Abstract
In contrast to relapse, the mechanisms of multiple sclerosis (MS) disease progression are less understood and appear not to be exclusively inflammatory in nature. In this pilot study we investigated the relationship between disturbed CNS energy metabolism and MS disease progression. We tested the hypothesis that cerebrospinal fluid (CSF) concentrations of sorbitol, fructose, and lactate, all metabolites of extra-mitochondrial glucose metabolism, would be elevated in secondary progressive (SP) MS patients and would be associated with worsening neurologic disability. We measured metabolite concentrations by gas chromatographic/mass spectrometric and enzymatic methods in archived CSF samples from 85 MS patients [31 relapsing-remitting (RR) and 54 SP patients] and 18 healthy controls. We found that concentrations of all three metabolites, but not concentrations of glucose or myoinositol, were significantly increased in CSF from SP and, to a lesser degree, RR patients, compared to controls. Furthermore, CSF concentrations of sorbitol and fructose (polyol pathway metabolites), but not lactate (anaerobic glycolysis metabolite), correlated positively and significantly with Expanded Disability Status Scale (EDSS) score, an index of neurologic disability in MS patients. We conclude that extra-mitochondrial glucose metabolism is increased in MS patients and is associated with disease progression evidenced by increasing EDSS score. As extra-mitochondrial glucose metabolism increases with impaired mitochondrial metabolism of glucose, these findings implicate mitochondrial dysfunction in the pathogenesis of MS disease progression. CSF metabolic profiling may be useful in clarifying the role of mitochondrial pathology in progression and in targeting and monitoring therapies for disease progression that aim to preserve or boost mitochondrial glucose metabolism.
Keywords: Multiple sclerosis, metabolism, mitochondrial, polyol, lactate, glucose, energy, progression, degeneration
INTRODUCTION
Despite the success of immunomodulatory therapies in preventing relapse, most individuals with relapsing-remitting (RR) multiple sclerosis (MS) eventually develop secondary progressive (SP) disease with irreversible neurologic disability. This observation, plus research showing that progression is not affected by relapse, suggests that the pathophysiology of progression is not exclusively inflammatory in nature (1, 2).
Searching for new approaches to preventing disease progression, investigators have explored a possible role of disturbed CNS energy metabolism (3-6). This work has primarily focused on dysfunction of the mitochondrion, the cellular organelle most critical to energy metabolism. Mitochondrial disease mechanisms have been implicated in other neurodegenerative disorders and may be particularly relevant to MS because of similarities in neurologic symptoms between MS and Leber's Hereditary Optic Neuropathy (LHON), a demyelinating disorder caused by mitochondrial DNA mutations (7, 8).
In this study we investigated the relationship between disturbed CNS energy metabolism and MS disease progression by measuring cerebrospinal fluid (CSF) concentrations of metabolites from two extra-mitochondrial pathways of glucose metabolism, the polyol pathway (sorbitol and fructose) and the anaerobic glycolytic pathway (lactate), in MS patients at different disease stages (RR and SP) and in healthy controls. This was a hypothesis-driven, rather than an unbiased metabolomic, study. We focused on two extra-mitochondrial pathways of glucose metabolism whose activity would be expected to increase with mitochondrial dysfunction.
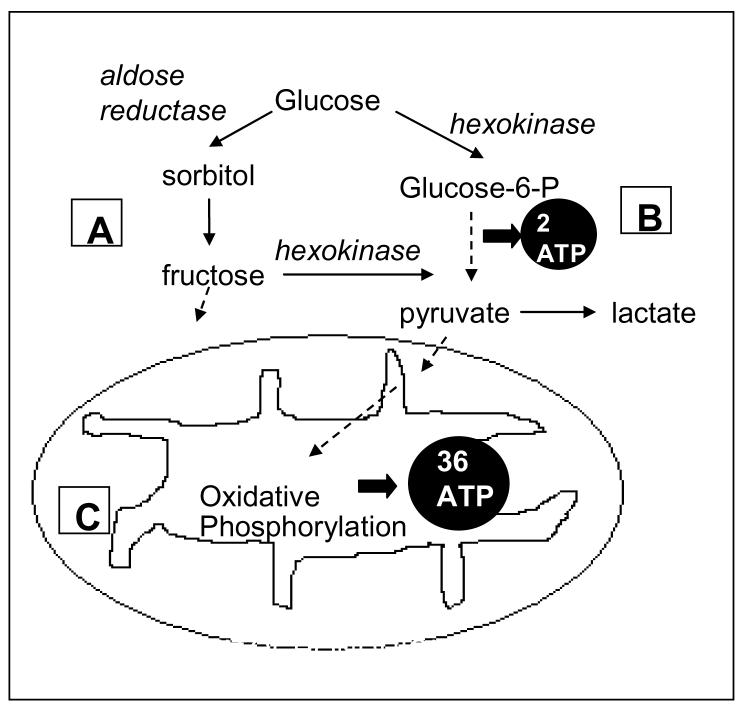
The first is the polyol pathway, a minor metabolic pathway of glucose running parallel to glycolysis, whose activity is increased in certain pathological states including hyperglycemia, mitochondrial dysfunction, and rare inborn errors of polyol metabolism (Figure 1A). Previous investigators have reported elevated CSF concentrations of either or both of the polyol pathway metabolites of glucose, sorbitol and fructose, in MS patients (9-11). There are no reports, however, that specify whether the elevation was specific to RR or to progressive MS. Based on preliminary data showing a greater increase in CSF polyol pathway metabolite concentrations in progressive (primary and secondary) compared to RR patients (unpublished data), we hypothesized that increased CNS polyol pathway activity contributes to disease progression. Increased polyol pathway activity has been linked to demyelinating axonal disease in diabetes mellitus and in disorders caused by rare inborn errors of polyol metabolism (12-14). Polyol pathway mediated disease mechanisms include osmotic stress, due to intracellular accumulation of polyols, as well as oxidative and ischemic cellular stresses (15).
Figure 1.
Mitochondrial and two extra-mitochondrial pathways of glucose metabolism: (A) the polyol pathway, (B) anaerobic glycolysis, and (C) mitochondrial oxidative phosphorylation. Pathways are highly simplified.
Anaerobic glycolysis was the other pathway studied (Figure 1B). We assessed activity of this pathway by measuring CSF concentration of its end product, lactate. CSF lactate concentration is a routinely used biomarker of mitochondrial disorders (16). CSF lactate increases in mitochondrial disorders because glucose is shunted primarily to anaerobic glycolysis when mitochondrial oxidative glucose metabolism falters (17). Only two molecules of adenosine triphosphate (ATP) are produced for every molecule of glucose metabolized anaerobically to lactate compared to thirty-six ATP produced through mitochondrial oxidative phosphorylation (Figure 1C). The dysfunction of mitochondrial oxidative phosphorylation either inherited or acquired through inflammation-induced mitochondrial damage, results in a cellular energy deficit, loss of mitochondrial transmembrane potential, and ultimately apoptotic cell death in individuals with mitochondrial disorders and in animal models of MS (18, 19). These changes are hypothesized to play a role in the neuroaxonal degeneration underlying MS progression (3, 5).
We hypothesized that CSF concentration of sorbitol, fructose, and lactate would be elevated in SP patients and would be associated with worsening neurologic disability, suggesting a relationship between increased activity of extra-mitochondrial pathways of glucose metabolism and disease progression.
SUBJECTS AND METHODS
Subjects and samples
We used archived samples with anonymous identifiers; therefore, this study received an Institutional Review Board exemption from full Board review and informed consent. CSF samples with matching serum samples from individuals with MS (n=105) were obtained from the Human Brain and Spinal Fluid Resource Center (VA West Los Angeles Healthcare Center, 11301 Wilshire Blvd. Los Angeles, CA 90073). RR and SP samples were categorized as RR remitted, RR relapsed, SP stationary, and SP relapsed, based on diagnoses made at the time of lumbar puncture by attending neurologists specializing in MS according to previously reported diagnostic criteria (20). Healthy control (HC) CSF samples (n=18) were obtained from the archives of the National Institute of Mental Health (NIMH) Clinical Neuroendocrinology Branch, Bethesda, Maryland.
Of the 105 MS samples, 20 were removed from the final data set for the following reasons: 12 because more than one came from the same patient at different disease stages; 3 due to non-physiologic metabolite concentration values; and 5 for hyperglycemia, defined as a random serum glucose ≥ 7.78 mM (140mg/dl). Inclusion of multiple samples from the same individual would have violated the assumptions of our statistical tests; therefore, we chose, arbitrarily, to include the sample obtained earliest in the disease course. Hyperglycemia, possibly indicative of diabetes mellitus, would confound the relationship between MS diagnosis and CNS glucose metabolism. Final analyses included data from CSF and serum samples of 85 MS patients [31 RR (22 remitted, 9 relapsed) and 54 SP (37 stationary, 17 relapsed)] and CSF from 18 healthy controls. Current medication information was available for 50 MS subjects; of these, only one subject each from the RR and SP groups was found to be taking immunomodulatory medication at the time of lumbar puncture. In both cases the medication was a corticosteroid administered by mouth, and both subjects had random serum glucose <7.78 mM. CSF samples were kept on ice until storage at −80°C. Blood was obtained in tubes without additives, and then centrifuged to obtain serum, which was stored at −80°C. All samples were packed with dry ice for shipment to the University of Maryland Department of Psychiatry where they were stored at −80°C until assay.
Biochemical methods
CSF sorbitol, fructose, and myoinositol concentrations were measured by a previously reported gas chromatography-mass spectrometry (GC-MS) method using [13C]6 sorbitol, [13C]6 fructose, and [2H]6 myoinositol (Isotec, Inc., Miamisburg, OH) as internal standards and an electron impact GC-MS system (Hewlett-Packard 5971B, Fullerton, CA) with a 12m × 0.25mm HP-5MS column (21). Myoinositol was included for comparison to sorbitol and fructose because it is a polyol that is not produced by the polyol pathway of glucose metabolism. Selected ion monitoring was performed at mass values 275 and 280, 210 and 214, and 289 and 293 for fragments of endogenous fructose, myoinositol, and sorbitol, and their heavier internal standard isotopes, respectively. Endogenous concentrations were calculated from the mass area ratios (corrected for natural abundance) and the known amount of internal standard added. Measurements were made in duplicate and blind to sample identity. Average intra- and inter-assay coefficients of variation (CV) were 3.8% and 5.3%, respectively.
We measured CSF and serum glucose and CSF lactate concentrations using a YSI 2300 STAT Plus Glucose & Lactate Analyzer (YSI, Inc., Yellow Springs, OH) according to manufacturer directions. Average intra- and inter-assay CVs were 2.4% and 3.2% for glucose, respectively, and 2.6 % and 3.7% for lactate, respectively. These measurements were also made in duplicate and blind to sample identity. The upper limit of the normal range for CSF lactate concentration was calculated to be two standard deviations (SD) above our healthy control mean per investigators of mitochondrial disorders (16, 17). Serum glucose concentrations for the 18 healthy control samples from the USA National Institute of Mental Health (NIMH) Clinical Neuroendocrinology Branch were obtained from the NIH Department of Laboratory Medicine where they had been determined by the standard glucose oxidase method.
Statistical methods
Data distributions were assessed for significant difference from a normal distribution using the Kolmogorov-Smirnov test. If data were distributed normally, we used parametric statistics; otherwise, we log10-transformed data or used nonparametric statistics. We used Pearson's r and Spearman's rho to assess correlations. We compared group means by Student's t test (with Levene's test for equality of variances) or one-way analysis of variance (ANOVA), followed by pair-wise comparisons by Dunnett's T3 test. Non parametric group comparisons were by the Kruskal-Wallis test followed by pair-wise comparisons by Wilcoxon rank test derived Z statistic with alpha Bonferroni-corrected to p=0.017 for multiple comparisons. We used correlations and dichotomous group comparisons to assess potentially confounding relationships between demographic or clinical variables and metabolites. If we found significant relationships between demographic or clinical variables and metabolites, we compared group mean metabolite concentrations by analysis of covariance (ANCOVA) using these variables as covariates. Covariates that did not have significant between-subjects effects in the ANCOVA were dropped from analyses. Categorical variables were analyzed using Pearson's chi square (χ2) test. All tests were two-tailed, and alpha was set at p=0.05 unless specified. We used SPSS version 12.0 (SPSS, Inc., Chicago, IL) for most analyses.
RESULTS
Subject characteristics and CSF indices
As MS subjects were recruited from a USA Veterans Affairs Medical Center, they were predominantly male, as were controls (Table 1). Groups differed significantly by age, with controls younger than RR and SP MS subjects. In terms of neurologic disability, Expanded Disability Status Scale (EDSS) scores of SP patients were significantly greater than scores of RR patients, indicating, as expected, greater neurologic disability in SP patients. CSF white blood cell (WBC) and red blood cell (RBC) counts were significantly greater in RR patients than in SP patients. RR and SP patients did not differ on indices of blood-brain barrier permeability (CSF/serum albumin ratio) and of acute CNS inflammation (IgG Index).
Table 1.
Subject and sample characteristics by subject group
| Variablea | Subject group | Analysisb | ||
|---|---|---|---|---|
| HC (N=18) |
RR MS (N=31) |
SP MS (N=54) |
||
| Age (years) | 36.7± 8.4 | 45.2 ± 14.2 | 49.9 ± 10.3 | F=9.23, df=2, 100, p<0.001 RR > HC (p=0.035) SP >HC (p<0.001) |
| Gender (% male) | 66.7 | 74.2 | 79.6 | χ2=1.29, df=2, P=0.524 |
| Expanded Disability Status Scale (EDSS) score |
N/A | 5.2 ± 1.7 (N=9) |
6.5 ± 0.92 (N=21) |
t=2.24, df=10, p=0.048 |
| CSF RBC count per mm3 |
2.7 (3.1) | 3.7 (1.5) (N=13) |
1.7 (2.7) (N=37) |
Z=2.58, p=0.010 |
| CSF WBC count per mm3 |
0.8 (1.7) | 2.1 (4.3) (N=14) |
0.7 (1.1) (N=37) |
χ2=7.26, df=2, p=0.027 RR>SP (p=0.011)c |
| CSF (mg/l)/ Serum (g/l) albumin ratio |
N/A | 6.4 ± 3.4 (N=16) |
5.7 ± 1.7 (N=43) |
t=0.886, df=18, p=0.388d |
| IgG Index | N/A | 0.96 ± 0.67 (N=16) |
0.97 ± 0.49 (N=43) |
t=0.45, df=57, p=0.964 |
Abbreviations: HC=Healthy Controls, RR MS=Relapsing-remitting MS patients, SP MS=Secondary progressive MS patients, RBC=red blood cell, WBC=white blood cell.
Values are means ± standard deviation or medians (interquartile range) unless specified.
Group distributions are compared by ANOVA with post hoc pairwise comparisons by Dunnett's T3 test or by Kruskall-Wallis test with post hoc Wilcoxon rank tests, unless otherwise specified. On variables without HC data, RR and SP MS groups are compared by Student's t test.
Alpha Bonferroni-corrected to p=0.017. P values uncorrected.
Groups differed significantly on Levene's test for equality of variances; t, df, and p values adjusted accordingly.
Glucose and metabolite concentrations
Before comparing groups on glucose and metabolite concentrations, we assessed potentially confounding relationships of age, gender, and cell counts to glucose and metabolite concentrations in the full 103-subject data set. Age correlated positively and significantly with CSF lactate, sorbitol, and fructose concentrations, as did log10- transformed CSF cell counts with myoinositol concentration. When groups were compared by ANCOVA, however, neither variable had significant between-subjects effects (p≥0.066). This was also the case for male gender (p>0.130), which initially appeared to be associated with higher CSF glucose and lactate concentrations. The only confounding relationship that survived analysis by ANCOVA was a positive relationship between CSF WBC and CSF myoinositol concentration (F=10.3, df=1, 65, p=0.002). There were no significant correlations between blood cell counts and CSF concentrations of glucose sorbitol, fructose, or lactate (p≥0.237).
The 85-sample MS data set was assessed for confounding relationships between CSF glucose and metabolites and the following variables: relapse, IgG Index, and CSF/serum albumin ratio. We found IgG Index to be inversely related to myoinositol concentration (F=4.94, df=1, 56, p=0.030). Relapse was significantly and positively related to serum glucose concentration (F=6.78, df=1, 74, p=0.011). No CSF metabolite correlated significantly with CSF/serum albumin ratio (p≥0.467) or IgG Index (p≥0.123).
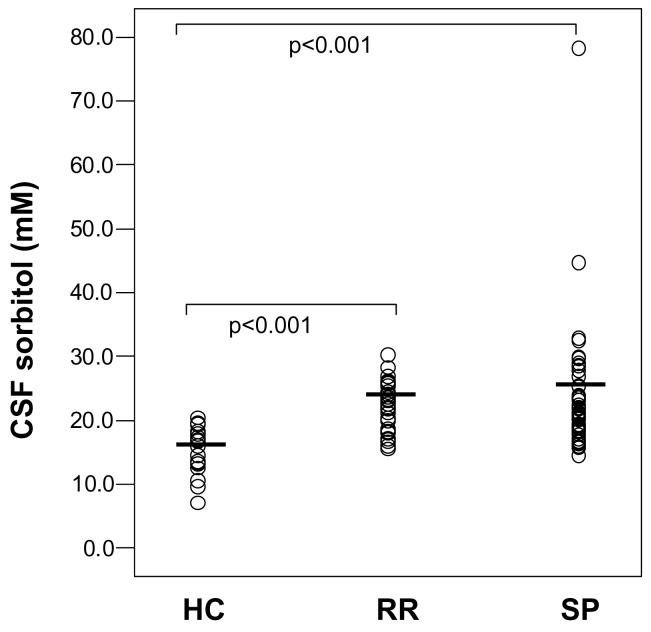
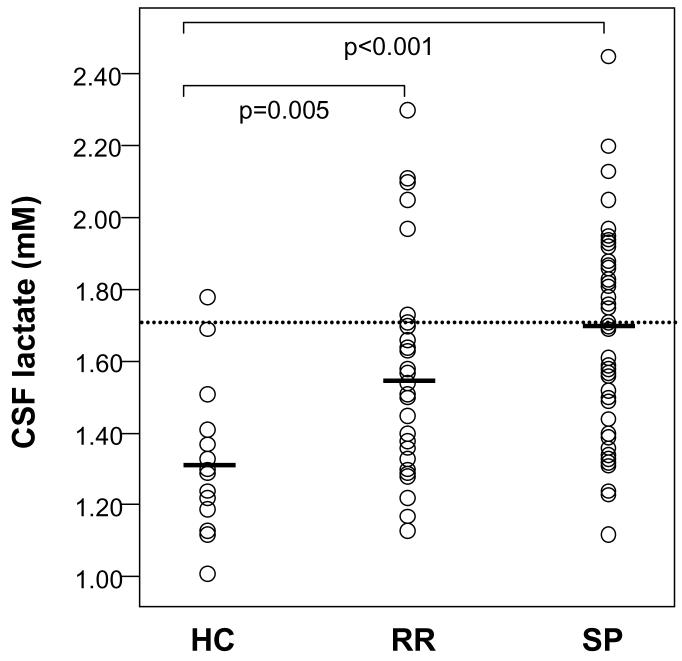
Comparing groups on glucose and metabolite concentrations, we found that CSF concentrations of sorbitol, fructose, and lactate were significantly elevated in RR and SP subjects compared to controls (Table 2, Figures 2 and 3). However, groups did not differ significantly on glucose concentrations or on WBC-adjusted myoinositol concentrations. ANCOVA comparing RR and SP groups on serum glucose concentration with relapse as covariate also showed no significant difference between groups (F=0.073, df=1, 74, p=0.787). Groups also differed significantly (χ2=11.7, df=2, p=0.003) in terms of proportions of subjects with CSF lactate elevated 2 SD above the healthy control mean (1.69 mM): healthy controls (1/18 or 5.6 %); RR (8/31 or 25.8 %), and SP (25/54 or 46.3 %). Although there were no significant differences between RR and SP patients on CSF glucose or any glucose metabolite, SP patients had consistently higher CSF concentrations of lactate and polyol pathway metabolites than did RR patients.
Table 2.
CSF and serum concentrations of glucose and metabolites
| Variablea | Subject group | |||
|---|---|---|---|---|
| HC N=18 |
RR N=31 |
SP N=54 |
Analysis | |
| Glucose CSF (mM) |
3.24 ± 0.36 | 3.46 ± 0.48 | 3.51 ± 0.78 | F=1.32, df=2, 100, p=0.271 |
| Glucose Serum (mM) |
4.58 ± 0.48 | 4.54 ± 1.0 | 4.31 ± 0.99 | F=0.87, df=2, 100, p=0.422 |
| Lactate CSF (mM) |
1.31 ± 0.19 | 1.55 ± 0.30 | 1.67 ± 0.29 | F=11.2, df=2, 100, p<0.001 SP>HC (p<0.001) RR>HC (p=0.005) |
| Sorbitol CSF (μM) |
15.6 ± 3.9 | 22.0 ± 3.7 | 23.4 ± 9.4 | F=14.0, df=2, 100, p=0.001 SP>HC (p<0.001) RR>HC (p<0.001) |
| Fructose CSF (μM) |
156 ± 50 | 209 ± 85 | 227 ± 87 | F=5.27, df=2, 100, p=0.007 SP>HC (p<0.001) RR>HC (p=0.017) |
| Myoinositol CSF (μM) |
150 ± 29 | 178 ± 42 | 165 ± 43 | F=2.72, df=2, 100, p=0.070 F=1.77, df=2, 63, p=0.179b |
HC=Healthy Controls, RR MS=Relapsing-remitting MS patients, SP MS=Secondary progressive MS patients, WBC=white blood cell.
Values are means ± standard deviation.
ANCOVA with CSF WBC count as covariate.
Figure 2.
Scatter plots of CSF sorbitol concentration data distributions for Healthy control subjects (HC, n=18), Relapsing-remitting MS (RR MS, n=31), and Secondary progressive MS subjects (SP MS, n=54). Horizontal bars represent group means.
Figure 3.
Scatter plots of CSF lactate concentration data distributions for Healthy control subjects (HC, n=18), Relapsing-remitting MS (RR MS, n=31), and Secondary progressive MS subjects (SP MS, n=54). Horizontal bars represent group means. Dotted line represents upper cut-off of normal clinical range.
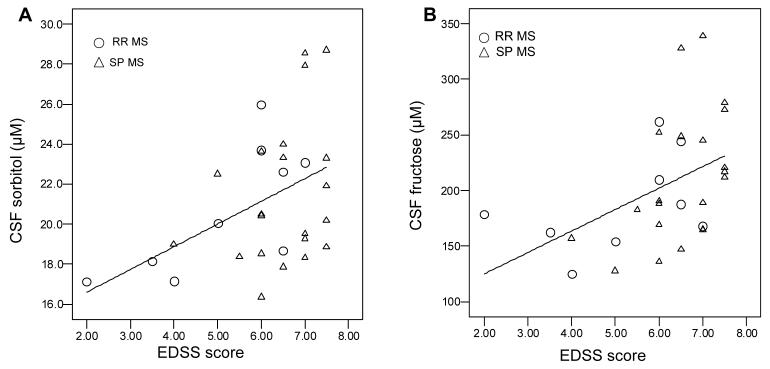
In terms of the relationship of metabolite concentrations to neurologic disability, EDSS score correlated significantly with CSF sorbitol (r=0.437, p=0.016) and fructose (r=0.460, p=0.011) (Figure 4), but not with lactate or myoinositol concentrations (p≥0.123). CSF lactate concentration correlated positively with sorbitol and fructose concentrations in RR (r=0.523, p=0.003 and r=0.298, p=0.104, respectively) and SP (r=0.407, p=0.002 and r=0.093, p=0.503, respectively) patients, but negatively in healthy controls (r=−0.492, p=0.038 and r=−0.319, p=0.196, respectively).
Figure 4.
Scatter plots of CSF sorbitol (A) and fructose (B) concentrations by neurologic disability measured by Expanded Disability Status Scale (EDSS) score. Subjects are 9 individuals with relapsing remitting (RR) and 21 with secondary progressive (SP) MS.
DISCUSSION
In this pilot study we investigated the relationship between disturbed CNS energy metabolism and MS disease progression. Our major findings were that CSF concentrations of sorbitol, fructose, and lactate were elevated in RR and SP MS patients compared to healthy controls and that CSF sorbitol and fructose correlated positively and significantly with neurologic disability (EDSS score) in MS patients. Taken together, these findings support an association between increased activity of extra-mitochondrial pathways of glucose metabolism and MS disease progression that begins during the RR stage of disease. We discuss these findings and others below in relation to CNS glucose metabolism, metabolism in MS, mitochondrial disorders, study limitations, and relevance to the pathophysiology and treatment of MS.
CSF concentrations of sorbitol, fructose and lactate reflect CNS, rather than whole body metabolism, because all three molecules are polar, have no specific transporter, and therefore do not readily cross the blood-brain barrier (22-26). Moreover, lack of correlation of CSF metabolite concentrations with CSF blood cell counts and CSF/serum albumin ratio, suggests that CSF concentrations of polyol pathway metabolites and lactate were not significantly influenced by blood cell metabolism (in vivo or in vitro) or by movement of metabolites across a leaky blood-brain-barrier.
Our measurements of CSF polyol pathway metabolites in healthy controls and in MS patients are consistent with values reported in the literature (9-11, 23, 27). Although published healthy control CSF fructose concentrations are not available, healthy control means ± SD for CSF sorbitol of 13.0 ±3.5, and 13.8±3.4 μM reported by other investigators using the same technique are similar to ours (23, 27). The increased concentrations we found are consistent with the only other report of CSF sorbitol concentration (11) and with the two reports of CSF fructose concentration (9, 10) in MS patients. Interestingly, Lutz and colleagues (2007) found that fructose was elevated in MS patients independent of the number of gadolinium enhanced (actively inflamed) plaques (9), suggesting that increased polyol pathway activity is independent of acute inflammation. This is consistent with our not finding a significant relationship of polyol pathway metabolites with IgG Index, CSF WBC, or relapse.
Increased CSF concentrations of polyol pathway metabolites are not unique to MS. They have also been observed in patients with rare inborn errors of metabolism, diabetes mellitus, bipolar disorder, and unipolar major depression (14, 21, 28-30). However, this is not the case for patients with numerous other CNS disorders including Down's syndrome, Alzheimer's disease, brain tumors, and stroke (24, 27, 31, 32). Therefore, increased CNS polyol pathway activity does not appear to be a nonspecific response to CNS disease.
The healthy control mean ± SD for CSF lactate that we report is similar to means ± SD of 1.21± 0.182 and 1.39 ± 0.106 mM (16, 33) reported by investigators of mitochondrial disorders. It is crucial to point out that most studies, including MS studies, that have reported CSF lactate concentrations in disease states have not used samples from healthy control subjects for comparison. Rather, they have used samples from patients being investigated for a neurologic disorder other than the disorder being studied. This is the likely reason that most reported “normal ranges” for lactate are actually supra-normal. This is particularly true for “normal ranges” reported in studies of meningitis, where relatively high CSF lactate concentrations are used as cutoffs in distinguishing bacterial from non-bacterial meningitis. Comparing our MS patients to our healthy controls, approximately one quarter of RR patients and nearly half of all SP patients had elevated CSF lactate levels.
In comparing our findings to other reports of CSF lactate concentrations in MS patients, it is important to acknowledge that the literature is inconsistent. We believe that the major reason for inconsistency is that studies lacked a healthy control group. Whether investigators found decreases, increases or no difference in MS patient CSF lactate compared to controls depended on the comparison values of their neurologic controls. Investigators who found a decrease or no significant difference in MS patient CSF lactate levels compared to controls used neurologic patient controls and reported supra-normal control means of 2.2 (34) and 2.07 mM (35), respectively. This was also the case for Lutz and colleagues (2007) who reported significantly elevated CSF lactate only in MS patients with, but not without, active inflammatory plaques compared to neurologic controls. Their control neurologic group had a CSF lactate level exceeding 2.00 mM (9). In contrast, Nicoli and colleagues (1996) found elevated CSF mean lactate level in 13 RR and 6 primary progressive patients (1.96 mM) compared to a neurologic patient control group mean of 1.68 mM (10). Jongen and colleagues (1997), although they had no control group, found elevated CSF lactate in 16 SP patients (1.785 mM) compared to 17 RR patients (1.660 mM), which came close to statistical significance (p=0.08) (36). Therefore, our findings are not at odds with the literature, because all studies that found CSF lactate in MS patients to be decreased or not significantly different relative to controls used as controls neurologic patients with mean CSF lactate levels exceeding 2.00 mM.
There are at least two, non-mutually exclusive, hypotheses relating elevated CSF lactate to MS pathophysiology. The first is that elevated CSF lactate results from the glycolysis of activated leukocytes in active inflammatory plaques. This hypothesis was suggested by Simone and colleagues (1996) and is consistent with their finding that CSF lactate correlated positively with the number of CSF mononuclear cells and with the presence of gadolinium enhancing plaques on MRI in RR patients in relapse (37). It is also supported by the report of a strong positive correlation (rho=0.691) between CSF lactate and the number of inflammatory plaques (9). Since we did not observe a correlation between lactate and WBC count or IgG index, our data do not support this hypothesis. However, our study was limited by a lack of neuroimaging data and differential WBC counts.
The second hypothesis is that increased CSF lactate, and increased CSF polyol pathway metabolites, are both related to mitochondrial dysfunction. Under normal homeostatic conditions, CNS lactate is produced through anaerobic glycolysis by neurons to meet acutely increased energy demands during activation and by astrocytes to be shuttled to neurons as a precursor to oxidative metabolism by mitochondria (38). In mitochondrial disorders, oxidative phosphorylation is impaired, resulting in an accumulation of lactate. Molecular and neuroimaging studies have shown that both anaerobic glycolysis and polyol pathway activity are increased in individuals with mitochondrial disorders (39-41). The positive correlation between CSF concentrations of polyol pathway metabolites and lactate in our MS patients, but not in our healthy controls, is consistent with this hypothesis.
Based on MS-like mitochondrial disorders, such as LHON, investigators have searched for polymorphisms of mitochondrial DNA in individuals with MS. Although there are reports of mitochondrial DNA polymorphisms in particular individuals and subgroups of MS patients (42, 43), it is considered unlikely that mitochondrial DNA mutations are widely associated with typical forms of MS (44). However, a relationship between MS disease progression and acquired mitochondrial dysfunction secondary to inflammation-induced damage to the mitochondrion has been hypothesized (5). Lu and colleagues (2000) have reported evidence of oxidative damage to mitochondrial DNA and decreased activity of mitochondrial metabolic enzymes in chronic active lesions of MS (5). These findings and a postmortem study of chronically demyelinated lesions in motor cortex (3), suggest that mitochondrial impairment leading to disturbed energy metabolism could contribute to neuroaxonal degeneration underlying disease progression.
Our findings indicate that disturbed energy metabolism precedes the diagnosis of progressive disease and, therefore, could be causally related to disease progression. However, we did not find a significant correlation between CSF lactate level and neurologic disability as we had for CSF polyol pathway metabolite levels. In contrast, Kaufmann and colleagues (2004), using proton MR spectroscopy to measure intraventricular lactate in vivo, found that CSF lactate concentration correlated significantly with neurological impairment in patients with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) (45). The reason for this is unclear, and our review of the literature found no MS studies that examined this relationship. One possibility is that CSF lactate level has greater day-to-day variation than do CSF polyol pathway metabolite levels and therefore does not accurately reflect a long term measure of disability such as EDSS score.
Although metabolites of the anaerobic glycolytic and polyol pathways may be important biomarkers of mitochondrial dysfunction in MS, it is unclear whether increased activity of these pathways and accumulation of their metabolites contributes directly to neuroaxonal degeneration in MS. Increased polyol pathway activity is associated with demyelinating disease of peripheral nervous system axons in diabetes mellitus and with demyelinating CNS disease caused by rare inborn errors of polyol metabolism (13, 14, 21, 28-30). However, CSF polyol concentrations in patients with leukoencephalopathies related to inborn errors of polyol metabolism are more than 100 times that of healthy controls (14), far greater than the elevations seen in individuals with MS. Accumulation of lactate can cause direct cellular damage by lowering pH. Interestingly, Friese and colleagues (2007) have hypothesized that reduced pH secondary to lactate accumulation could be related to axonal degeneration observed in the MS animal model, experimental autoimmune encephalitis (46). This effect is thought to be mediated by an acid-sensing ion channel that opens when pH drops, allowing excessive amounts of Na+ and Ca++ to enter axons.
It is important to mention the following study limitations. First, the imprecise group matching on age is an inherent limitation of this retrospective study, and could have contributed to metabolic differences among groups. However, the results of our ANCOVA showing no significant effect of age on group comparisons of metabolite concentrations suggest that group differences cannot be explained on the basis of age differences. Second, although subjects were excluded for elevated serum glucose levels, subjects were not fasting at the time serum and CSF samples were obtained. Third, clinical information including CSF cell counts, IgG Index, medications at time of lumbar puncture, and CSF/serum albumin ratio were available for only approximately two-thirds of the MS patient sample. Finally, although the mean EDSS score of SP patients was significantly greater than RR patients, the groups differed by only 1.3 points. The RR score was relatively high and may have contributed to our not finding significant differences in lactate or polyol pathway metabolites between RR and SP groups.
CONCLUSION
The findings of this pilot study indicate increased activity of extra-mitochondrial pathways of CNS glucose metabolism in individuals with MS. These alterations in energy metabolism may be related directly or indirectly to the pathogenesis of MS disease progression. CSF metabolic profiles might be useful as biomarkers to target and monitor the effects of future therapies aimed at preventing MS disease progression by protecting mitochondria or boosting mitochondrial energy metabolism. Further, preferably prospective, study is needed to characterize the temporal relationship between changes in CSF and brain metabolic profile and evidence of neuroaxonal degeneration and increasing neurologic disability in individuals with MS. This information would be helpful in determining whether the metabolic changes observed in this study contribute to or are a consequence of MS disease progression.
ACKNOWLEDGEMENTS
The authors thank the individuals who generously donated their CSF; Lisa McFarland, M.S. for GC-MS technical assistance; Andrew P. Goldberg, M.D. and the Baltimore VAMC GRECC for extended loan of the GC-MS system; Roland Martin, M.D. and Laura Tranquill, M.S. of the NINDS Neuroimmunology Branch, for assistance in providing CSF samples for a preliminary study; W.W. Tourtelotte, M.D., Ph.D. and staff at the Human Brain and Spinal Fluid Resource Center for assistance with samples and clinical information; and the National Multiple Sclerosis Society for funding this study with a Pilot Research Project Award (PP0932) to W.T.R. This research was also supported in part by the Intramural Research Program of the NIH, NINDS.
SOURCE OF SUPPORT FOR THE STUDY
This study was funded by a National Multiple Sclerosis Society Pilot Research Project Award (PP0932) to W.T.R. This research was also supported in part by the Intramural Research Program of the NIH, NINDS, which provided CSF samples for a preliminary study, but no funding.
REFERENCES
- 1.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 2.McDonald WI. Relapse, remission, and progression in multiple sclerosis. N Engl J Med. 2000;343:1486–7. doi: 10.1056/NEJM200011163432010. [DOI] [PubMed] [Google Scholar]
- 3.Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–89. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 4.Kalman B, Leist TP. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromolecular Med. 2003;3:147–58. doi: 10.1385/NMM:3:3:147. [DOI] [PubMed] [Google Scholar]
- 5.Lu F, Selak M, O'Connor J, Croul S, Lorenzana C, Butunoi C, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 6.Waxman SG. Ions, energy and axonal injury: towards a molecular neurology of multiple sclerosis. Trends Mol Med. 2006;12:192–5. doi: 10.1016/j.molmed.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Kalman B, Lublin FD, Alder H. Mitochondrial DNA mutations in multiple sclerosis. Mult Scler. 1995;1:32–6. doi: 10.1177/135245859500100106. [DOI] [PubMed] [Google Scholar]
- 8.Lees F, Macdonald AM, Turner JW. Leber's Disease with Symptoms Resembling Disseminated Sclerosis. J Neurol Neurosurg Psychiatry. 1964;27:415–21. doi: 10.1136/jnnp.27.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutz NW, Viola A, Malikova I, Confort-Gouny S, Audoin B, Ranjeva JP, et al. Inflammatory multiple-sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PLoS ONE. 2007;2:e595. doi: 10.1371/journal.pone.0000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicoli F, Vion-Dury J, Confort-Gouny S, Maillet S, Gastaut JL, Cozzone PJ. Cerebrospinal fluid metabolic profiles in multiple sclerosis and degenerative dementias obtained by high resolution proton magnetic resonance spectroscopy. C R Acad Sci III. 1996;319:623–31. [PubMed] [Google Scholar]
- 11.Smith SL, Novotny M, Karmen A. Elevation of certain polyols in the cerebrospinal fluid of patients with multiple sclerosis. J Chromatogr. 1984;336:351–5. doi: 10.1016/s0378-4347(00)85159-2. [DOI] [PubMed] [Google Scholar]
- 12.Berry GT, Hunter JV, Wang Z, Dreha S, Mazur A, Brooks DG, et al. In vivo evidence of brain galactitol accumulation in an infant with galactosemia and encephalopathy. J Pediatr. 2001;138:260–2. doi: 10.1067/mpd.2001.110423. [DOI] [PubMed] [Google Scholar]
- 13.Gabbay KH. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annu Rev Med. 1975;26:521–36. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- 14.van der Knaap MS, Wevers RA, Struys EA, Verhoeven NM, Pouwels PJ, Engelke UF, et al. Leukoencephalopathy associated with a disturbance in the metabolism of polyols. Ann Neurol. 1999;46:925–8. doi: 10.1002/1531-8249(199912)46:6<925::aid-ana18>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Stevens MJ, Feldman EL, Greene DA. The aetiology of diabetic neuropathy: the combined roles of metabolic and vascular defects. Diabet Med. 1995;12:566–79. doi: 10.1111/j.1464-5491.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 16.Finsterer J. Cerebrospinal-fluid lactate in adult mitochondriopathy with and without encephalopathy. Acta Med Austriaca. 2001;28:152–5. doi: 10.1046/j.1563-2571.2001.01036.x. [DOI] [PubMed] [Google Scholar]
- 17.Seyama K, Suzuki K, Mizuno Y, Yoshida M, Tanaka M, Ozawa T. Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes with special reference to the mechanism of cerebral manifestations. Acta Neurol Scand. 1989;80:561–8. doi: 10.1111/j.1600-0404.1989.tb03927.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalman B, Laitinen K, Komoly S. The involvement of mitochondria in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;188:1–12. doi: 10.1016/j.jneuroim.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem. 2006;281:31950–62. doi: 10.1074/jbc.M603717200. [DOI] [PubMed] [Google Scholar]
- 20.Rose AS, Kuzma JW, Kurtzke JF, Namerow NS, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20:1–59. doi: 10.1212/wnl.20.5_part_2.1. [DOI] [PubMed] [Google Scholar]
- 21.Regenold WT, Kling MA, Hauser P. Elevated sorbitol concentration in the cerebrospinal fluid of patients with mood disorders. Psychoneuroendocrinology. 2000;25:593–606. doi: 10.1016/s0306-4530(00)00012-3. [DOI] [PubMed] [Google Scholar]
- 22.Davson H, Welch K, Segal MB. Physiology and pathophysiology of the cerebrospinal fluid. Churchill Livingstone; Edinburgh ; New York: 1987. [Google Scholar]
- 23.Shetty HU, Holloway HW, Rapoport SI. Capillary gas chromatography combined with ion trap detection for quantitative profiling of polyols in cerebrospinal fluid and plasma. Anal Biochem. 1995;224:279–85. doi: 10.1006/abio.1995.1041. [DOI] [PubMed] [Google Scholar]
- 24.Shetty HU, Holloway HW, Schapiro MB. Cerebrospinal fluid and plasma distribution of myo-inositol and other polyols in Alzheimer disease. Clin Chem. 1996;42:298–302. [PubMed] [Google Scholar]
- 25.Wray HL, Winegrad AI. Free fructose in human cerebrospinal fluid. Diabetologia. 1966;2:82–5. doi: 10.1007/BF00423014. [DOI] [PubMed] [Google Scholar]
- 26.Watson MA, Scott MG. Clinical utility of biochemical analysis of cerebrospinal fluid. Clin Chem. 1995;41:343–60. [PubMed] [Google Scholar]
- 27.Shetty HU, Schapiro MB, Holloway HW, Rapoport SI. Polyol profiles in Down syndrome. myo-Inositol, specifically, is elevated in the cerebrospinal fluid. J Clin Invest. 1995;95:542–6. doi: 10.1172/JCI117696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry GT. The role of polyols in the pathophysiology of hypergalactosemia. Eur J Pediatr. 1995;154:S53–64. doi: 10.1007/BF02143805. [DOI] [PubMed] [Google Scholar]
- 29.Regenold WT, Hisley KC, Obuchowski A, Lefkowitz DM, Marano C, Hauser P. Relationship of white matter hyperintensities to cerebrospinal fluid glucose polyol pathway metabolites—a pilot study in treatment-resistant affective disorder patients. J Affective Disorders. 2005;85:341–50. doi: 10.1016/j.jad.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Servo C. Sorbitol and myoinositol levels in the cerebrospinal fluid of diabetic patients. Acta Endocrinol Suppl. 1980;238:133–7. [PubMed] [Google Scholar]
- 31.Servo C, Palo J, Pitkanen E. Gas chromatographic separation and mass spectrometric identification of polyols in human cerebrospinal fluid and plasma. Acta Neurol Scand. 1977;56:104–10. doi: 10.1111/j.1600-0404.1977.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe S, Kamiyama J, Chigasaki H, Yoshioka S. Polyol content of cerebrospinal fluid in brain-tumor patients. J Neurosurg. 1989;70:183–9. doi: 10.3171/jns.1989.70.2.0183. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Ujike H, Wada K, Tsuji T. Cerebrospinal fluid lactate and pyruvate concentrations in patients with Parkinson's disease and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) J Neurol Neurosurg Psychiatry. 1997;62:290. doi: 10.1136/jnnp.62.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aasly J, Garseth M, Sonnewald U, Zwart JA, White LR, Unsgard G. Cerebrospinal fluid lactate and glutamine are reduced in multiple sclerosis. Acta Neurol Scand. 1997;95:9–12. doi: 10.1111/j.1600-0404.1997.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 35.Lynch J, Peeling J, Auty A, Sutherland GR. Nuclear magnetic resonance study of cerebrospinal fluid from patients with multiple sclerosis. Can J Neurol Sci. 1993;20:194–8. [PubMed] [Google Scholar]
- 36.Jongen PJ, Lamers KJ, Doesburg WH, Lemmens WA, Hommes OR. Cerebrospinal fluid analysis differentiates between relapsing-remitting and secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997;63:446–51. doi: 10.1136/jnnp.63.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simone IL, Federico F, Trojano M, Tortorella C, Liguori M, Giannini P, et al. High resolution proton MR spectroscopy of cerebrospinal fluid in MS patients. Comparison with biochemical changes in demyelinating plaques. J Neurol Sci. 1996;144:182–90. doi: 10.1016/s0022-510x(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 38.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–63. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frackowiak RS, Herold S, Petty RK, Morgan-Hughes JA. The cerebral metabolism of glucose and oxygen measured with positron tomography in patients with mitochondrial diseases. Brain. 1988;111(Pt 5):1009–24. doi: 10.1093/brain/111.5.1009. [DOI] [PubMed] [Google Scholar]
- 40.Danielson SR, Carelli V, Tan G, Martinuzzi A, Schapira AH, Savontaus ML, et al. Isolation of transcriptomal changes attributable to LHON mutations and the cybridization process. Brain. 2005;128:1026–37. doi: 10.1093/brain/awh447. [DOI] [PubMed] [Google Scholar]
- 41.Rango M, Bozzali M, Prelle A, Scarlato G, Bresolin N. Brain activation in normal subjects and in patients affected by mitochondrial disease without clinical central nervous system involvement: a phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 2001;21:85–91. doi: 10.1097/00004647-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Mihailova SM, Ivanova MI, Quin LM, Naumova EJ. Mitochondrial DNA variants in Bulgarian patients affected by multiple sclerosis. Eur J Neurol. 2007;14:44–7. doi: 10.1111/j.1468-1331.2006.01541.x. [DOI] [PubMed] [Google Scholar]
- 43.Olsen NK, Hansen AW, Norby S, Edal AL, Jorgensen JR, Rosenberg T. Leber's hereditary optic neuropathy associated with a disorder indistinguishable from multiple sclerosis in a male harbouring the mitochondrial DNA 11778 mutation. Acta Neurol Scand. 1995;91:326–9. doi: 10.1111/j.1600-0404.1995.tb07016.x. [DOI] [PubMed] [Google Scholar]
- 44.Kalman B, Alder H. Is the mitochondrial DNA involved in determining susceptibility to multiple sclerosis? Acta Neurol Scand. 1998;98:232–7. doi: 10.1111/j.1600-0404.1998.tb07301.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann P, Shungu DC, Sano MC, Jhung S, Engelstad K, Mitsis E, et al. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology. 2004;62:1297–302. doi: 10.1212/01.wnl.0000120557.83907.a8. [DOI] [PubMed] [Google Scholar]
- 46.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–9. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]