Abstract
Rationale
Serotonin transporter (SERT) knockout (−/−) mice have an altered phenotype in adulthood, including high baseline anxiety and depressive-like behaviors, associated with increased baseline extracellular serotonin levels throughout life.
Objectives
To examine the effects of increases in serotonin following administration of the serotonin precursor 5-hydroxy-L-tryptophan (5-HTP) in SERT wildtype (+/+), heterozygous (+/−) and −/− mice.
Results
5-HTP increased serotonin in all five brain areas examined, with ~2–5-fold increases in SERT +/+ and +/− mice, and greater 4.5–11.7-fold increases in SERT −/− mice. Behaviorally, 5-HTP induced exaggerated serotonin syndrome behaviors in SERT −/− mice, with similar effects in male and female mice. Studies suggest promiscuous serotonin uptake by the dopamine transporter (DAT) in SERT −/− mice, and here, the DAT blocker GBR 12909 enhanced 5-HTP-induced behaviors in SERT −/− mice. Physiologically, 5-HTP induced exaggerated temperature effects in SERT-deficient mice. The 5-HT1A antagonist WAY 100635 decreased 5-HTP-induced hypothermia in SERT +/+ and +/− mice, with no effect in SERT −/− mice, whereas the 5-HT7 antagonist SB 269970 decreased this exaggerated response in SERT −/− mice only. WAY 100635 and SB 269970 together completely blocked 5-HTP-induced hypothermia in SERT +/− and −/− mice.
Conclusions
These studies demonstrate that SERT −/− mice have exaggerated neurochemical, behavioral and physiological responses to further increases in serotonin, and provide the first evidence of intact 5-HT7 receptor function in SERT −/− mice, with interesting interactions between 5-HT1A and 5-HT7 receptors. As roles for 5-HT7 receptors in anxiety and depression were recently established, the current findings have implications for understanding the high anxiety and depressive-like phenotype of SERT-deficient mice.
Keywords: High-performance liquid chromatography (HPLC), Temperature, Serotonin syndrome behaviors, Dopamine transporter (DAT), 5-hydroxy-L-tryptophan (5-HTP), 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY 100635), 5-carboxamidotryptamine maleate (5-CT), SB 269970, GBR 12909
Introduction
Alterations in the serotoninergic system have been implicated in depression and other mood and anxiety disorders, including obsessive compulsive disorder (OCD) (Caspi et al. 2003; Hu et al. 2006; Lesch et al. 1996; Murphy and Lesch 2008; Owens and Nemeroff 1998). The serotonin transporter (SERT; 5-HTT) is the main mechanism for removal of serotonin from the synapse, thus maintaining homeostatic levels of serotonin in the extracellular space (Blakely et al. 1991; Hariri and Holmes 2006). SERT is the site of action for the leading treatment choice for depression and anxiety disorders, the selective serotonin reuptake inhibitors (SSRIs) (Heydorn 1999; Jones and Blackburn 2002; Murphy et al. 1998), which increase extracellular levels of serotonin by blocking SERT.
Alterations in serotonin levels during early development in rodents have behavioral consequences in adulthood (Ansorge et al. 2008; Ansorge et al. 2004). SERT heterozygous (+/−) and knockout (−/−) mice have marked increases in extracellular serotonin concentrations compared to their SERT wildtype (+/+) littermates (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004). SERT −/− mice display high baseline anxiety-like behaviors (Ansorge et al. 2004; Holmes et al. 2003a; Kalueff et al. 2007), high reactivity to stress (Li et al. 1999; Tjurmina et al. 2002; Wellman et al. 2007) and a depressive-like phenotype, including increased immobility in the tail suspension test and alterations in REM sleep (Alexandre et al. 2006; Holmes et al. 2002; Popa et al. 2008; Wisor et al. 2003; Zhao et al. 2006). Some aspects of the SERT −/− phenotype are “rescued” by early postnatal serotonin depletion via treatment with the serotonin synthesis inhibitor para-chlorophenylalanine (PCPA) (Alexandre et al. 2006; Persico et al. 2001), and a similar phenotype to that of SERT −/− mice is induced in SERT +/+ mice via early postnatal SSRI treatment (Ansorge et al. 2008; Ansorge et al. 2004; Popa et al. 2008; Xu et al. 2004). Thus, increased serotonin during early critical periods of development is implicated in the SERT −/− phenotype. These alterations are specific to serotonin, as early postnatal blockade of the norepinephrine transporter (NET) does not induce similar phenotypic alterations in adult mice (Ansorge et al. 2008).
Increased activation of 5-HT1A receptors by enhanced serotonin levels during development contributes to the phenotype of SERT −/− mice. SERT −/− mice have decreased 5-HT1A binding and function (Gobbi et al. 2001; Holmes et al. 2003b; Li et al. 2000; Li et al. 1999), and acute administration of the selective 5-HT1A antagonist WAY 100635 normalizes the high baseline anxiety-like behavior in adult SERT −/− mice (Holmes et al. 2003b). Further, early postnatal treatment with WAY 100635 rescues some, but not all, aspects of the adult SERT −/− mice phenotype (Alexandre et al. 2006).
5-HT7 receptors are expressed in early stages of development (Garcia-Alcocer et al. 2006; Vizuete et al. 1997), and recent studies have established important roles for 5-HT7 receptors in anxiety, depression, REM sleep and circadian rhythms (Bonaventure et al. 2007; Guscott et al. 2005; Hedlund et al. 2005; Wesolowska et al. 2006a; Wesolowska et al. 2006b; Wesolowska et al. 2007). Although all of these phenotypes are altered in SERT −/− mice, 5-HT7 receptor function has not yet been examined in SERT-deficient mice.
We recently showed that female SERT −/− mice have exaggerated serotonin syndrome behavioral responses to further increases in serotonin levels induced via administration of the serotonin precursor 5-hydroxy-L-tryptophan (5-HTP) or the monoamine oxidase-A/B inhibitor tranylcypromine (Fox et al. 2007a). In mice, these behaviors are predominantly mediated by postsynaptic 5-HT1A receptors (Goodwin et al. 1987a; Goodwin et al. 1987b; Goodwin et al. 1986; Lucki et al. 1984; Smith and Peroutka 1986; Yamada et al. 1988). SERT −/− mice also show evidence of these behaviors spontaneously, in the absence of drug (Fox et al. 2007a; Kalueff et al. 2007). We therefore hypothesized that these spontaneous behaviors were due to established elevated baseline extracellular levels of serotonin (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004), and by extension, that the exaggerated responses to 5-HTP and tranylcypromine were likely due to further increases in serotonin in SERT −/− mice (Fox et al. 2007a).
To further understand the consequences of lifelong elevations in serotonin, and the effects of further enhancements of serotonin in adulthood, we assessed the neurochemical, behavioral and physiological effects of 5-HTP administration in SERT +/+, +/− and −/− mice. First, using high-performance liquid chromatography (HPLC), we examined the effects of 5-HTP on brain tissue levels of serotonin, on serotonin’s major metabolite, 5-hydroxyindoleacetic acid (5-HIAA), as well as on serotonin turnover ratios in SERT +/+, +/− and −/− mice. For behavioral assessments, we examined possible sex differences in 5-HTP-induced behaviors in male and female SERT +/+, +/− and −/− mice, as previous studies show sex differences in presynaptic 5-HT1A function in SERT-deficient mice (Bouali et al. 2003; Holmes et al. 2003a; Li et al. 2000; Li et al. 1999). Further, as the dopamine transporter (DAT) has been shown to take up serotonin in SERT −/− mice, but not in SERT +/+ or +/− mice (Mossner et al. 2006; Pan et al. 2001; Schmitt et al. 2003; Shen et al. 2004; Zhou et al. 2002), we assessed the effects of DAT blockade on 5-HTP-induced serotonin syndrome behaviors in SERT −/− mice.
As a physiological measure, we assessed the temperature effects of 5-HTP in SERT +/+, +/− and −/− mice. Presynaptic 5-HT1A (Bill et al. 1991; Goodwin et al. 1985; Martin et al. 1992) and 5-HT7 receptors (Faure et al. 2006; Guscott et al. 2003; Hedlund et al. 2003; Hedlund et al. 2004) regulate hypothermic responses in mice, and as mentioned, presynaptic 5-HT1A receptors are downregulated in SERT −/− mice; however to date, 5-HT7 receptor function has not been examined in SERT-deficient mice. We therefore examined roles for 5-HT1A and 5-HT7 receptors, and their interactions, in hypothermic responses to 5-HTP and to two 5-HT1A/7 agonists, 5-CT and 8-OH-DPAT, in SERT-deficient mice.
Methods
Animals
Subjects were male and female SERT +/+, +/− and −/− mice produced by homologous recombination in ES cells as previously described (Bengel et al. 1998), and currently the product of 19–23 heterozygous backcrosses on a C57BL/6J genetic background. Animals were approximately 20–35 g in weight at the beginning of the experiments, and were housed in groups of 3–5 animals per cage with food and water available ad libitum. Animals were maintained on a 12-h light:12-h dark cycle (lights on at 0600 h) in a facility approved by the American Association for Accreditation of Laboratory Animal Care. On test days, animals were moved to the testing room in their home cage 1 h prior to testing, and all experiments were carried out between 1000 h and 1300 h. Multiple testing was performed in most animals, and there was a minimum of one week between tests to allow for drug washout. Mice were not retested following administration of either tranylcypromine or clorgyline, as these are irreversible inhibitors of MAO. All experiments adhered to the guidelines of the National Institutes of Health, and were approved by the National Institute of Mental Health Animal Care and Use Committee.
Drugs
5-HTP, trans-2-phenylcyclopropylamine hydrochloride (tranylcypromine), N-methyl-N-propargyl-3-(2,4-dichlorophenoxy)propylamine hydrochloride (clorgyline), 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) and N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY 100635) were obtained from Sigma Chemical Company (St. Louis, MO). 5-carboxamidotryptamine maleate (5-CT), SB 269970 hydrochloride and GBR 12909 dihydrochloride were obtained from Tocris Bioscience (Ellisville, MO). 5-HTP (5 mg/ml or 10 mg/ml) was prepared as an emulsion in a vehicle of 5% Tween-80 in distilled water; clorgyline (0.2 mg/ml), 8-OH-DPAT (0.1 mg/ml), WAY 100635 (0.1 mg/ml), 5-CT (0.01 mg/ml) and GBR 12909 (1 mg/ml) were dissolved in saline; tranylcypromine (0.1 mg/kg) and SB 269970 (0.2 mg/mg or 0.6 mg/ml) were prepared in distilled water, and the latter was frozen until immediately before use. All drugs were administered by intraperitoneal (ip) injection.
Analysis of brain region monoamines and metabolites
Thirty min following administration of 5-HTP (80 mg/kg) or its vehicle, SERT +/+, +/− and −/− mice were killed via cervical dislocation. Brains were removed immediately and dissected on a glass plate set in ice. Following removal of hypothalamus and frontal cortex, brains were bisected sagitally to expose and dissect hippocampus and striatum from each hemisphere, followed by isolation of brainstem containing pons and medulla (Bengel et al. 1998). Brain samples were stored at −80 °C before HPLC using electrochemical detection (ECD), as previously described (Andrews and Murphy 1993).
HPLC analysis assessing concentrations of monoamines and their precursors and metabolites was performed as previously described (Kim et al. 2005). Specifically, brain tissue samples were homogenized in 200–250 μl of 0.1 N HC1O4 using sonication and centrifuged at 7200 × g (12 000 rpm) for 10 min. For quantification of monoamines in the supernatant, an Axxichrom ODS C18 (5 μm, 25 cm × 0.46 cm) analytical column, an ESA Coulochem II detector with analytical cell (E1 = 100 mV, E2 = 300 mV; Model 5014) and a guard cell (100 mV; Model 5020) were set up with an ESA solvent pump (Model 582) and a Gibson autosampler (Model 231) fitted with a 50 μl sample loop. The mobile phase was composed 8.6 mM heptane sulfonic acid, 0.3% phosphoric acid, 0.27% triethylamine, 0.34 mM EDTA and 12% acetonitrile delivered at a flow rate of 0.6 ml/min. Samples were prepared using 100 μl of the supernatant with the addition of an internal standard (50 μl of 1 μM N-methyl serotonin for monoamines). A 55 μl aliquot of this mixture was injected onto the analytical column for HPLC-EC analysis.
Serotonin syndrome behaviors
Serotonin syndrome behaviors induced by 5-HTP (80 mg/kg) were evaluated in male and female SERT +/+, +/− and −/− mice (n = 4–6 per group). In a separate study, SERT −/− mice were administered vehicle or the DAT blocker GBR 12909 (20 mg/kg) followed 30 min later by 5-HTP (40 mg/kg ) (n = 6 per group), and serotonin syndrome behaviors were assessed. In both studies, the first drug was administered after 15 min of habituation in a large Plexiglass cylinder. Behavioral assessments were made based on previous methods (Fox et al. 2007a; Izumi et al. 2006; Kennett et al. 1985). Behaviors associated with the rodent serotonin syndrome were recorded for five 1-min periods starting 5 min after drug administration. In each assessment period, the following behaviors were recorded: intermittent behaviors included head weaving, forepaw treading and backward movement (scored on a scale of 0 to 4; 0 = absent, 1 = present once, 2 = present several times, 3 = present frequently, 4 = present continuously); continuous behaviors included hind limb abduction, Straub tail, tremor and low body posture (scored on a scale of 0 to 4; 0 = absent, 1 = perceptible, 2 = weak, 3 = medium, 4 = maximal). The scores from the five 1-min periods were summed together for each behavior. Overall serotonin syndrome scores were calculated for each 5-min assessment (sum of scores for all intermittent and continuous behaviors) (Fox et al. 2007a; Jacobs 1976). Assessments were performed by observers blind to the genotype of the mice and/or the drug condition.
Temperature
Temperature was assessed using a digital thermometer (Model BAT 12, RET-3 probe, Physitemp Instruments, Clifton, NJ). The probe was inserted approximately 2 cm into the rectum of the mouse, using mild tail restraint to hold the mouse in place when necessary. Following the first temperature assessment, animals were placed individually into large Plexiglass containers in a room with an ambient temperature of 21 ± 1 °C. Rectal temperature was measured every 15 min beginning 15 min prior to drug administration.
In an initial dose-response study, SERT +/+, +/− and −/− mice were administered 20, 40, 80 or 160 mg/kg 5-HTP (n = 4–11 per group). In all other studies, a dose of 80 mg/kg 5-HTP (except where noted) was used, determined as optimal based on the dose-response studies. Temperature change induced by 5-HTP (80 mg/kg) was compared between male and female SERT +/+, +/− and −/− mice (n = 4–6 per group). In a separate study, the effects of GBR 12909 (20 mg/kg) on temperature change following 5-HTP (40 mg/kg) in SERT −/− mice was examined (n = 6 per group). In SERT +/+ mice, the effects of pretreatment with a monoamine oxidase inhibitor (MAOI), either tranylcypromine (0.5 or 1 mg/kg; n = 5–6 per group) or clorgyline (1.2 mg/kg; n = 5), 60 min prior to 5-HTP (80 mg/kg) on temperature change were examined, and comparisons were made to SERT +/+ mice administered 5-HTP alone (n = 8).
In three separate studies, we examined the effects of pretreatment 30 min earlier with the 5-HT1A antagonist WAY 100635 (1 mg/kg) and the 5-HT7 antagonist SB 269970 (3 or 12 mg/kg), administered alone or in combination, on temperature change induced by 80 mg/kg 5-HTP (n = 4 per group; 3 mg/kg SB 269970), 0.1 mg/kg 5-CT (n = 6–8 per group; 12 mg/kg SB 269970) or 2 mg/kg 8-OH-DPAT (n = 5–14 per group; WAY 100635 only) in female SERT +/+, +/− and −/− mice. The dose of 5-CT (0.1 mg/kg) was selected based on pilot dose-response studies examining the hypothermic effects of five doses of 5-CT (0.1, 0.5, 1, 1.5 and 3 mg/kg); temperature change was greatest following 3 mg/kg over a 105 min period (data not shown). Finally, we assessed the effects of pretreatment 30 min earlier with SB 269970 (3 or 12 mg/kg) on the hypothermic response to 8-OH-DPAT (2 mg/kg; n = 5–6 per group) in purchased wildtype C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME).
Head twitches
Following 15 min of habituation in a Plexiglass cylinder, SERT +/+, +/− and −/− mice were administered either vehicle or WAY 100635 (1 mg/kg ) (n = 4–11 per group). Head twitches, a response mediated by 5-HT2A receptors (Gonzalez-Maeso et al. 2003; Moya et al. 2007; Willins and Meltzer 1997), were counted for five 1-min periods starting 5 min after drug administration, and were summed over these five periods.
Statistical analyses
For each experiment, data were analyzed using one-way or two-way (genotype × drug condition) analyses of variance (ANOVAs). Post-hoc comparisons between genotypes or between drug conditions were conducted using Tukey HSD pair-wise comparisons or by t-tests when only two groups were compared. Significance was based on p < 0.05.
Results
Effects of 5-HTP on tissue serotonin levels, 5-HIAA levels and serotonin turnover rates (5-HIAA:serotonin ratios)
We first examined the effects of 80 mg/kg 5-HTP versus its vehicle on tissue levels of serotonin and its major metabolite 5-HIAA, and the ratio of 5-HIAA to serotonin (an index of serotonin turnover rate). Comparisons of vehicle-treated mice confirmed previous findings of lower baseline tissue serotonin levels in SERT −/− mice compared to SERT +/+ mice in all brain areas examined (all p’s < 0.0001, based on pre-planned Tukey post hoc comparisons to confirm earlier baseline findings (Bengel et al. 1998; Kim et al. 2005; Sheridan et al. 1999)).
Thirty min after 5-HTP, tissue serotonin levels were increased compared to vehicle (baseline) in all five brain areas examined, regardless of genotype (Table 1). Compared to baseline serotonin levels in brain stem, frontal cortex, hippocampus and hypothalamus, 5-HTP increased serotonin levels 2.1- to 2.5-fold in SERT +/+ mice, 2.0- to 2.9-fold in SERT +/− mice, and to a greater extent, 4.4- to 6.8-fold, in SERT −/− mice. Of particular note, 5-HTP-induced serotonin increases in striatum were highest, with 4.9-fold increases in SERT +/+ mice, 5.1-fold increases in SERT +/− mice, and greater 11.7-fold increases in SERT −/− mice.
Table 1.
Tissue levels of serotonin, 5-HIAA and serotonin turnover rates (5-HIAA:serotonin) 30 min after 5-HTP (80 mg/kg) or its vehicle in SERT +/+, +/− and −/− mice.
| Measurement (pg/mg tissue) Brain area
|
Drug condition | Two-way ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle
|
5-HTP
|
Genotype
|
Drug condition
|
Interaction
|
||||||||
| SERT +/+
|
SERT +/−
|
SERT −/−
|
SERT +/+
|
SERT +/−
|
SERT −/−
|
F2,31 |
p-value
|
F1,31 |
p-value
|
F2,31 |
p-value
|
|
| Serotonin | ||||||||||||
| Brain stem | 961 ± 22 | 976 ± 62 | 284 ± 54#### | 2498 ± 252 | 2611 ± 205 | 1922 ± 265 | 57.59 | < 0.0001 | 769.63 | < 0.0001 | 0.33 | NS |
| Frontal cortex | 763 ± 55 | 811 ± 65 | 243 ± 14#### | 1595 ± 214 | 1600 ± 197 | 1071 ± 56 | 70.22 | < 0.0001 | 370.86 | < 0.0001 | 0.12 | NS |
| Hippocampus | 780 ± 67 | 741 ± 92 | 247 ± 32#### | 2024 ±515 | 2005 ± 288 | 1432 ± 223 | 17.59 | < 0.0001 | 202.08 | < 0.0001 | 0.07 | NS |
| Hypothalamus | 1799 ± 199 | 1790 ± 225 | 580 ± 144#### | 5237 ± 628 | 5251 ± 446 | 3688 ± 322 | 56.88 | < 0.0001 | 751.03 | < 0.0001 | 0.87 | NS |
| Striatum | 715 ± 50 | 737 ± 106 | 307 ± 109#### | 3505 ± 399 | 3771 ± 647 | 3579 ± 498 | 2.00 | NS (0.15) | 561.97 | < 0.0001 | 1.15 | NS |
| 5-HIAA | ||||||||||||
| Brain stem | 884 ± 168 | 591 ± 162 | 508 ± 133 | 5292 ± 563 | 5645 ± 496 | 5070 ± 622 | 2.34 | NS (0.11) | 1165.88 | < 0.0001 | 2.07 | NS |
| Frontal cortex | 385 ± 97 | 292 ± 84 | 221 ± 62 | 3209 ± 277 | 3132 ± 447 | 2713 ± 171 | 5.99 | 0.006 | 1153.74 | < 0.0001 | 1.98 | NS |
| Hippocampus | 795 ± 191 | 512 ±129 | 618 ±534 | 4172 ± 351 | 4081 ± 384 | 3617 ± 331 | 3.33 | 0.049 | 839.53 | < 0.0001 | 2.15 | NS |
| Hypothalamus | 999 ± 209 | 675 ± 233 | 600 ± 174 | 7236 ± 1055 | 7110 ± 746 | 6370 ± 571 | 3.43 | 0.045 | 966.50 | < 0.0001 | 1.00 | NS |
| Striatum | 589 ± 223 | 483 ± 125 | 417 ± 171 | 5512 ±449 | 5713 ± 313 | 5269 ± 680 | 1.59 | NS | 1630.89 | < 0.0001 | 0.9 | NS |
| Serotonin turnover | ||||||||||||
| Brain stem | 92 ± 16 | 60 ± 14 | 178 ± 31**** | 212 ± 17++++ | 216 ± 13++++ | 264 ± 11++++**** | 73.12 | < 0.0001 | 407.19 | < 0.0001 | 11.63 | < 0.0001 |
| Frontal cortex | 51 ± 13 | 36 ± 8 | 90 ± 22 | 203 ± 19 | 196 ± 12 | 254 ± 20 | 40.82 | < 0.0001 | 869.76 | < 0.0001 | 0.38 | NS |
| Hippocampus | 101 ± 20 | 68 ± 10 | 244 ± 194 | 214 ±42 | 208 ± 38 | 259 ± 50 | 6.28 | 0.005 | 10.25 | 0.003 | 1.85 | NS |
| Hypothalamus | 55 ± 9 | 37 ± 9 | 103 ± 17**** | 138 ± 10++++ | 135 ± 5++++ | 173 ± 9++++**** | 84.38 | < 0.0001 | 588.16 | < 0.0001 | 5.75 | 0.008 |
| Striatum | 83 ± 33 | 65 ± 11 | 134 ± 23** | 159 ± 20++ | 155 ± 24++++ | 148 ± 17 | 6.18 | 0.006 | 65.32 | < 0.0001 | 9.85 | < 0.0001 |
Data represent the mean ± SEM. NS, not significant.
p < 0.0001 compared to SERT +/+ mice in the same drug condition, based on pre-planned comparisons (Tukey post hoc tests) to confirm previous findings (Kim et al. 2005);
p < 0.01
p < 0.0001 compared to SERT +/+ mice in the same drug condition
p < 0.01
p < 0.0001 compared to vehicle-treated mice of the same genotype (Tukey post hoc tests for significant interactions).
5-HTP also increased 5-HIAA levels in all brain areas examined compared to vehicle-treated mice, regardless of genotype (Table 1). Further, serotonin turnover rates were increased in 5-HTP-treated mice compared to vehicle-treated mice in all brain areas examined regardless of genotype, with 1.9- to 4.0-fold increases turnover rates in SERT +/+ mice, 2.4- to 5.4-fold increases in SERT +/− mice, and 1.1- to 2.8-fold increases in SERT −/− mice (Table 1). There were also significant genotype × drug interactions for brain stem, hypothalamus and striatum (Table 1). Specifically, serotonin turnover rates were significantly higher in 5-HTP-treated mice of all three genotypes in each of these brain areas (p’s < 0.001), except for SERT −/− mice in striatum. There were no differences in serotonin turnover rates between SERT +/+ and +/− mice. In vehicle-treated mice, serotonin turnover rates in SERT −/− mice were 1.6- to 1.9-fold higher compared to SERT +/+ mice in each of these brain areas (p’s < 0.006). In 5-HTP-treated mice, serotonin turnover rates were ~1.3-fold higher in SERT −/− mice compared to SERT +/+ mice in brain stem and hypothalamus (p’s < 0.0001), with no genotype differences in striatum.
Effects of 5-HTP on catecholamines
5-HTP decreased norepinephrine levels in all genotypes in brain stem, hippocampus and hypothalamus (p’s < 0.008), with no change in the frontal cortex (striatum not examined) (data not shown). 5-HTP also decreased dopamine in striatum (p = 0.001), with a trend toward decreased DOPAC (p = 0.074) in mice of all three genotypes compared to vehicle-treated mice (data not shown). There were no significant genotype × drug condition interactions for norepinephrine, dopamine or DOPAC for any of the brain areas examined.
Serotonin syndrome behaviors
We previously showed that female SERT −/− mice have exaggerated serotonin syndrome behavioral responses to 5-HTP relative to SERT +/+ mice (Fox et al. 2007a). As other studies have shown sex differences in presynaptic 5-HT1A function in SERT-deficient mice, we compared 5-HTP-induced behaviors in male and female SERT +/+, +/− and −/− mice in order to examine possible sex differences in postsynaptic 5-HT1A function in these mice. For serotonin syndrome behaviors overall, we again found that SERT −/− mice had exaggerated behavioral responses to 5-HTP compared to SERT +/+ mice (p < 0.0001 regardless of sex), with no differences between male and female mice (Table 2). Data for individual behaviors are also presented in Table 2.
Table 2.
Overall and individual serotonin syndrome behaviors in male and female SERT +/+, +/− and −/− mice administered 5-HTP (80 mg/kg)
| Sex
|
Two-way ANOVA
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin syndrome behavior(s)
|
Males
|
Females
|
Genotype
|
Sex
|
Interaction
|
|||||||
| SERT+/+
|
SERT+/−
|
SERT−/−
|
SERT+/+
|
SERT-+/−
|
SERT−/−
|
F2,31 |
p |
F1,31 |
p |
F2,31 |
p |
|
| Overall | 16.8±4.7 | 15.9±4.3 | 46.1±7.2 | 15.0±5.3 | 15.4±5.0 | 44.6±4.2 | 108.58 | <0.0001 | 0.42 | NS | 0.04 | NS |
| Headweaving | 7.3±1.3 | 7.2±2.7 | 15.2±2.7 | 7.5±2.7 | 8.2±3.8 | 15.6±5.4 | 17.66 | <0.0001 | 0.19 | NS | 0.04 | NS |
| Forepaw treading | 5.0±3.6 | 4.0±2.2 | 5.2±1.3 | 4.8±2.4 | 5.7±2.1 | 8.4±4.8 | 1.54 | NS | 2.09 | NS | 0.80 | NS |
| Backward movement | 0.0±0.0 | 0.2±0.4 | 2.4±0.9 | 0.3±0.5 | 0.0±0.0 | 1.6±0.1 | 6.88 | 0.004 | 0.24 | NS | 0.36 | NS |
| Hind limb abduction | 2.1±0.6 | 1.8±1.0 | 10.0±3.7 | 0.9±0.6 | 0.5±0.3 | 7.2±2.7 | 47.17 | −0.0001 | 6.23 | 0.02 | 0.50 | NS |
| Straub tail | 0.4±0.5 | 0.3±0.4 | 0.2±0.3 | 0.0±0.0 | 0.0±0.0 | 0.1±0.2 | 0.12 | NS | 4.96 | 0.04 | 0.50 | NS |
| Tremor | 0.5±0.4 | 0.6±0.4 | 2.6±1.9 | 0.1±0.3 | 0.0±0.0 | 2.4±1.4 | 15.94 | <0.0001 | 1.08 | NS | 0.10 | NS |
| Low posture | 1.5±0.4 | 1.9±0.4 | 10.5±2.3 | 1.5±0.6 | 1.1±0.7 | 9.3±1.6 | 155.86 | <0.0001 | 2.22 | NS | 0.55 | NS |
Data represent the mean ± SEM
NS not significant
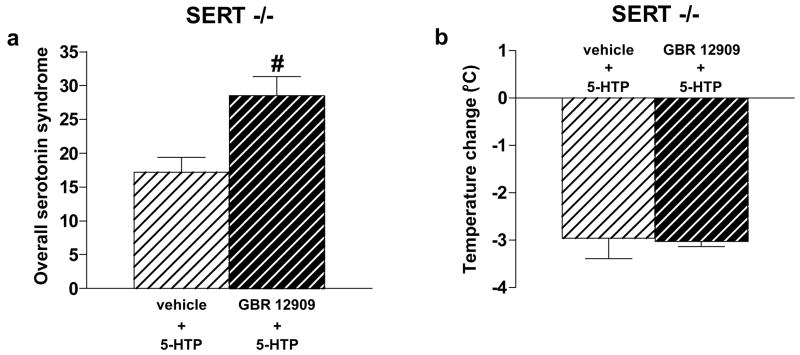
As mentioned, DAT promiscuously takes up serotonin in SERT −/− mice, but not in SERT +/+ or +/− mice (Mossner et al. 2006; Pan et al. 2001; Schmitt et al. 2003; Shen et al. 2004; Zhou et al. 2002). Thus, we assessed the effects of the DAT blocker GBR 12909 on 5-HTP-induced serotonin syndrome behaviors in SERT −/− mice. Pretreatment with GBR 12909 significantly increased 5-HTP-induced serotonin syndrome behaviors overall in SERT −/− mice (p = 0.01) (Figure 1a).
Figure 1.
Effect of the DAT blocker GBR 12909 on 5-HTP-induced serotonin syndrome behaviors and hypothermia in SERT −/− mice. In SERT −/− mice, pretreatment with the DAT blocker GBR 12909 (20 mg/kg) 30 min earlier increased serotonin syndrome behaviors overall induced by 5-HTP (40 mg/kg) (a), whereas pretreatment with this same dose of GBR 12909 had no effect on 5-HTP-induced hypothermia 30 min after 5-HTP (b). Data represent the mean ± SEM. # p < 0.05.
5-HTP-induced hypothermia
We next examined the effects of 5-HTP on temperature in SERT +/+, +/− and −/− mice. 5-HTP decreased temperature in mice of all three SERT genotypes over a range of doses and over time (Figure 2a–c). Comparisons between the genotypes of temperature change were made at 30 min following 5-HTP, the time point corresponding to the HPLC assessments (above) and the end of the serotonin syndrome behavioral assessments described here and previously (Fox et al. 2007a). There were significant main effects of genotype (F2,56 = 58.69, p < 0.0001) and dose (F3,56 = 150.53, p < 0.0001), and a significant genotype × dose interaction (F6,56 = 6.18, p < 0.0001). SERT +/− mice had greater changes in temperature following 80 mg/kg 5-HTP (p = 0.022), and SERT −/− mice had greater changes in temperature following administration of 40, 80 and 160 mg/kg 5-HTP (p’s < 0.0001), compared to SERT +/+ mice administered these same doses (Figure 2d). There were no differences in 5-HTP-induced hypothermia between male and female mice (data not shown). Further, pretreatment with the DAT blocker GBR 12909 had no effect on 5-HTP-induced hypothermia in SERT −/− mice (Figure 1b).
Figure 2.
Dose- and time-response curves for 5-HTP-induced hypothermia. A range of doses of the serotonin precursor 5-HTP decreased temperature in SERT +/+ (a), +/− (b) and −/− (c) mice over time. Comparisons between the genotypes were made for temperature change 30 min after 5-HTP; SERT +/− mice administered 80 mg/kg 5-HTP, and SERT −/− mice administered 40, 80 and 160 mg/kg 5-HTP, had greater changes in temperature compared to SERT +/+ mice administered these same doses (d). Data represent the mean ± SEM. * p < 0.05 and **** p < 0.0001 compared to SERT +/+ mice administered the same dose of 5-HTP.
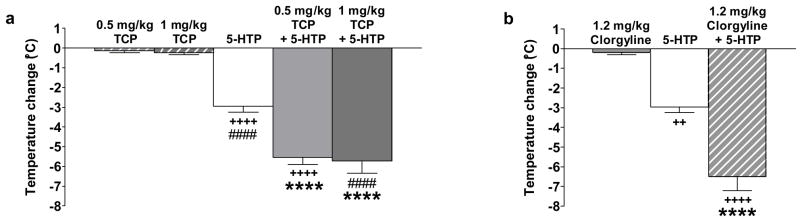
Effects of MAOI pretreatment on 5-HTP-induced hypothermia in SERT +/+ mice
Although administration of a single serotonin-enhancing drug increased serotonin syndrome behaviors in SERT −/− mice, a combination of two serotonin-enhancing drugs was required to induce serotonin syndrome behaviors in SERT +/+ mice (Fox et al. 2007a). To determine the effects of additional increases in serotonin levels in SERT +/+ mice, we next examined the effects of pretreatment with a MAOI on 5-HTP-induced temperature change in SERT +/+ mice. There were significant main effects of drug condition for both the nonselective MAO-A/B inhibitor tranylcypromine (F4,29 = 51.61, p < 0.0001) and the MAO-A-selective inhibitor clorgyline (F2,15 = 48.65, p < 0.0001). Administered alone, neither tranylcypromine (Figure 3a) nor clorgyline (Figure 3b) had an effect on temperature in SERT +/+ mice. However, pretreatment with either tranylcypromine (p’s < 0.0001) or clorgyline (p < 0.0001) enhanced the temperature change in SERT +/+ mice induced by 80 mg/kg 5-HTP, similar to the changes in SERT −/− mice administered this same dose of 5-HTP alone (−7.05 ± 0.80 °C) (Figure 2d).
Figure 3.
Effects of MAOI pretreatment on 5-HTP-induced temperature change in SERT +/+ mice. Pretreatment with tranylcypromine (TCP) (0.5 or 1 mg/kg) (a) or clorgyline (1.2 mg/kg) (b) enhanced the hypothermic response to 5-HTP (80 mg/kg) 30 min after administration, a change similar to that observed in SERT −/− mice administered 5-HTP alone (−7.05 ± 0.80 °C; Figure 2c,d). Data represent the mean ± SEM. **** p < 0.0001 compared to mice administered this same dose of 5-HTP alone; ++ p < 0.01, ++++ p < 0.0001 compared to mice administered 0.5 mg/kg tranylcypromine alone (a) or clorgyline alone (b); #### p < 0.0001 compared to mice administered 1 mg/kg tranylcypromine alone.
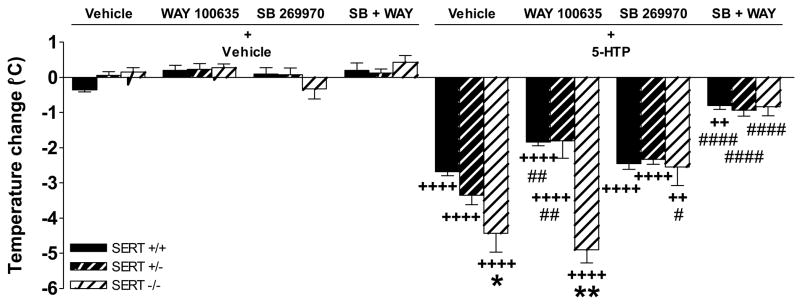
Effects of WAY 100635 and SB 269970 pretreatment on 5-HTP-induced temperature change
To assess possible roles for 5-HT1A and 5-HT7 receptors in the exaggerated temperature response to 5-HTP, mice were pretreated with the selective 5-HT1A antagonist WAY 100635, the selective 5-HT7 antagonist SB 269970, or the combination of WAY 100635 and SB 269970. There were significant main effects for genotype (F2,72 = 11.72, p < 0.0001) and drug condition (F11,72 = 106.49, p < 0.0001), and a significant genotype × drug condition interaction (F14,72 = 7.56, p < 0.0001). 5-HTP again decreased temperature in mice of all three genotypes compared to their respective controls administered vehicle alone (p’s < 0.0001), with an exaggerated response in SERT −/− mice (p = 0.017), and a trend toward an increased response in SERT +/− mice (p = 0.067), compared to SERT +/+ mice (Figure 4). Pretreatment with WAY 100635 partially decreased 5-HTP-induced temperature change in SERT +/+ and +/− mice (p’s < 0.003 compared to 5-HTP alone), with no effect in SERT −/− mice. Pretreatment with SB 269970 decreased 5-HTP-induced temperature change in SERT −/− mice (p = 0.013 compared to 5-HTP alone), and there was a trend toward a decrease in SERT +/− mice (p = 0.094 compared to 5-HTP alone), with no effect in SERT +/+ mice (NS compared to 5-HTP alone). Pretreatment with the combination of WAY 100635 and SB 269970 decreased 5-HTP-induced hypothermia in mice of all three genotypes (p’s < 0.0001 compared to 5-HTP alone), and completely blocked 5-HTP-induced hypothermia in SERT +/− and −/− mice (NS compared to the combination of WAY 100635 and SB 269970 administered alone).
Figure 4.
Effects of pretreatment with WAY 100635 and SB 269970 on 5-HTP-induced temperature change. 5-HTP (80 mg/kg) again induced an exaggerated temperature change in SERT −/− mice 30 min after administration, with a trend toward significance in SERT +/− mice (p = 0.067) compared to SERT +/+ mice. Pretreatment with the 5-HT1A antagonist WAY 100635 (1 mg/kg) decreased 5-HTP-induced hypothermia in SERT +/+ and +/− mice, with no effect in SERT −/− mice. Conversely, in SERT −/− mice, pretreatment with the 5-HT7 antagonist SB 269970 (3 mg/kg) decreased the temperature change induced by 5-HTP, down to levels in SERT +/+ mice in the same drug condition, with a trend toward a decrease in SERT +/− mice (p = 0.094), whereas SB 269970 administered alone had no effect alone on 5-HTP-induced hypothermia in SERT +/+ mice. Pretreatment with the combination WAY 100635 (1 mg/kg) and SB 269970 (3 mg/kg) eliminated 5-HTP-induced hypothermia in SERT +/− and −/− mice, down to levels of their counterparts administered this combination of pretreatment drugs alone (i.e. in the absence of 5-HTP). Data represent the mean ± SEM. * p < 0.05, ** p < 0.01 compared to SERT +/+ mice in the same drug condition; ++ p < 0.01, ++++ p < 0.0001 compared to mice of the same genotype treated with the pretreatment drug(s) only; # p < 0.05, #### p < 0.0001 compared to mice of the same genotype administered vehicle plus 5-HTP.
5-CT-induced hypothermia and the effects of pretreatment with WAY 100635 and SB 269970
Recent studies show that 5-CT-induced hypothermia is at least partially mediated by 5-HT7 receptors (Guscott et al. 2003; Hagan et al. 2000; Hedlund et al. 2003). Thus, to further examine 5-HT1A and 5-HT7 receptors in temperature regulation in SERT-deficient mice, we examined the temperature effects of the 5-HT1A/7 agonist 5-CT. 5-CT decreased temperature in SERT +/+, +/− and −/− mice over time (Figure 5a). In a separate study, we compared the temperature change 30 min following administration of 5-CT in SERT-deficient mice pretreated with WAY 100635, SB 269970, or their combination. There were significant main effects for genotype (F2,130 = 3.53, p = 0.032) and drug condition (F7,130 = 71.13, p < 0.0001), but the genotype × drug condition interaction was not significant (F14,130 = 0.81, NS). Regardless of genotype, 5-CT induced a significant temperature change compared to vehicle (Figure 5b) (p < 0.0001). This effect was decreased by pretreatment with either WAY 100635 or SB 269970 each administered alone (p’s < 0.0001 compared to 5-CT alone), and was completely blocked by the combination of WAY 100635 and SB 269970 (p < 0.0001 versus 5-CT alone; NS compared to the combination of WAY 100635 and SB 269970 administered alone).
Figure 5.
5-CT-induced temperature change and the effects of pretreatment with WAY 100635 and SB 269970. 5-CT (0.1 mg/kg) decreased temperature in SERT +/+, +/− and −/− mice over time (a). Thirty min after drug administration, 5-CT (0.1 mg/kg) significantly decreased temperature, with no differences between the genotypes (b). Regardless of genotype, 5-CT-induced hypothermia was decreased by pretreatment with either WAY 100635 (1 mg/kg) or SB 269970 (12 mg/kg) each administered alone, and was completely blocked by pretreatment with the combination of WAY 100635 (1 mg/kg) and SB 269970 (12 mg/kg). Data represent the mean ± SEM. ++++ < 0.0001 compared to the pretreatment drug(s) alone, regardless of genotype; #### p < 0.0001 compared to mice treated with vehicle plus 5-CT, regardless of genotype.
8-OH-DPAT-induced hypothermia and the effects of pretreatment with WAY 100635 or SB 269970
As mentioned, SERT −/− mice have blunted or absent hypothermic responses to 8-OH-DPAT (Bouali et al. 2003; Holmes et al. 2003a; Li et al. 2000; Li et al. 1999), an effect mediated by presynaptic 5-HT1A receptors (Bill et al. 1991; Goodwin et al. 1985; Martin et al. 1992). Recently, we showed that 8-OH-DPAT, at a dose of 2 mg/kg ip, induced similar serotonin syndrome behaviors in SERT +/+, +/− and −/− mice, an effect mediated by postsynaptic 5-HT1A receptors (Fox et al. 2007a; Goodwin et al. 1987a; Goodwin et al. 1987b; Goodwin et al. 1986; Lucki et al. 1984; Smith and Peroutka 1986; Yamada et al. 1988). For direct comparison to our previous studies, which were suggestive of intact postsynaptic 5-HT1A function, we assessed the hypothermic response to 2 mg/kg ip 8-OH-DPAT in SERT +/+, +/− and −/− mice. There were significant main effects for genotype (F2,98 = 6.33, p = 0.003) and drug condition (F3,98 = 33.44, p < 0.0001), and a significant genotype × drug condition interaction (F6,98 = 2.596, p = 0.023). 8-OH-DPAT decreased temperature in SERT +/+ and +/− mice compared to vehicle (p’s < 0.0001), with no effect in SERT −/− mice (Figure 6a). This effect of 8-OH-DPAT in SERT +/+ and +/− mice was completely blocked by pretreatment with WAY 100635 (p’s < 0.003 compared to 8-OH-DPAT; NS compared to WAY 100635 alone). Taken together with our previous findings, the absence of a hypothermic response and an intact behavioral response to 8-OH-DPAT (2 mg/kg ip) in SERT −/− mice, suggests that although presynaptic 5-HT1A function is altered, postsynaptic 5-HT1A receptors are functionally intact in SERT −/− mice.
Figure 6.
8-OH-DPAT-induced temperature change and the effects of pretreatment with WAY 100635 or SB 269970. 8-OH-DPAT (2 mg/kg) decreased temperature in SERT +/+ and +/− mice compared to vehicle, an effect that was blocked by pretreatment with WAY 100635 (1 mg/kg), whereas 8-OH-DPAT had no effect on temperature in SERT −/− mice (a). Pretreatment with a low (3 mg/kg) or a high (12 mg/kg) dose of SB 269970 (SB) had no effect on 8-OH-DPAT-induced hypothermia in purchased C57BL/6J wildtype mice (b). Data represent the mean ± SEM. * p < 0.05 compared to SERT +/+ mice in the same drug condition; + p < 0.05, ++++ p < 0.0001 compared to mice of the same genotype administered the pretreatment drug(s) only; ## p < 0.01 compared to mice of the same genotype treated with vehicle plus 8-OH-DPAT.
Recent studies show that 8-OH-DPAT-induced hypothermia is partially mediated by 5-HT7 receptors, an effect which may be dose-dependent (Bonaventure et al. 2002; Faure et al. 2006; Hedlund et al. 2004). To confirm that the hypothermic response to 2 mg/kg ip 8-OH-DPAT, which had no effect in SERT −/− mice, is mediated by presynaptic 5-HT1A receptors and not 5-HT7 receptors, we examined the effects of pretreatment with a low (3 mg/kg) and a high (12 mg/kg ip) dose of the selective 5-HT7 antagonist SB 269970 on 8-OH-DPAT-induced hypothermia in purchased C57BL/6J mice. There was a significant main effect of drug condition (F5,31 = 55.61, p < 0.0001). Again, 8-OH-DPAT induced a hypothermic response compared to vehicle, and pretreatment with either dose of SB 269970 had no effect on this response (Figure 6b).
Head twitches
During the above-mentioned temperature studies, we noted that the selective 5-HT1A antagonist WAY 100635 induced the head twitch response in SERT −/− mice, an effect mediated by 5-HT2A receptors (Gonzalez-Maeso et al. 2003; Moya et al. 2007; Willins and Meltzer 1997). To further examine this effect, we assessed the number of head twitches in SERT +/+, +/− and −/− mice administered WAY 100635 (1 mg/kg) or its vehicle. There were significant main effects for genotype (F2,38 = 30.38, p < 0.0001) and drug condition (F12,38 = 99.68, p < 0.0001), and a significant genotype × drug condition interaction (F12,38 = 34.79, p < 0.0001). There were no differences between SERT +/+, +/− and −/− mice administered vehicle, suggesting equal head twitches at baseline, which were few (Figure 7). WAY 100635 increased the number of head twitches in SERT +/− and −/− mice compared to their vehicle-treated counterparts (p’s < 0.008), with a trend toward an increase in SERT +/+ mice (p = 0.082). In WAY 100635-treated mice, SERT −/− mice had significantly more head twitches than did SERT +/+ mice (p = 0.001).
Figure 7.
WAY 100635-induced head twitches. The selective 5-HT1A antagonist WAY 100635 (1 mg/kg) induced head twitches in SERT +/− and −/− mice, with a trend toward an increase in SERT +/+ mice compared to vehicle (p = 0.082). This effect was exaggerated in SERT −/− mice compared to SERT +/+ mice. Data represent the mean ± SEM. ** p < 0.01 compared to SERT +/+ mice in the same drug condition; ++ p < 0.01, ++++ p < 0.0001 compared to mice of the same genotype administered vehicle.
Discussion
SERT −/− mice have enhanced baseline extracellular fluid serotonin levels (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004), and lifelong exposure to these enhanced levels of serotonin results in a high-anxiety and depressive-like phenotype in adult SERT −/− mice (Alexandre et al. 2006; Ansorge et al. 2008; Ansorge et al. 2004; Holmes et al. 2003a; Holmes et al. 2002; Kalueff et al. 2007; Popa et al. 2008; Wisor et al. 2003; Zhao et al. 2006). In the current studies, we examined the neurochemical, behavioral and physiological effects of further increases in serotonin in SERT +/+, +/− and −/− mice that were produced by administration of the serotonin precursor 5-HTP.
Examinations of tissue serotonin levels in vehicle-treated mice confirm previous reports of decreased baseline tissue serotonin and its major metabolite, 5-HIAA, in addition to increased serotonin turnover and synthesis rates, in SERT −/− mice (Table 1) (Bengel et al. 1998; Kim et al. 2005; Sheridan et al. 1999). Thirty minutes following 5-HTP, mice of all three SERT genotypes had significantly higher levels of serotonin and 5-HIAA compared to vehicle-treated mice in all brain areas examined (Table 1). In 5-HTP-treated mice, the increases in serotonin versus baseline levels were higher in SERT −/− mice in all brain areas examined (~4.5 to 11.7-fold increases versus 2.1- to 2.5-fold increases in SERT +/+ and +/− mice), with the highest fold-increases in striatum for mice of all three genotypes. Importantly, the timing of these assessments and the dose of 5-HTP correspond to the dose and timing which induced exaggerated behavioral and temperature responses in SERT −/− mice.
Confirming our previous findings, SERT −/− mice had exaggerated serotonin syndrome behavioral responses to 5-HTP (Fox et al. 2007a). As mentioned, SERT −/− mice also display serotonin syndrome behaviors at baseline in the absence of drug (Fox et al. 2007a; Kalueff et al. 2007), and we postulated that these baseline behaviors were due to elevated baseline extracellular serotonin levels (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004). The current neurochemical studies show that 5-HTP increased tissue serotonin levels in mice of all three SERT genotypes, with higher fold-increases versus baseline in SERT −/− mice.
Once released, the effects of increased tissue serotonin levels following 5-HTP administration appear exaggerated in SERT −/− mice. Several factors may explain these responses. First, serotonin cannot be taken back up into the presynaptic cell, as SERT −/− mice lack SERT, the primary mechanism of removal of serotonin from the synapse (Blakely et al. 1991), transcribing only a truncated, non-functional protein (Bengel et al. 1998; Ravary et al. 2001). Further, SERT −/− mice have decreased clearance of serotonin (Montanez et al. 2003) and decreased presynaptic 5-HT1A autoreceptor function (Gobbi et al. 2001; Li et al. 2000; Li et al. 1999), which when activated serve as a negative feedback loop for serotonin synthesis (Hoyer et al. 1994; Sharp et al. 2007). These homeostatic alterations in SERT −/− mice result in ~6-fold increases in extracellular fluid serotonin at baseline (Fabre et al. 2000; Mathews et al. 2004; Shen et al. 2004). Thus, in SERT −/− mice, the 5-HTP-induced increases in serotonin shown here likely result in further enhanced extracellular levels of serotonin, which, acting on downstream receptors, result in the exaggerated behavioral and temperature responses currently reported.
Previous examinations of SERT −/− mice show sex differences in presynaptic 5-HT1A receptor function, including assessments of 8-OH-DPAT-induced hypothermia (Bouali et al. 2003; Holmes et al. 2003a; Li et al. 2000; Li et al. 1999). We found no differences in the exaggerated serotonin syndrome behavioral responses to 5-HTP between male and female SERT −/− mice (Table 2), behaviors mediated by postsynaptic 5-HT1A receptors (Goodwin et al. 1987a; Goodwin et al. 1987b; Goodwin et al. 1986; Lucki et al. 1984; Smith and Peroutka 1986; Yamada et al. 1988). This suggests that postsynaptic 5-HT1A function, in contrast to presynaptic 5-HT1A function, is intact in both male and female SERT −/− mice.
In further behavioral assessments in SERT −/− mice, pretreatment with the DAT blocker GBR 12909 increased 5-HTP-induced serotonin syndrome behaviors (Figure 1a), although GBR 12909 did not affect 5-HTP-induced temperature change (Figure 1b). Although there are no changes in DAT expression in SERT −/− mice (Sora et al. 2001), DAT takes up serotonin in SERT −/− mice (but not in SERT +/+ or +/− mice), potentially providing a partial compensatory mechanism for exaggerated levels of serotonin in mice lacking SERT (Mossner et al. 2006; Murphy and Lesch 2008; Pan et al. 2001; Schmitt et al. 2003; Shen et al. 2004; Zhou et al. 2002). DAT is highly expressed in striatum (Bannon et al. 1998), and in the current studies, the fold increases in serotonin following a high dose of 5-HTP were largest in striatum in mice of all three genotypes, with 11.7-fold increases in SERT −/− mice (versus ~5-fold increases in SERT +/+ and +/− mice in striatum) (Table 1). Although serotonin turnover rates in striatum were increased in SERT +/+ and +/− mice administered 5-HTP compared to their vehicle-treated counterparts, this was not the case in SERT −/− mice in striatum. These findings might underlie enhancing effects of GBR 12909 pretreatment on 5-HTP, and together, the current findings support and are consistent with the proposed compensatory activity of DAT in SERT −/− mice (Zhou et al. 2002).
Providing further physiological evidence of exaggerated responses to 5-HTP, SERT +/− and −/− mice had enhanced hypothermic responses to various doses of 5-HTP (Figure 2), including 80 mg/kg, the dose assessed in the current HPLC and behavioral analyses. In SERT +/+ mice, pretreatment with a MAOI (tranylcypromine or clorgyline), drugs which decrease serotonin metabolism and therefore increase serotonin levels (Baker et al. 1984; McKim et al. 1983), increased the temperature change induced by 80 mg/kg 5-HTP (Figure 3), a change which was similar to that observed in SERT −/− mice administered 5-HTP alone (Figure 2c,d). These findings are in accord with our previous report of exaggerated serotonin syndrome behavioral responses to 5-HTP alone in SERT −/− mice, and increased serotonin syndrome behaviors in SERT +/+ mice administered a MAOI plus 5-HTP (Fox et al. 2007a), and lend support to the conclusion that the exaggerated responses observed in SERT −/− mice are due to enhanced serotonin levels following 5-HTP alone.
Pretreatment with WAY 100635 attenuated 5-HTP-induced hypothermia in SERT +/+ and +/− mice, with no effect in SERT −/− mice (Figure 4), indicating that 5-HT1A receptors do not mediate the exaggerated hypothermic response to 5-HTP in SERT −/− mice. Further, 8-OH-DAPT had no hypothermic effects in SERT −/− mice, consistent with previous reports of reduced expression and function of these 5-HT1A receptors (Bouali et al. 2003; Holmes et al. 2003b; Li et al. 2000; Li et al. 1999). Together with our earlier finding of intact behavioral responses to this same dose of 8-OH-DPAT (2 mg/kg ip), mediated by postsynaptic 5-HT1A receptors, these results confirm decreased presynaptic 5-HT1A function, but intact postsynaptic 5-HT1A function, in SERT-deficient mice, as suggested previously (Holmes et al. 2003b).
Research using antagonists selective for 5-HT7 receptors and 5-HT7 knockout mice has established a role for 5-HT7 receptors in murine temperature regulation (Faure et al. 2006; Guscott et al. 2003; Hedlund et al. 2003). Recent studies show that the hypothermic effects of 5-CT, previously thought to be mediated by 5-HT1 receptors, are in fact mediated by 5-HT7 receptors. For example, 5-CT-induced hypothermia is blocked by the 5-HT7 antagonists SB 269970 and SB 258719 in mice (Guscott et al. 2003) and by SB 269970 in guinea pigs (Hagan et al. 2000), whereas antagonists at various 5-HT1 receptors have no effect on 5-CT-induced hypothermia, including WAY 100635 (5-HT1A), pindolol (5-HT1A/B) and GR 127935 and GR 125743 (5-HT1B/D) (Guscott et al. 2003; Hagan et al. 2000). Further, 5-CT did not alter temperature in 5-HT7 knockout mice (Guscott et al. 2003; Hedlund et al. 2003), although a high dose, 3 mg/kg, did have a blunted hypothermic effect (Hedlund et al. 2003).
In these first assessments of 5-HT7 receptor function in SERT-deficient mice, we show that 5-CT induced similar hypothermic responses in mice of all three SERT genotypes, an effect that was partially decreased by pretreatment with SB 269970 (Figure 5). Further, SB 269970 attenuated the exaggerated hypothermic response to 5-HTP in SERT −/− mice, with a trend toward a decrease in SERT +/− mice, and no significant effect in SERT +/+ mice (Figure 4). These findings provide the first evidence of intact 5-HT7 receptor function in SERT −/− mice.
Recent studies also show that 8-OH-DPAT has effects at 5-HT7 receptors in a dose-dependent manner, including experiments in 5-HT1A knockout mice and their littermates (Bonaventure et al. 2002). 5-HT7 knockout mice have a diminished hypothermic response to 8-OH-DPAT, an effect that was blocked by WAY 100635, suggesting that this response is partially mediated by 5-HT1A receptors in addition to 5-HT7 receptors (Hedlund et al. 2004). In 5-HT7 wildtype mice, SB 269970 inhibited the hypothermic effects of a low dose, but not higher doses, of 8-OH-DPAT, with no effect in 5-HT7 knockout mice (Hedlund et al. 2004). Other recent research indicates that the selective 5-HT7 antagonist SB 269970 dose-dependently attenuates 8-OH-DPAT-induced hypothermia in rats (Faure et al. 2006; Hedlund et al. 2004), an effect that was additive to the attenuating effects of WAY 100635 (Faure et al. 2006). These findings suggest that 8-OH-DPAT might also act on 5-HT7 receptors and not solely at 5-HT1A receptors, as previously thought.
As mentioned, early postnatal treatment with WAY 100635 rescued several aspects of the SERT −/− phenotype, including depressive-like behaviors on the tail suspension test in male and female SERT −/− mice (Alexandre et al. 2006). Interestingly, the decreased or absent hypothermic responses to 8-OH-DPAT in SERT −/− mice were only slightly reversed via early postnatal WAY 100635 treatment in female SERT −/− mice, with no effect in male SERT −/− mice (Alexandre et al. 2006). Thus, it is possible this dysfunctional hypothermic response in SERT −/− mice is due to alterations in 5-HT7 receptors. However, in the current studies, 8-OH-DPAT again had no hypothermic effect in SERT −/− mice (Figure 6a), and neither a low nor a high dose of SB 269970, doses effective in decreasing 5-HTP and 5-CT-induced hypothermia respectively, altered 8-OH-DPAT-induced hypothermia in wildtype mice (Figure 6b). Together, these findings suggest that 2 mg/kg ip 8-OH-DPAT is selective for 5-HT1A receptors, and that 5-HT7 receptors are intact in SERT −/− mice.
Interestingly, pretreatment with the combination of SB 269970 and WAY 100635 completely blocked 5-HTP-induced hypothermia in both SERT +/− and −/− mice. These findings are of particular note as WAY 100635 alone did not affect 5-HTP-induced hypothermia in SERT −/− mice, and the attenuating effects of SB 269970 were enhanced in an additive or super-additive manner by the addition of WAY 100635 (Figure 4). Additionally, pretreatment with WAY 100635 decreased 5-CT-induced hypothermia in mice of all three SERT genotypes, and again, the combination of SB 269970 and WAY 100635 completely blocked 5-CT-induced hypothermia (Figure 5). An earlier report described similar additive effects of these two antagonists in blocking the hypothermic effects of 8-OH-DPAT in rats (Faure et al. 2006). In our previous report, SB 269970 had no effect on serotonin syndrome behaviors administered alone, and did not increase the blockade of these behaviors by WAY 100635 in SERT −/− mice, suggesting no interaction between postsynaptic 5-HT1A and 5-HT7 receptors (Fox et al. 2007a). The current findings do, however, suggest an interesting interaction between presynaptic 5-HT1A receptors and 5-HT7 receptors which will require further research, including examinations in SERT +/− and −/− mice which, as noted, have altered presynaptic 5-HT1A function.
Of further note, WAY 100635 administered alone induced head twitches in SERT −/− mice (Figure 7), a response mediated by 5-HT2A receptors (Gonzalez-Maeso et al. 2003; Moya et al. 2007; Willins and Meltzer 1997). This is particularly interesting in light of reports of decreased 5-HT2A function in SERT −/− mice, including decreased head twitch responses to the 5-HT2A/2C agonist, (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), and decreased 5-HT2A -mediated activation of the PLA2 arachidonic acid signaling pathway in SERT +/− and −/− mice (Basselin et al. under review; Qu et al. 2005). It is also interesting to note that WAY 100635 has been shown to attenuate increased levels of anxiety and to induce hyperthermia in SERT −/− mice, with no effects in SERT +/+ or +/− mice (Holmes et al. 2003a). These findings suggest that changes in 5-HT1A receptors in SERT −/− mice are more complex than previously thought. We are currently examining the mechanisms underlying WAY 100635-induced head twitches in SERT-deficient mice.
In the current studies, we report exaggerated behavioral and hypothermic responses to 5-HTP in SERT −/− mice, and show that these exaggerated responses are associated with enhanced increases in tissue serotonin over baseline levels in all five brain regions studied. In addition to confirming decreased or absent presynaptic 5-HT1A receptor function and intact postsynaptic 5-HT1A receptor function in SERT −/− mice, the current studies are the first to assess 5-HT7 receptors in SERT-deficient mice. 5-HT7 receptors have recently been implicated in anxiety, depression, circadian rhythms and REM sleep, all of which are altered in SERT −/− mice (Fox et al. 2007b; Murphy and Lesch 2008). As some, but not all, aspects of the phenotype of SERT −/− mice are rescued by early postnatal treatment with WAY 100635, it will be interesting to see if early postnatal blockade of 5-HT7 receptors will affect the development of the high-anxiety and depressive-like phenotype of SERT −/− mice. It is of significance to investigate the role of 5-HT7 receptors in these phenotypic alterations in SERT-deficient mice, in regard to implications for the treatment of anxiety and affective disorders. In addition, when the 5-HT7 antagonist SB 269970 was given together with the 5-HT1A antagonist WAY 100635, the abolishment of the temperature response to 5-HTP revealed an unexpected additive or super-additive effect of these two antagonists, a finding also requiring further investigation.
In humans, functional polymorphisms in SERT, such as the 5-HTTLPR plus SNPs within it, rs25531 and rs25532, can alter SERT expression and function by ~50% in lymphocytes, platelets and brain (Hu et al. 2006; Lesch et al. 1996; Murphy et al. 2004; Murphy and Lesch 2008; Praschak-Rieder et al. 2007), equal to that of SERT +/− mice. Individuals with the lesser expressing form of these SERT variants have been shown to have elevations in trait anxiety and increased susceptibility to depression associated with major life events (Caspi et al. 2003; Uher and McGuffin 2008). Further, these individuals are poor responders to SSRIs (Hu et al. 2006; Murphy et al. 2004; Serretti et al. 2005). As such, investigations of the role of 5-HT7 receptors in the high anxiety and depressive-like phenotype of SERT-deficient mice is of importance, especially in light of recent reports that 5-HT7 blockade enhances the antidepressant-like effects of SSRIs (Bonaventure et al. 2007).
Acknowledgments
This research was supported by the NIMH Intramural Research program.
References
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–64. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews AM, Murphy DL. Sustained depletion of cortical and hippocampal serotonin and norepinephrine but not striatal dopamine by 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP): a comparative study with 2′-CH3-MPTP and MPTP. J Neurochem. 1993;60:1167–70. doi: 10.1111/j.1471-4159.1993.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–81. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Baker GB, LeGatt DF, Coutts RT, Dewhurst WG. Rat brain concentrations of 5-hydroxytryptamine following acute and chronic administration of MAO-inhibiting antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:653–6. doi: 10.1016/0278-5846(84)90030-7. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Sacchetti P, Granneman JG. Watson SJ, editor. The dopamine transporter: potential involvement in neuropsychiatric disorders. Psychopharmacology: The Fourth Generation of Progress. 1998 online http://www.acnp.org/G4/toc.htm.
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol. 1991;103:1857–64. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, Miller K, Lovenberg TW, Atack J, Dugovic C. Selective blockade of 5-HT7 receptors enhances 5-HT transmission, antidepressant-like behavior and REM sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.119404. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Nepomuceno D, Kwok A, Chai W, Langlois X, Hen R, Stark K, Carruthers N, Lovenberg TW. Reconsideration of 5-hydroxytryptamine (5-HT)(7) receptor distribution using [(3)H]5-carboxamidotryptamine and [(3)H]8-hydroxy-2-(di-n-propylamino)tetraline: analysis in brain of 5-HT(1A) knockout and 5-HT(1A/1B) double-knockout mice. J Pharmacol Exp Ther. 2002;302:240–8. doi: 10.1124/jpet.302.1.240. [DOI] [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–12. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Faure C, Mnie-Filali O, Scarna H, Debonnel G, Haddjeri N. Effects of the 5-HT7 receptor antagonist SB-269970 on rat hormonal and temperature responses to the 5-HT1A/7 receptor agonist 8-OH-DPAT. Neurosci Lett. 2006;404:122–6. doi: 10.1016/j.neulet.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Fox M, Jensen C, Gallagher P, Murphy D. Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology. 2007a;53:643–656. doi: 10.1016/j.neuropharm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007b;195:147–66. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Alcocer G, Segura LC, Garcia Pena M, Martinez-Torres A, Miledi R. Ontogenetic distribution of 5-HT2C, 5-HT5A, and 5-HT7 receptors in the rat hippocampus. Gene Expr. 2006;13:53–7. doi: 10.3727/000000006783991935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–95. [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–43. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). A model of presynaptic 5-HT1 function. Neuropharmacology. 1985;24:1187–94. doi: 10.1016/0028-3908(85)90153-4. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. Attenuation by electroconvulsive shock and antidepressant drugs of the 5-HT1A receptor-mediated hypothermia and serotonin syndrome produced by 8-OH-DPAT in the rat. Psychopharmacology (Berl) 1987a;91:500–5. doi: 10.1007/BF00216018. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR, Heal DJ. The pharmacology of the behavioural and hypothermic responses of rats to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) Psychopharmacology (Berl) 1987b;91:506–11. doi: 10.1007/BF00216019. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Wood AJ, Green AR. The enhancement by lithium of the 5-HT1A mediated serotonin syndrome produced by 8-OH-DPAT in the rat: evidence for a post-synaptic mechanism. Psychopharmacology (Berl) 1986;90:488–93. doi: 10.1007/BF00174066. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Guscott MR, Egan E, Cook GP, Stanton JA, Beer MS, Rosahl TW, Hartmann S, Kulagowski J, McAllister G, Fone KC, Hutson PH. The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacology. 2003;44:1031–7. doi: 10.1016/s0028-3908(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol. 2000;130:539–48. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1375–80. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–7. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–32. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Heydorn WE. Paroxetine: a review of its pharmacology, pharmacokinetics and utility in the treatment of a variety of psychiatric disorders. Expert Opin Investig Drugs. 1999;8:417–41. doi: 10.1517/13543784.8.4.417. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003a;2:365–80. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003b;28:2077–88. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–23. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Iwamoto N, Kitaichi Y, Kato A, Inoue T, Koyama T. Effects of co-administration of a selective serotonin reuptake inhibitor and monoamine oxidase inhibitors on 5-HT-related behavior in rats. Eur J Pharmacol. 2006;532:258–64. doi: 10.1016/j.ejphar.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. An animal behavior model for studying central serotonergic synapses. Life Sci. 1976;19:777–85. doi: 10.1016/0024-3205(76)90303-9. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–68. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dickinson SL, Curzon G. Central serotonergic responses and behavioural adaptation to repeated immobilisation: the effect of the corticosterone synthesis inhibitor metyrapone. Eur J Pharmacol. 1985;119:143–52. doi: 10.1016/0014-2999(85)90290-0. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–95. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–9. [PubMed] [Google Scholar]
- Martin KF, Phillips I, Hearson M, Prow MR, Heal DJ. Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br J Pharmacol. 1992;107:15–21. doi: 10.1111/j.1476-5381.1992.tb14457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–81. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- McKim RH, Calverly DG, Dewhurst WG, Baker GB. Regional concentrations of cerebral amines: effects of tranylcypromine and phenelzine. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:783–6. doi: 10.1016/0278-5846(83)90066-0. [DOI] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–9. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Mossner R, Simantov R, Marx A, Lesch KP, Seif I. Aberrant accumulation of serotonin in dopaminergic neurons. Neurosci Lett. 2006;401:49–54. doi: 10.1016/j.neulet.2006.02.081. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–61. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B. Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry. 1998;59(Suppl 15):4–12. [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–23. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The serotonin transporter and depression. Depress Anxiety. 1998;8(Suppl 1):5–12. [PubMed] [Google Scholar]
- Pan Y, Gembom E, Peng W, Lesch KP, Mossner R, Simantov R. Plasticity in serotonin uptake in primary neuronal cultures of serotonin transporter knockout mice. Brain Res Dev Brain Res. 2001;126:125–9. doi: 10.1016/s0165-3806(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–73. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–54. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol Psychiatry. 2007;62:327–31. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Ravary A, Muzerelle A, Darmon M, Murphy DL, Moessner R, Lesch KP, Gaspar P. Abnormal trafficking and subcellular localization of an N-terminally truncated serotonin transporter protein. Eur J Neurosci. 2001;13:1349–62. doi: 10.1046/j.0953-816x.2001.1511.x. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71:701–9. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- Serretti A, Benedetti F, Zanardi R, Smeraldi E. The influence of Serotonin Transporter Promoter Polymorphism (SERTPR) and other polymorphisms of the serotonin pathway on the efficacy of antidepressant treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1074–84. doi: 10.1016/j.pnpbp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–36. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–9. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Sheridan D, Wichems C, Murphy D, Andrews A. The effects of PCPA on monoamine neurotransmitter levels in mice with a disruption of the serotonin transporter gene. 29th Annual Meeting of the Society for Neuroscience.1999. [Google Scholar]
- Smith LM, Peroutka SJ. Differential effects of 5-hydroxytryptamine1a selective drugs on the 5-HT behavioral syndrome. Pharmacol Biochem Behav. 1986;24:1513–9. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–6. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–46. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Traiffort E, Vargas C, Machado A, Cano J. Expression of 5-HT7 receptor mRNA in rat brain during postnatal development. Neurosci Lett. 1997;227:53–6. doi: 10.1016/s0304-3940(97)00302-9. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–91. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K. Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol. 2006a;553:185–90. doi: 10.1016/j.ejphar.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006b;51:578–86. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Tatarczynska E, Nikiforuk A, Chojnacka-Wojcik E. Enhancement of the anti-immobility action of antidepressants by a selective 5-HT(7) receptor antagonist in the forced swimming test in mice. Eur J Pharmacol. 2007;555:43–7. doi: 10.1016/j.ejphar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, Edgar DM. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14:233–8. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res. 2004;150:151–61. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Horisaka K. The behavioural effects of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in mice. Eur J Pharmacol. 1988;154:299–304. doi: 10.1016/0014-2999(88)90205-1. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, Murphy DL, Lanthorn TH, Holmes A. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140:321–34. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Lesch KP, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–19. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]