Abstract
Depressed individuals display biased attention for emotional information when stimuli are presented for relatively “long” (e.g., 1 second) durations. The current study examined whether attentional biases are sustained over a much longer period. Specifically, clinically depressed and never depressed young adults simultaneously viewed images from four emotion categories (sad, threat, positive, neutral) for 30 seconds while line of visual gaze was assessed. Depressed individuals spent significantly more time viewing dysphoric images and less time viewing positive images than their never depressed counterparts. Time course analyses indicated that these biases were maintained over the course of the trial. Results suggest that depressed participants’ attentional biases for dysphoric information are sustained for relatively long periods even when other emotional stimuli are present. Mood congruent information-processing biases appear to be a robust feature of depression and may have an important role in the maintenance of the disorder.
Keywords: Cognitive bias, information processing, depression maintenance, attention, eye movements
Cognitive theories of depression postulate that depressed individuals are characterized by negative biases in information processing (e.g., Beck, 1976). In general, these models propose that biases in attention, perception, and memory serve to maintain a major depressive episode. In regard to attention, depressed individuals are expected to selectively attend to negative stimuli and filter out positive stimuli. This bias, in turn, is thought to contribute to the maintenance of the disorder.
Numerous studies of attentional biases in depression have been conducted. Despite initial null findings (e.g., Mogg, Bradley, Williams, & Mathews 1993; MacLeod, Mathews, & Tata, 1986), more recent work has documented an association between depression and biased attention. For instance, Gotlib et al. (2004) reported an attentional bias for sad facial expressions in a clinically depressed sample using a dot probe task (Gotlib, Krasnoperova, Yue, & Joorman, 2004). Gotlib et al. (2004) and Joormann and Gotlib (2007) subsequently replicated this finding. Caseras, Garner, Bradley, and Mogg (2007) provided further evidence that dysphoric individuals maintained their gaze longer on negative pictures than non-dysphoric people.
One explanation for why this recent work has more consistently found an attentional bias for negative stimuli involves the stimulus duration. Early work tended to use relatively brief stimulus durations (e.g., ≤500ms). More recent work has used longer stimulus durations (e.g., 1000ms). Given this set of findings, Mogg and Bradley (2005) have suggested that depression is not associated with an initial orienting bias, but instead attentional biases are more likely to be observed under conditions that allow or encourage elaborative processing (i.e., longer stimulus durations). Indeed, this is consistent with other conceptualizations that point to sustained processing as a critical factor for the maintenance of depression (e.g., Siegle, Granholm, Ingram, & Matt, 2001).
To date, few studies of biased attention have used assessments that measure sustained processing of emotional stimuli. Commonly used dot probe tasks obtain a “snapshot” of biased processing, as an attentional bias is measured by reaction time immediately following the offset of the stimulus. If the probe is consistently detected more quickly when it replaces dysphoric stimuli, a bias for dysphoric stimuli is inferred. Use of multiple stimulus durations can assess the time course of sustained processing; however, assessing more than two or three stimulus durations often becomes difficult due to subject burden. Therefore, reaction time tasks may provide an excellent assessment of early processing but yield a somewhat incomplete picture of sustained information processing.
To overcome this issue, a relatively new paradigm has been proposed in which tracking of eye movements provides a relatively continuous index of information processing (Hermans, Vansteenwegen, & Eelen, 1999; Isaacowitz, 2005). Under normal viewing conditions, individuals typically direct their line of gaze towards stimuli that attract their attention (Jonides, 1981). While attention can be moved without eye movements under certain laboratory conditions, the converse is not true; there is an obligatory shift in attention prior to every eye movement (Kowler, Anderson, Dosher, & Blaser, 1995). Shifts in gaze position thus closely follow and are guided by shifts in attentional focus. Further, measuring eye movements avoids problems of slow and variable motor responses observed in some psychiatric conditions (Mathews, Ridgeway, & Williamson, 1996). Visual attention is not typically confounded by such motor-slowing deficits. Most importantly, eye tracking methodology may be ideally suited for measuring biased attention in depression, as line of visual gaze can be assessed relatively continuously (e.g., sampled every 16ms) across relatively long periods of time.
Only one small study of sustained information processing using eye tracking methodology has been completed to date with depressed individuals. Eizenman et al. (2003) used eye tracking methodology to study the visual scanning pattern of depressed (n = 8) and non-depressed (n = 9) individuals. Participants were instructed to scan and re-scan four complex emotional images that were presented simultaneously for 10.5 seconds. These images depicted dysphoric, threat, positive, and neutral themes. Depressed participants spent significantly more time attending to dysphoric images than controls. This study also demonstrated that the average glance duration for dysphoric images was significantly longer for depressed individuals suggesting difficultly shifting their attention away from dysphoric stimuli.
Given that only one study to date has examined attentional biases in clinically depressed individuals using eye tracking methodology, we thought it was important to replicate the Eizenman et al. (2003) findings. However, we also wanted to expand on this previous work by examining the time course of biased attention. Particularly, it is important to understand whether a depressed individual’s selective attention for mood congruent stimuli increases, decreases, or is sustained over time. This should provide important insight into the nature and pervasiveness of biased attention in major depressive disorder.
Using a similar eye tracking paradigm as Eizenman et al. (2003), we obtained a relatively continuous measurement of selective attention using eye tracking methodology while extending the stimulus presentation time. Additionally, participants were instructed to look freely at the images with no constraints, as if they were watching television or viewing images in a photo album. These modifications were implemented to encourage naturalistic information processing, as in other eye tracking studies (Isaacowitz, 2005). Finally, in addition to examining the percentage of time participants attended to each stimulus category over time, we also examined whether depression groups differed in number of fixations per stimulus category, glance duration for each stimulus type, and location of first fixation to better characterize negatively biased attention in depression.
Method
Participants
Participants were eighty-six young adults between the ages of 18 and 21, either currently experiencing a major depressive episode or with no history of major depression. These participants were recruited from a large university and surrounding community in the southwestern United States. Students (n = 83) participated in exchange for credit in their introductory psychology course while community participants (n = 3) were compensated monetarily ($20) for their time. A two-step procedure was used to recruit participants. All participants were initially screened using the short form of the Beck Depression Inventory (BDI-SF) to assess for the presence or absence of depressive symptoms. Individuals with scores less than four and greater than fourteen were recruited for the study.
Upon arrival for the study, individuals completed a diagnostic interview and self-report assessments of current depression. Inclusion criteria for the depressed group was a diagnosis of current major depressive disorder (MDD) in the absence of past mania or hypomania and a score of fifteen or higher on the Beck Depression Inventory-II (BDI-II). Inclusion in the never depressed group (ND) required the absence of any current or past mood disorder and a BDI-II score less than nine at the time of testing. Consistency between interview-rated and self-reported depressive symptoms (BDI-II score) was used to ensure that individuals with moderate self-reported depressive symptoms were not included in the nondepressed group and that individuals with few self-reported symptoms of depression were not included in the depressed group.
Participants were excluded for the following reasons: past depressive episode (never depressed group only, n = 8), MDD in partial remission (n = 1), Bipolar II Disorder (n = 1), lack of agreement between SCID diagnosis and BDI-II score (n = 11), and difficulty obtaining stable eye tracking (e.g. unacceptable head movement, interference from glasses, eyelid occluded the pupil; n = 5). Therefore, the final sample consisted of sixty participants (32 men and 28 women; age M = 18.82, SD = 0.90); fifteen met diagnostic criteria of major depressive disorder (BDI-II M = 29.53, SD = 8.77; BAI M = 22.87, SD = 4.48; 60% female) and forty-five were never depressed participants (BDI-II M = 2.71, SD = 2.05; BAI M = 22.87, SD = 12.68; 47% female). The depressed and never-depressed groups did not differ in terms of gender distribution χ2 (N = 45) = 2.20, p = 15.
Assessments
Diagnostic assessment
Major depressive disorder (MDD) was diagnosed with the Mood Disorders Module of the Structured Clinical Interview for the DSM-IV Diagnoses – Patient Version (SCID; First, Spitzer, Gibbon, & Williams., 1998). Two trained assessors conducted all interviews. Three advanced doctoral trainees acted as independent assessors and each rated twenty percent of all interviews (n = 15). Agreement on MDD diagnosis was very good (kappas [κ] = .85 – 1.0).
Self-report measures of depression and anxiety
Participants completed the Beck Depression Inventory-II (BDI-II: Beck, Brown, & Steer, 1996) and the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988), two widely used self-report measures of depression and anxiety. Each questionnaire consists of 21 items that measure the presence and severity of cognitive, affective, motivational, and physiological symptoms of depression or anxiety. Past reports indicate adequate reliability and validity of these measures (Beck, Steer, & Garbin, 1988; Fydrich, Dowdall, & Chambless, 1992).
Eye Tracking Paradigm
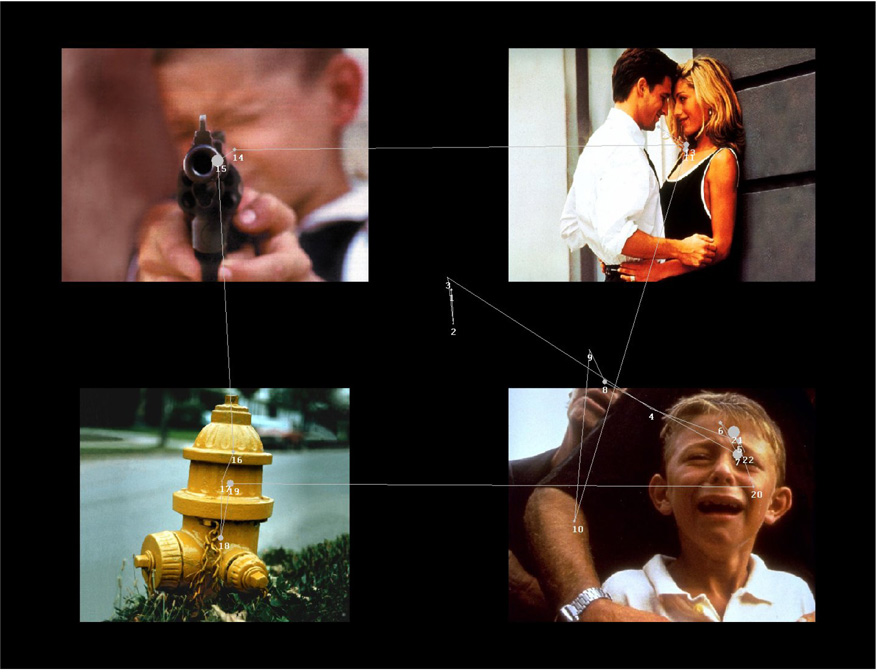
This task involved simultaneous presentation of four images selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005).1 Images from this picture system are well standardized and used extensively in studies of psychopathology. Each slide contained 4 images, one of 12 images selected from each of the following four categories: dysphoric, threat, neutral, and positive (see Figure 1).
Figure 1.
This trial was randomly selected from a depressed participant. Fixations from this trial are superimposed on this image. Larger diameter of fixations indicates longer fixation duration. Numbers next to each fixation indicates sequence in which fixations occurred. Lines connecting fixations are intended to highlight the fixation sequence, but do not represent the actual scan path.
The IAPS images have been systematically rated for valence but not for specific emotion categories, such as dysphoric and threat. We conducted a pilot study to categorize the negative images into dysphoric and threat categories.2 The IAPS stimuli have previously been rated on nine point scales for valence (unpleasant = 1 to pleasant = 9) and arousal (calm = 1 to excitement = 9). Valence ratings for the threatening and dysphoric images ranged from 2 to 4, whereas positive images were rated from 6 to 8. Neutral images had valence ratings of approximately 5.
The mean valence ratings for positive (M = 7.3, SD = 0.4) and neutral (M = 5.1, SD = 0.2) images were significantly different from the other three categories (ts > 13.1, ps < .001), whereas the valence ratings for dysphoric (M = 2.4, SD = 0.4) and threatening (M = 2.6, SD = 0.6) images did not differ from each other (t = .58, ns). However, threatening (M = 6.7, SD = 0.6) images did have a significantly greater mean arousal rating than dysphoric (M = 4.9, SD = 0.5), positive (M = 4.6, SD = 0.7), or neutral (M = 2.8, SD = 0.3) images (ts > 7.8, ps < .001). In addition, neutral images had a significantly lower mean arousal rating than dysphoric, positive, or threatening images (ts > 7.9, ps < .001), whereas the dysphoric and positive images did not differ from each other in arousal (ts = 1.4, ns).
For each eye tracking trial, the location of each image was randomly selected, with the constraint that each stimulus category must occur in each of the four positions three times across 12 trials. Eight filler trials with four neutral images were presented to obscure the nature of the task. A total of 20 trials (12 study + 8 filler) were presented in a new random order for each participant. Each trial began with a 1000 millisecond centrally presented fixation cross, followed by presentation of stimuli for 30 seconds.
Eye Tracking System
Line of visual gaze was assessed using a remote optics eye tracking system model R6 from Applied Science Laboratories. Head location was fixed using a chin rest and forehead bar. The direction of gaze, measured with x and y coordinates, was sampled every 16.7 milliseconds (60Hz). Eye movements that were stable for more than 100ms within a 1° of visual angle were classified as a fixation. Areas of interest (AOIs) were also identified for each trial and corresponded with the total area for each of the four images. Thus, it was possible to determine location of first fixation, number of fixations, and total time spent viewing each of the four images presented during each trial (see Figure 1). E-Prime software was used to present the stimuli and to automate the recording of eye location with the eye tracker software.
Procedure
After signing an informed consent form, participants completed a demographics form, the BDI-II, BAI, and the Mood Disorders Module from the Structured Clinical Interview for DSM-IV (SCID; First et al., 1998). Participants were then escorted to the eye tracking room and seated in a height adjustable chair and placed their chin in a chin rest. The chin rest was positioned so that participants’ eyes were level with the middle of the 17-inch monitor on which the stimuli were presented. This ensured that all participants’ eyes were in the same location relative to the camera and the monitor. Camera adjustments were made to best capture the participant’s right eye, and a 9-point calibration was completed to confirm that the eye tracker was recording line of visual gaze within 1° of visual angle for each calibration point. Calibration was repeated until this criterion was met.
Once calibration was successful, participants were instructed via the computer screen to view the images naturally, as if they were watching television. The only constraint was that they were to view the images at all times during each trial. Importantly, all instructions emphasized that the study was measuring associations between pupil dilation (a secondary measure that was not relevant to this study) and emotional images. Tracking of eye movements was not mentioned in order to minimize demand effects. Further, participants were instructed to look at the fixation cross prior to each trial in order to standardize the starting location of their gaze. The experimenter (located in an adjacent room) then monitored the stimulus presentation and eye tracking throughout each trial.
Data Analysis Plan
Our primary analysis examined whether depression groups differentially attended to each stimulus category over time. To do so, we divided each 30 second trial into six 5-second segments. Within each five-second segment we computed percent time attending to each stimulus category. We then used a mixed-plot ANOVA to examine whether depression groups differentially attended to stimuli from each category over time. Secondary analyses examined whether depression groups differentially attended to stimuli category across several additional eye tracking outcomes: (a) number of fixations per category, (b) mean glance duration, and (d) location of first fixation.
Results
Percent Time Attending to Stimuli
A 6 (time segment: 0 – 5, 5 – 10, 10 – 15, 15 – 20, 20 – 25, 25 – 30) × 4 (stimulus valence: dysphoric, threat, positive, neutral) ×2 (depression group: MDD, ND) mixed-plot analysis of variance examined whether depression group was associated with differential attention to stimuli category over time. Analyses indicated a significant main effect for valence, F(3, 174) = 11.47, p < .001, η2 = .17, that was qualified by a valence × depression group interaction, F(3, 174) = 3.21, p = .03, η2 = .05. There was also a significant main effect for time, F(5, 290) = 11.62, p < .001, η2 = .18, that was qualified by a valence × time interaction, F(15, 870) = 2.92, p < .001, η2 = .05. The time×depression group, F(5, 290) = 1.21, p = .32, η2 = .02, and valence ×time × depression group, F(15, 870) = 1.21, p = .26, η2 = .02, interactions were not statistically significant. 3
We examined the significant stimuli valence × depression group interaction by comparing depression groups within each stimulus category. The depressed group spent a significantly greater percentage of time attending to dysphoric stimuli, F(1, 58) = 7.83, p = .007, η2 = .12 and less time attending to positive stimuli, F(1, 58) = 4.92, p = .03, η2 = .08, than never-depressed participants (see Table 1). There were no group differences for threatening stimuli, F(1, 58) = 0.06, p = .82, η2 = .00, and neutral stimuli, F(1, 54) = 0.00, p = .99, η2 = .00. The absence of a significant stimuli valence × depression group × time interaction suggests that the observed group differences for dysphoric and positive stimuli did not significantly change over time.
Table 1.
Mean (standard deviation) for total fixation time, number of fixations, glance duration, and location of first fixation for each stimulus category for depressed (D) and never-depressed (ND) groups.
| Stimulus Category | Fixation Time (% of total) | Number of Fixations (% of total) | Glance Duration (sec) | Location of First Fixation (% of total) | ||||
|---|---|---|---|---|---|---|---|---|
| ND | D | ND | D | ND | D | ND | D | |
| Dysphoric | 23.17 | 27.98 | 24.04 | 27.97 | .42 | .45 | 21.36 | 24.11 |
| (5.75) | (6.10) | (4.47) | (4.81) | (.08) | (.10) | (9.80) | (13.04) | |
| Threat | 27.28 | 27.91 | 26.08 | 26.39 | .44 | .46 | 31.69 | 30.50 |
| (7.62) | (8.47) | (6.49) | (6.68) | (.08) | (.08) | (11.36) | (8.99) | |
| Positive | 30.62 | 25.01 | 29.08 | 25.45 | .44 | .43 | 27.45 | 26.65 |
| (8.94) | (6.16) | (6.82) | (5.41) | (.09) | (06) | (11.56) | (13.50) | |
| Neutral | 18.92 | 19.09 | 20.79 | 20.19 | .42 | .44 | 18.97 | 18.07 |
| (5.58) | (5.23) | (4.41) | (4.51) | (.11) | (.15) | (9.94) | (10.60) | |
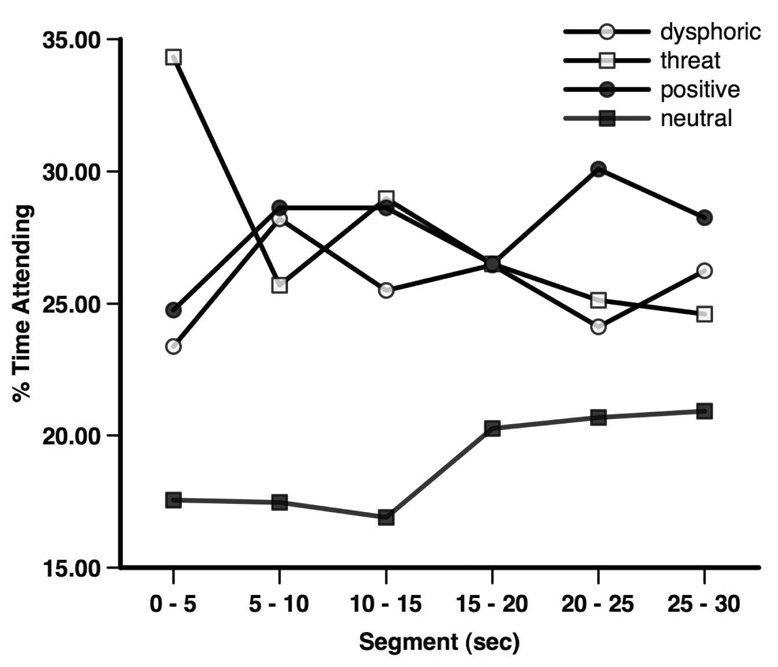
We also examined the time × valence interaction by examining change over time within each stimulus category (see Figure 2). For dysphoric stimuli, there was no significant effect for time, F(5, 295) = 1.71, p = .13, η2 = .03; attention towards dysphoric stimuli was relatively stable over time. For threatening stimuli, there were significant linear, F(1, 59) = 28.19, p < .001, η2 = .32, quadratic, F(1, 59) = 5.78, p = .02, η2 = .09, and cubic, F(1, 59) = 4.35, p = .04, η2 = .07, effects for time. Attention to threatening stimuli strongly decreased over time; however, this linear decrease was attenuated somewhat during the middle segment of the trial. Attention to positive stimuli had a significant linear, F(1, 59) = 7.12, p = .01, η2 = .11, and quadratic, F(1, 59) = 5.17, p = .03, η2 = .08, association with time; percent time attending to positive stimuli increased over time but then decreased towards the end of the trial. Percent time attending to neutral stimuli had a significant linear association with time, F(1, 59) = 10.45, p = .002, η2 = .15; attention towards neutral stimuli increased over time.
Figure 2.
Percent time attending to each stimulus category across five-second segments.
Percent Fixations per Stimulus Category
A 4 (valence: dysphoric, threat, positive, neutral) × 2 (depression group: depressed, never-depressed) mixed-plot ANOVA examined whether depression groups differed in percentage of fixations per stimulus category. There was a significant valence effect, F(3, 174) = 10.05, p < .001, η2 = .15, and a significant valence × group interaction, F(3, 174) = 2.60, p = .05, η2 = .04. Follow-up simple effects between group comparisons revealed that the depressed groups had a significantly greater percentage of fixations for dysphoric stimuli, F(1, 58) = 8.33, p = .005, η2 = .13, and marginally fewer fixations for positive stimuli, F(1, 58) = 3.50, p = .06, η2 = .06, than never-depressed participants (see Table 1). No group differences were observed for threat, F(1, 58) = 0.88, p = .88, η2 = .00, or neutral stimuli, F(1, 58) = 0.21, p = .65, η2 = .00.
Mean Glance Duration
A 4 (valence: dysphoric, threat, positive, neutral) × 2 (depression group: depressed, never-depressed) mixed-plot ANOVA examined whether depression groups differed in glance duration (i.e., mean fixation duration) by stimulus category. Analyses indicated a non-significant effect for valence, F(3, 174) = 0.97, p = .41, η2 = .02, and a non-significant valence × depression group interaction, F(3, 174) = 1.06, p = .37, η2 = .02. Mean glance duration for depressed and never-depressed participants did not significantly differ across stimulus categories (see Table 1).
Location of First Fixation
A 4 (valence: dysphoric, threat, positive, neutral) × 2 (depression group: depressed, never-depressed) mixed-plot ANOVA examined whether depression groups differed in location of first fixation across stimulus categories. Analyses revealed a significant main effect for valence, F(3, 174) = 8.32, p < .001, η2 = .13, and a non-significant interaction between valence and depression group, F(3, 174) = 0.25, p = .86, η2 = .00. LSD follow-up comparisons indicated that participants’ first fixations were more likely to be directed towards threat and positive stimuli than neutral and dysphoric stimuli (ps < .05). Likelihood of first fixation being directed towards threat stimuli and positive stimuli did not differ (p = .16).
Discussion
We examined selective attention for emotional stimuli in clinically depressed and never depressed young adults. Uniquely, we also examined the unfolding of selective attention over time in these two groups. Depressed individuals spent more time attending to dysphoric stimuli than their never depressed counterparts. This group difference was relatively consistent over the course of the 30-second trial; that is, time did not moderate the association between depression group status and stimulus valence.
The number of fixations directed at dysphoric stimuli, rather than longer glance durations, appeared to be responsible for the depression group differences in total time spent viewing dysphoric images. This repeated turning of attention towards the dysphoric stimuli likely reflects sustained or elaborative processing of the stimuli. Each fixation represents a relatively small proportion of each stimulus; repeated fixations within a stimulus may be required to comprehensively process the image. In contrast, the never depressed group spent significantly more time attending to and had a greater number of fixations for positive stimuli than depressed participants, suggesting more sustained processing of the positive images among the never depressed group. Finally, there were no group differences for location of first fixation. All participants tended to initially view the threatening and positive images more often than dysphoric or neutral images.
These findings are consistent with cognitive models (e.g., Beck, 1976), which posit that depressed individuals display biased attention for dysphoric stimuli. Our study provides an important replication of the only other eye tracking study to examine biased attention in a sample of clinically depressed individuals. Taken together, these studies provide a robust demonstration of depressed individuals’ biased attention for dysphoric information and loss of bias for positive information that characterizes never-depressed individuals. More importantly, however, our study expands upon previous work by demonstrating that these group differences are sustained; that is, they are relatively consistent over time. The vast majority of research has examined information processing biases among depressed individuals early (e.g., < 1500 ms) in the processing of emotional stimuli. We examined the course of processing over a 30-second trial (30,000 ms) and found that depressed individuals more consistently attended to dysphoric stimuli than their never-depressed counterparts. This evidence further bolsters the argument that sustained processing of dysphoric stimuli likely contributes to the maintenance of major depressive disorder.
This study also provides a relatively strong test of biased processing because dysphoric stimuli were presented simultaneously with other emotional stimuli. Most prior research has examined processing biases with pairs of stimuli (e.g., neutral-emotional word pairs). Our data suggest that the presentation of emotionally evocative stimuli does not disrupt biased attention for dysphoric stimuli—depressed individuals attend to dysphoric stimuli even in the presence of other emotional information including other negative information (i.e., threat stimuli).
Although depressed individuals show specific differences in their processing of dysphoric and positive stimuli, these differences were not evident in the very earliest stages of information processing. There were no group differences for which stimulus category participants viewed first. Whereas anxious individuals show a tendency to orient their eye movements toward threat stimuli (Mogg, Millar, & Bradley, 2000), results from this and other (Caseras et al., 2007) work suggest that depressed individuals do not show such a bias.
Information processing biases are thus more likely to occur in the elaborative stages of information processing. This is consistent with other research which has shown that depressed individuals tend to ruminate about dysphoric topics (Nolen-Hoeksema, 2000), have more difficulty inhibiting dysphoric stimuli (Goeleven, De Raedt, Baert, & Koster, 2006), and experience more intrusive negative thoughts (Beevers, Wenzlaff, Hayes, & Scott, 1999). It may be that anxiety is characterized by vigilance for threat-related stimuli in the early stages of processing followed by the avoidance of threatening stimuli (Rinck & Becker, 2006), whereas depression involves processing biases during more elaborative stages of information processing (Mogg & Bradley, 2005). Future work should examine whether difficulty disengaging attention underlies this sustained processing bias in depression (e.g., Koster, De Raedt, Goeleven, Franck, & Crombez, 2005).
Several limitations of this study should be noted. We studied young adults, which limits generalizability to older depressed populations. Although we conducted clinical interviews to diagnose major depressive disorder, we did not complete full SCID interviews (i.e., only the Mood Disorders Module). Therefore, it is possible that participants may have had other undiagnosed disorders. Our sample size for the depressed group was also relatively small, although it was more than twice the size of the only other eye tracking study of selective attention with clinically depressed participants (Eizenman et al., 2003). Larger sample sizes in future work will allow for more statistical power to detect smaller differences in attentional biases over time. Finally, although the use of complex images that vary in content may have reduced our experimental control somewhat, they do have the added benefit of increased realism and perhaps generalizability.
In conclusion, the current study suggests that depressed young adults significantly differ from their never depressed counterparts in how they attend to emotional information. Depressed individuals are more likely to attend to dysphoric images and less likely to attend to positive images than never depressed people. The current results also suggest that these biases are sustained over time relatively long periods of time. That is, there were no significant effects for time, suggesting that the biases were evident throughout the majority of each 30-second trial. Given these results, future longitudinal research is needed to determine whether sustained processing of dysphoric information is associated with time to depression remission and whether it increases vulnerability for experiencing a depressive episode. We believe that eye tracking methodology may provide a sensitive and robust assessment of biased processing that is ideal for this important work.
Acknowledgments
Preparation of this article was facilitated by a grant (R01MH076897) from the National Institute of Mental Health to Christopher Beevers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The following IAPS images were used: Dysphoric – 2141, 2205, 2276, 2455, 2700, 2703, 2799, 2900, 3230, 9220, 9421, and 9530; Threat – 1120, 1300, 2811, 3500, 6260, 6312, 6313, 6350, 6510, 6560, 6562, and 6821; Positive – 1340, 2091, 2165, 2208, 2224, 2299, 2339, 2340, 2501, 4599, 4700, and 8461; Neutral – 2038, 2102, 2393, 2397, 2745, 2850, 5500, 5731, 7009, 7041, 7080, and 7185; Neutral filler – 2235, 2396, 2514, 2880, 5390, 5740, 7000, 7004, 7010, 7053, 7090, 7100, 7187, 7235, 7547, and 7950.
We conducted a pilot study to identify which emotion category best described the negative images. Ninety undergraduate students were asked to rate how “sad” and “threatening” each of the 24 negative images was on a 9-point scale (not at all = 1 to extremely = 9). Presentation order of the images was randomized across participants and presentation order for the rating categories were counterbalanced. As expected, the dysphoric images were rated as significantly more sad than threatening images (M = 6.73, SD = 1.89; M = 4.15, SD = 2.69, respectively), t(89) = 13.4, p < .001. Further, the threatening images were rated as significantly more threatening than dysphoric images (M = 7.38, SD = 1.86; M = 2.71, SD = 2.11, respectively), t(89) = −21.6, p < .001.
We completed this same analysis controlling for anxiety symptoms measured with the total score on the BAI. Anxiety symptoms did not significantly interact with any of the main effects or interactions in the previous analysis (Fs < 1, ns). Further, although the previously observed interaction between depression group status and stimulus valence was attenuated somewhat, F(3, 162) = 1.70, p = .16, which is not surprising given the strong correlation between the BAI and BDI (r = .8), the form of the interaction was very similar to analyses without the BAI covariate. Therefore, anxiety symptoms do not appear to be primarily responsible for the observed effects for depression group status.
References
- Beck AT. Cognitive therapy and the emotional disorders. New York: New American Library; 1976. [Google Scholar]
- Beck AT, Brown AT, Steer RA. Psychological Corporation. San Antonio: TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beevers CG, Wenzlaff RM, Hayes AM, Scott WD. Depression and the ironic effects of thought suppression: Therapeutic strategies for improving mental control. Clinical Psychology: Science and Practice. 1999;6:133–148. [Google Scholar]
- Caseras X, Garner M, Bradley BP, Mogg K. Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology. 2007;116(3):491–497. doi: 10.1037/0021-843X.116.3.491. [DOI] [PubMed] [Google Scholar]
- Eizenman M, Yu L, Grupp L, Eizenman E, Ellenbogen M, Germar M, Levitan RD. A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Research. 2003;118:117–128. doi: 10.1016/s0165-1781(03)00068-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders-Patient Edition (SCID-I/P, 11/2002 revision) New York: New York State Psychiatric Institute; 1998. Biometrics Research. [Google Scholar]
- Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. Journal of Anxiety Disorders. 1992;6:55–61. [Google Scholar]
- Goeleven E, De Raedt R, Baert S, Koster EHW. Deficient inhibition of emotional information in depression. Journal of Affective Disorders. 2006;93:149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joorman J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113(1):127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Hermans D, Vansteenwegen D, Eelen P. Eye movement registration as a continuous index of attention deployment: Data from a group of spider anxious students. Cognition & Emotion. 1999;13:419. [Google Scholar]
- Isaacowitz DM. The gaze of the optimist. Personality and Social Psychology Bulletin. 2005;31:407. doi: 10.1177/0146167204271599. [DOI] [PubMed] [Google Scholar]
- Jonides J. Voluntary versus automatic control over the mind's eye movements. In: Long J, Baddeley AD, editors. Attention and performance ix. Hillsdale: Guilford; 1981. pp. 187–203. [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-congruent attentional bias in dysphoria: Maintained attention to and impaired disengagement from negative information. Emotion. 2005;5:446. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;35:1897. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2005. Technical Report A-6. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behaviour Research & Therapy. 1996;34:695. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy & Research. 2005;29:29–45. [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Rinck M, Becker ES. Spider fearful individuals attend to threat, then quickly avoid it: Evidence from eye movements. Journal of Abnormal Psychology. 2006;115:231–238. doi: 10.1037/0021-843X.115.2.231. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49(7):624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]