Abstract
35S-labeled Pseudomonas aeruginosa isolates were shown to bind to neutral glycosphingolipids (NGSLs) of rabbit corneal epithelia in culture by a thin-layer chromatogram overlay procedure. The lipids of the corneal epithelial cells grown in culture were extracted and partitioned into a chloroform-rich lower phase containing NGSLs and an aqueous upper phase containing gangliosides. By using a dot-blot assay, at least six times more radiolabeled P. aeruginosa isolates were shown to bind to the lipids in the lower phase compared with those in the upper phase. Thin-layer chromatography of the lower-phase lipids followed by staining with an orcinol spray revealed at least 10 NGSL components and several fast-migrating, nonglycosylated neutral lipid components (including cholesterol). 35S-labeled P. aeruginosa was shown to bind to NGSL components 1, 2, 5, 6, and 9. P. aeruginosa-reactive NGSL components 6 and 9 migrated with chromatographic mobilities similar to those of the standards ceramide trihexoside (CT) and ceramide monohexoside, respectively. Components 1 and 2 migrated slightly ahead of asialo GM1, and component 5 migrated faster than globoside but slower than CT. Among the various standards tested, P. aeruginosa bound to asialo GM1 and, to a lesser extent, to ceramide dihexoside and CT but not to GM1, GD1A, GM3, or ceramide monohexoside. It remains to be determined whether any of the five P. aeruginosa-reactive NGSL components of corneal epithelium identified in this study plays a role in the development of corneal infection. However, we have previously shown that component 9, one of the five P. aeruginosa-reactive NGSL components identified in this study, is present in significantly greater amounts in migrating epithelia than it is in nonmigrating epithelia (N. Panjwani, G. Michalopoulos, J. Song, G. Yogeeswaran, and J. Baum, Invest. Ophthalmol. Vis. Sci., in press). This may prove to be of biological significance because it is generally believed that traumatized (migrating) epithelia are more susceptible to infection than normal (nonmigrating) epithelia are.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balázs R., Brooksbank B. W., Patel A. J., Johnson A. L., Wilson D. A. Incorporation of ( 35 S) sulphate into brain constituents during development and the effects of thyroid hormone on myelination. Brain Res. 1971 Jul 23;30(2):273–293. doi: 10.1016/0006-8993(71)90079-5. [DOI] [PubMed] [Google Scholar]
- Baum J., Boruchoff S. A. Extended-wear contact lenses and pseudomonal corneal ulcers. Am J Ophthalmol. 1986 Mar 15;101(3):372–373. doi: 10.1016/0002-9394(86)90834-2. [DOI] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Brennan M. J., Joralmon R. A., Cisar J. O., Sandberg A. L. Binding of Actinomyces naeslundii to glycosphingolipids. Infect Immun. 1987 Feb;55(2):487–489. doi: 10.1128/iai.55.2.487-489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal C. D., Stromberg N., Nyberg G., Normark S., Karlsson K. A., So M. Pilin independent binding of Neisseria gonorrhoeae to immobilized glycolipids. Antonie Van Leeuwenhoek. 1987;53(6):425–430. doi: 10.1007/BF00415497. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Galentine P. G., Cohen E. J., Laibson P. R., Adams C. P., Michaud R., Arentsen J. J. Corneal ulcers associated with contact lens wear. Arch Ophthalmol. 1984 Jun;102(6):891–894. doi: 10.1001/archopht.1984.01040030711025. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Siddiqui B., Li Y. T., Li S. C., Hellerqvist C. G. Anomeric structure of globoside and ceramide grihexoside of human erythrocytes and hamster fibroblasts. J Biol Chem. 1971 Apr 10;246(7):2271–2277. [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M. M., Strejc M., Berk R. S. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987 Dec;28(12):1978–1985. [PubMed] [Google Scholar]
- Hazlett L. D., Moon M., Berk R. S. In vivo identification of sialic acid as the ocular receptor for Pseudomonas aeruginosa. Infect Immun. 1986 Feb;51(2):687–689. doi: 10.1128/iai.51.2.687-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Wells P. A., Berk R. S. Scanning electron microscopy of the normal and experimentally infected ocular surface. Scan Electron Microsc. 1984;(Pt 3):1379–1389. [PubMed] [Google Scholar]
- Jones D. B. Early diagnosis and therapy of bacterial corneal ulcers. Int Ophthalmol Clin. 1973 Winter;13(4):1–29. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
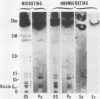
- Jumblatt M. M., Neufeld A. H. Beta-adrenergic and serotonergic responsiveness of rabbit corneal epithelial cells in culture. Invest Ophthalmol Vis Sci. 1983 Aug;24(8):1139–1143. [PubMed] [Google Scholar]
- Klotz S. A., Au Y. K., Misra R. P. A partial-thickness epithelial defect increases the adherence of Pseudomonas aeruginosa to the cornea. Invest Ophthalmol Vis Sci. 1989 Jun;30(6):1069–1074. [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H., Lomberg H., Gotschlich E., Hagberg L., Jodal U., Korhonen T., Samuelsson B. E., Schoolnik G., Svanborg-Edén C. Chemical and clinical studies on the interaction of Escherichia coli with host glycolipid receptors in urinary tract infection. Scand J Infect Dis Suppl. 1982;33:46–51. [PubMed] [Google Scholar]
- Mondino B. J., Weissman B. A., Farb M. D., Pettit T. H. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am J Ophthalmol. 1986 Jul 15;102(1):58–65. doi: 10.1016/0002-9394(86)90210-2. [DOI] [PubMed] [Google Scholar]
- Ramphal R., McNiece M. T., Polack F. M. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann Ophthalmol. 1981 Apr;13(4):421–425. [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Schein O., Hibberd P., Kenyon K. R. Contact lens complications: incidental or epidemic? Am J Ophthalmol. 1986 Jul 15;102(1):116–117. doi: 10.1016/0002-9394(86)90220-5. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S. J., Barza M., Gipson I. K. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Invest Ophthalmol Vis Sci. 1988 Mar;29(3):379–386. [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]