. The amount of A1C was determined by an enzyme-linked immunosorbant assay as described elsewhere, with an intra-assay CV of 2.5% (12).
Determination of RBP4 and transthyretin serum concentration.
The RBP4 and transthyretin serum levels were determined by a noncommercial enzyme-linked immunosorbant assay as previously described (8,13). For calibration of both assays, RBP4 and transthyretin standards obtained from human blood were used, respectively (N Protein Standard SL; Dade Behring, Marburg, Germany), representing the physiological (nontruncated) RBP4 and transthyretin forms.
Statistical analysis.
Statistical analysis was accomplished using SPSS (version 15.0; SPSS, Chicago). Results are expressed as medians and ranges. The data were compared using the nonparametric procedures Mann-Whitney U test and Kruskal-Wallis test as appropriate. Values of P < 0.05 were considered significant.
RESULTS
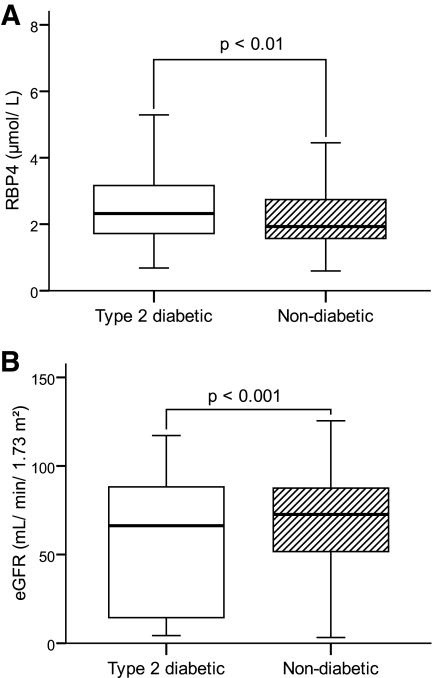
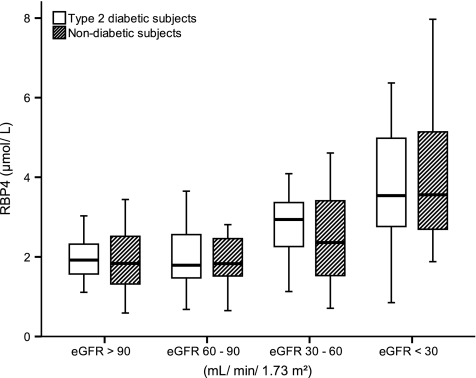
Anthropometric and clinical data were recorded by the attending physicians and are presented in Table 1. The whole type 2 diabetes cohort (n = 104) had elevated RBP4 serum levels in comparison with those of the whole nondiabetes cohort (n = 126) (median 2.65 μmol/l [range 0.68–8.03] vs. 2.01 μmol/l [0.59–7.97]; P = 0.001) (Fig. 1), confirming former results that RBP4 serum levels are related to type 2 diabetes (3–5). However, the diabetic subjects also revealed a reduction in kidney function, as indicated by a lower eGFR (56.0 ml/min per 1.73 m2 [4–117] for type 2 diabetic subjects vs. 74.1 ml/min per 1.73 m2 [3–126] for nondiabetic subjects; P < 0.001) (Fig. 1). Since the kidneys have a substantial influence on RBP4 metabolism (1,8), we tested whether the difference in RBP4 serum concentration between diabetic and nondiabetic subjects is consistent after stratifying for kidney function. To do so, we evaluated the RBP4 serum concentration of type 2 diabetic and nondiabetic subjects for all four groups individually; we were unable to detect any differences between the groups (P > 0.410 for all groups) (Fig. 2). Moreover, although there are no differences in RBP4 serum levels between type 2 diabetic and nondiabetic subjects with comparable eGFR, a gradual elevation of the RBP4 serum concentration was evident with progression of kidney injury (Fig. 2), resulting in the highest RBP4 levels for both type 2 diabetic and nondiabetic subjects measured in the group with eGFR <30 ml/min per 1.73 m2 in comparison with those of type 2 diabetic and nondiabetic subjects in all other groups (P < 0.01 for all groups). For the type 2 diabetic subjects, the difference in RBP4 serum concentration was also significant for the group with eGFR 30–60 ml/min per 1.73 m2 in comparison with those of the groups with eGFR >90 and 60–90 ml/min per 1.73 m2 (P < 0.01 for both). Furthermore, a significant inverse correlation between RBP4 serum concentration and eGFR was found (Spearman correlation coefficient rs = −0.452; P < 0.001). Finally, linear regression analysis with RBP4 as the dependent variable and including BMI, systolic and diastolic blood pressure, eGFR, and A1C (Table 2) revealed that eGFR (standardized β-coefficient = −0.482; P < 0.001) but not A1C (standardized β-coefficient = 0.093; P = 0.185) influcences RBP4 serum levels. This model was recalculated using either the presence of type 2 diabetes or fasting blood glucose instead of A1C; comparable with A1C, both parameters (presence of type 2 diabetes and fasting blood glucose) were not associated with RBP4 serum levels (P = 0.551 and P = 0.687, respectively).
TABLE 1.
Anthropometric and clinical data of the study population
| eGFR >90 ml/min per 1.73 m2 |
eGFR 60–90 ml/min per 1.73 m2 |
eGFR 30–60 ml/min per 1.73 m2 |
eGFR <30 ml/min per 1.73 m2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Nondiabetic | Type 2 diabetic | Nondiabetic | Type 2 diabetic | Nondiabetic | Type 2 diabetic | Nondiabetic | Type 2 diabetic | |
| n (male/female) | 29 (23/6) | 24 (17/7) | 54 (29/25) | 36 (18/18) | 27 (9/18) | 11 (5/6) | 16 (11/5) | 33 (19/14) |
| Age (years) | 62 (51–95) | 63 (50–84) | 60 (50–79) | 65 (48–82) | 67 (22–76) | 67 (56–72) | 60 (43–78) | 67 (39–75) |
| BMI (kg/m2) | 24.5 (17.9–36.7) | 31.8 (20.7–56.9)* | 25.5 (20.9–41.5) | 31.4 (21.2–47.7)† | 24.5 (14.8–36.9) | 32.0 (21.0–43.4)‡ | 25.0 (18.2–35.6) | 25.9 (17.5–39.1) |
| SBP (mmHg) | 132 (100–181) | 138 (109–171) | 130 (95–160) | 140 (110–184) | 136 (88–198) | 134 (109–166) | 153 (109–182) | 139 (68–192) |
| DBP (mmHg) | 77 (57–107) | 80 (62–98) | 74 (47–100) | 80 (55–95)† | 74 (46–116) | 75 (56–108) | 88 (54–107) | 70 (37–102)§ |
| Cholesterol (mmol/l) | 5.2 (3.6–6.6) | 5.1 (3.1–7.3) | 5.3 (3.5–10.5) | 5.2 (2.4–8.7) | 5.2 (2.1–8.3) | 6.0 (3.4–8.2) | 4.0 (2.8–7.1) | 4.1 (1.7–7.5) |
| HDL cholesterol (mmol/l) | 1.4 (0.7–2.1) | 1.2 (0.6–2.3) | 1.5 (0.7–2.8) | 1.3 (0.8–2.0)† | 1.6 (0.8–2.9) | 1.1 (07–1.6)‡ | 1.0 (0.6–2.5) | 0.9 (0.5–2.3) |
| LDL cholesterol (mmol/l) | 3.4 (2.6–4.7) | 3.3 (1.7–4.6) | 3.4 (2.0–8.0) | 3.3 (1.0–5.5) | 3.5 (1.1–5.6) | 3.7 (2.1–5.9) | 2.3 (20–5.6) | 2.2 (1.1–3.9) |
| Triglycerides (mmol/l) | 1.5 (0.6–11.4) | 1.6 (0.7–5.9) | 1.1 (0.5–3.5) | 1.4 (0.5–12.2)† | 1.5 (0.5–4.7) | 2.6 (1.5–7.3)‡ | 1.7 (0.5–11.4) | 2.2 (0.8–7.8) |
| FBG (mmol/l) | 5.1 (3.5–8.9) | 6.3 (4.7–10.1)* | 5.1 (3.8–11.3) | 6.5 (2.7–10.1)† | 4.7 (3.4–7.3) | 7.1 (4.0–21.1)‡ | 5.0 (3.7–8.2) | 5.8 (4.0–21.7)§ |
| A1C (%) | 5.6 (4.6–6.6) | 6.0 (5.3–10.3)* | 5.5 (4.4–6.9) | 6.4 (5.1–9.6)† | 5.7 (4.2–7.4) | 6.6 (5.7–8.7)‡ | 5.6 (4.5–6.8) | 7.4 (5.3–8.8)§ |
| eGFR (ml/min per 1.73 m2) | 100 (90–126) | 100 (90–117) | 77 (60–89) | 76 (62–88) | 51 (30–59) | 46 (33–56) | 12 (3–29) | 9 (4–29) |
| TTR (μmol/l) | 4.72 (1.01–11.2) | 5.17 (1.94–12.1) | 5.31 (056–9.98) | 4.22 (0.61–9.03) | 4.44 (0.83–12.1) | 5.08 (1.81–6.85) | 4.20 (1.75–10.4) | 4.23 (0.65–9.74) |
Data are medians (range) unless otherwise indicated.
Significant difference, with P < 0.05, between type 2 diabetic and nondiabetic subjects with an eGFR >90 ml/min per 1.73 m2.
Significant difference, with P < 0.05, between type 2 diabetic and nondiabetic subjects with an eGFR 60–90 ml/min per 1.73 m2.
Significant difference, with P < 0.05, between type 2 diabetic and nondiabetic subjects with an eGFR 30–60 ml/min per 1.73 m2.
Significant difference, with P < 0.05, between type 2 diabetic and nondiabetic subjects with an eGFR <30 ml/min per 1.73 m2. DBP, diastolic blood pressure; FBG, fasting blood glucose; SBP, systolic blood pressure; TTR, transthyretin.
FIG. 1.
RBP4 serum concentration (A) and eGFR (B) in type 2 diabetic (n = 104) and nondiabetic (n = 126) subjects.
FIG. 2.
RBP4 serum concentration in type 2 diabetic and nondiabetic subjects dependent on eGFR.
TABLE 2.
Linear regression analysis using RBP4 as the dependent variable and including BMI, systolic and diastolic blood pressure, eGFR, and A1C as independent variables
| Correlation coefficient | Standardized β | SE | P | |
|---|---|---|---|---|
| A1C | 0.098 | 0.093 | 0.083 | 0.185 |
| BMI | −0.054 | −0.051 | 0.014 | 0.464 |
| SBP | −0.122 | −0.152 | 0.004 | 0.100 |
| DBP | 0.101 | 0.127 | 0.009 | 0.173 |
| eGFR | −0.476 | −0.482 | 0.003 | 0.000 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
DISCUSSION
Several studies demonstrated elevated RBP4 serum levels in subjects with obesity, insulin resistance, or type 2 diabetes (3–5,14,15). Although it is known that kidney dysfunction results in elevated RBP4 serum concentrations (1,6–8,16) and that not only diabetes but also the metabolic syndrome and obesity are related to kidney dysfunction (9,10,17–19), information about the kidney function (such as serum creatinine concentration, glomerular filtration rate, and proteinuria) are lacking in most of the studies concerning RBP4 in the complex pathogenesis of insulin resistance and type 2 diabetes. Therefore, the aim of this study was to assess the influence of kidney function on the RBP4 serum concentration in type 2 diabetic and nondiabetic subjects.
By comparing the RBP4 serum concentration of type 2 diabetic and nondiabetic subjects irrespective of kidney function, we were able to confirm former findings and detected elevated RBP4 serum levels in type 2 diabetic subjects (3,4). However, type 2 diabetic subjects also revealed a diminished eGFR, indicating a reduced kidney function. Therefore, we stratified all subjects according to eGFR to evaluate the influence of kidney function on RBP4 serum levels. After stratification, no further differences in the RBP4 serum concentration were detectable between diabetic and nondiabetic subjects for each eGFR group. However, a decline in eGFR was accompanied by a gradual elevation of RBP4 serum levels in both type 2 diabetic and nondiabetic subjects, which was confirmed by the results of linear regression analysis, indicating an influence of eGFR but not of the presence of type 2 diabetes or other parameters of diabetes (A1C, fasting serum glucose, and BMI) on RBP4 serum concentration.
These data indicate that elevation of RBP4 serum concentration is more likely to be caused by the presence of impaired kidney function rather than type 2 diabetes. Thus, previously described associations of type 2 diabetes, the metabolic syndrome, or obesity with elevated serum RBP4 levels might be explained by the concurrence of impaired kidney function (1,6–8), which is more commonly found in these subjects (9,10). Consequently, we suggest that future studies investigating the potential role of RBP4 in the pathogenesis of insulin resistance and/or type 2 diabetes consider kidney function as a potential confounder when interpreting the results. However, it remains to be elucidated whether the increased levels of RBP4 in states of impaired kidney function contribute to the manifestation of insulin resistance and type 2 diabetes.
Acknowledgments
J.S. was supported by a Heisenberg Professorship by the Deutsche Forschungsgemeinschaft (SP 716/2[hypen]1) and a Nachwuchsgruppe “Molekulare Ernährung” of the German Ministery of Education and Research (FKZ0315083).
We thank all participants of the study and Elisabeth Pilz for her skillfull technical assistance in determining RBP4 and transthyretin serum levels.
Published ahead of print at http://diabetes.diabetesjournals.org on 16 September 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Goodman DS: Plasma Retinol-Binding Protein. Orlando, Florida, New York Academic Press,1984
- 2.Blaner WS: Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev 10 :308 –316,1989 [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB: Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436 :356 –362,2005 [DOI] [PubMed] [Google Scholar]
- 4.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB: Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354 :2552 –2563,2006 [DOI] [PubMed] [Google Scholar]
- 5.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS: Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 29 :2457 –2461,2006 [DOI] [PubMed] [Google Scholar]
- 6.Bernard A, Vyskocyl A, Mahieu P, Lauwerys R: Effect of renal insufficiency on the concentration of free retinol-binding protein in urine and serum. Clin Chim Acta 171 :85 –93,1988 [DOI] [PubMed] [Google Scholar]
- 7.Scarpioni L, Dall'aglio PP, Poisetti PG, Buzio C: Retinol binding protein in serum and in urine of glomerular and tubular nephropathies. Clin Chim Acta 68 :107 –113,1976 [DOI] [PubMed] [Google Scholar]
- 8.Raila J, Henze A, Spranger J, Mohlig M, Pfeiffer AF, Schweigert FJ: Microalbuminuria is a major determinant of elevated plasma retinol-binding protein 4 in type 2 diabetic patients. Kidney Int 72 :505 –511,2007 [DOI] [PubMed] [Google Scholar]
- 9.Middleton RJ, Foley RN, Hegarty J, Cheung CM, McElduff P, Gibson JM, Kalra PA, O'Donoghue DJ, New JP: The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol Dial Transplant 21 :88 –92,2006 [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Pozzoni P, Del Vecchio L: Renal manifestations in the metabolic syndrome. J Am Soc Nephrol 17 (Suppl. 2):S81 –S85,2006 [DOI] [PubMed] [Google Scholar]
- 11.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39 (Suppl. 1):S1 –S266,2002 [PubMed] [Google Scholar]
- 12.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF: Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52 :812 –817,2003 [DOI] [PubMed] [Google Scholar]
- 13.Raila J, Wirth K, Chen F, Buscher U, Dudenhausen JW, Schweigert FJ: Excretion of vitamin A in urine of women during normal pregnancy and pregnancy complications. Ann Nutr Metab 48 :357 –364,2004 [DOI] [PubMed] [Google Scholar]
- 14.Lee DC, Lee JW, Im JA: Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism 56 :327 –331,2007 [DOI] [PubMed] [Google Scholar]
- 15.Janke J, Engeli S, Boschmann M, Adams F, Böhnke J, Luft FC, Sharma AM, Jordan J: Retinol-binding protein 4 in human obesity. Diabetes 55 :2805 –2810,2006 [DOI] [PubMed] [Google Scholar]
- 16.Stewart WK, Fleming LW: Plasma retinol and retinol binding protein concentrations in patients on maintenance haemodialysis with and without vitamin A supplements. Nephron 30 :15 –21,1982 [DOI] [PubMed] [Google Scholar]
- 17.Hollenberg NK: Obesity and the kidney: why is the kidney at risk? Kidney Int 71 :187 –188,2007 [DOI] [PubMed] [Google Scholar]
- 18.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, Sattar N, Zukowska-Szczechowska E, Dominiczak AF: Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 71 :816 –821,2007 [DOI] [PubMed] [Google Scholar]
- 19.New JP, Middleton RJ, Klebe B, Farmer CK, de Lusignan S, Stevens PE, O'Donoghue DJ: Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet Med 24 :364 –369,2007 [DOI] [PubMed] [Google Scholar]