Abstract
Cells of the human retinal pigment epithelium (RPE) have a regular epithelial cell shape within the tissue in situ, but for reasons that remain elusive the RPE shows an incomplete and variable ability to re-develop an epithelial phenotype after propagation in vitro. In other epithelial cell cultures, formation of an adherens junction (AJ) composed of E-cadherin plays an important early inductive role in epithelial morphogenesis, but E-cadherin is largely absent from the RPE. In this review, the contribution of cadherins, both minor (E-cadherin) and major (N-cadherin), to RPE phenotype development is discussed. Emphasis is placed on the importance for future studies of actin cytoskeletal remodeling during assembly of the AJ, which in epithelial cells results in an actin organization that is characteristically zonular. Other markers of RPE phenotype that are used to gauge the maturation state of RPE cultures including tissue-specific protein expression, protein polarity, and pigmentation are described. An argument is made that RPE epithelial phenotype, cadherin-based cell–cell adhesion and melanization are linked by a common signaling pathway: the Wnt/β-catenin pathway. Analyzing this pathway and its intersecting signaling networks is suggested as a useful framework for dissecting the steps in RPE morphogenesis.
Also discussed is the effect of aging on RPE phenotype. Preliminary evidence is provided to suggest that light-induced sub-lethal oxidative stress to cultured ARPE-19 cells impairs organelle motility. Organelle translocation, which is mediated by stress-susceptible cytoskeletal scaffolds, is an essential process in cell phenotype development and retention. The observation of impaired organelle motility therefore raises the possibility that low levels of stress, which are believed to accompany RPE aging, may produce subtle disruptions of cell phenotype. Over time these would be expected to diminish the support functions performed by the RPE on behalf of photoreceptors, theoretically contributing to aging retinal disease such as age-related macular degeneration (AMD). Analyzing sub-lethal stress that produces declines in RPE functional efficiency rather than overt cell death is suggested as a useful future direction for understanding the effects of age on RPE organization and physiology. As for phenotype and pigmentation, a role for the Wnt/β-catenin pathway is also suggested in regulating the RPE response to oxidative stress. Exploration of this pathway in the RPE therefore may provide a unifying strategy for advancing our understanding of both RPE phenotype and the consequences of mild oxidative stress on RPE structure and function.
1. Introduction
The retinal pigment epithelium (RPE) displays the anatomic features of a typical monolayer epithelium with tightly packed cuboidal to columnar cells and apical microvilli. RPE cells also have a notably regular hexagonal organization in surface view and numerous cytoplasmic granules, including the pigment granules that help give the tissue its name. Many details have been added to the morphologic description of the RPE since the tissue was discovered more than a century ago (Wolfensberger, 1998), but a challenge that still remains is to determine how this characteristic RPE phenotype comes about.
The focus of this review is on whether and how the RPE cell reacquires a mature epithelial architecture following disruption of a previously established monolayer. (For issues relating to RPE specification during embryogenesis see reviews by Bharti et al. (2006) and Martinez-Morales et al. (2004)). To keep the emphasis on what might be called re-morphogenesis, the term ‘maturation,’ ‘epithelialization’ or ‘epithelial morphogenesis’ is used in preference to the term ‘differentiation’, which is commonly used when discussing embryonic development. The ability of the RPE to undergo re-morphogenesis is of considerable interest to both cell biologists and ophthalmologists. The former want to understand the molecular pathways that regulate cell-type-specific phenotypes, and the latter want to find ways to restore the structure of an RPE layer that is disrupted by injury or disease so that the tissue remains competent to fully support the retina.
A related topic that will also be discussed is whether normal aging reduces the organizational state of RPE cells. The RPE is believed to play a central role in retinal diseases of aging and many investigations have been conducted to examine RPE cell loss with age. Yet the hallmark of aging is gradual functional declines that take place over years rather than widespread cataclysmic cell death. Little is known, however, about the structure of the aging RPE or the mechanisms whereby age could cause phenotypic changes in the tissue. Even subtle modifications of cell shape or sub-cellular organization could have profound consequences for the efficient function of the tissue over time, and ultimately for the ability of the RPE to support the survival of adjacent photoreceptors.
In addressing the topic of phenotype development as it relates to the RPE, the role of cadherin adhesions is discussed in some detail, but the main goal here is to focus on the big picture of epithelial morphogenesis. Many rapidly advancing research fields, such as those relating to cell adhesion, sub-cellular molecular trafficking, tumor progression, oxidative stress and aging, are relevant for understanding epithelial cell phenotype development and retention. The intersection of these fields is providing an increasingly complicated and integrated view of epithelial cell shape determination. The intent here is to discuss how the RPE fits into this picture in an attempt to identify gaps in our understanding of the morphogenesis of this epithelial cell type, and perhaps to provide a framework for filling in those gaps. Selected concepts are invoked from several broad research areas inside and outside of ocular cell biology. Whenever possible, comprehensive recent reviews on those topics are cited rather than primary references to help direct the reader to the relevant literature.
2. Monolayer epithelial cell phenotype and the RPE
The defining morphologic feature of monolayer epithelial cells such as the RPE is compartmentalization of the cytoplasm along an apico-basal axis. A well-developed monolayer epithelium has organelles and cytoskeletal elements localized to specific sub-cellular positions. It also has a characteristic apical zonular band of cell–cell adhesion proteins associated with a prominent circumferential actin microfilament bundle, and plasma membrane molecules polarized to cell-type-specific apical, lateral or basal domains.
How epithelial cell topography is established and maintained has been the topic of many investigations, with the field skyrocketing in the latter part of the 1980's and early 1990's. At that time the major models for dissecting the events of epithelial morphogenesis were epithelial cell lines primarily from kidney and gut. Using these lines, development of an epithelial phenotype was shown to be critically dependent upon interactions between the actin microfilament and intermediate filament systems and the cell surface at sites of cell–substrate and cell–cell attachment (Hitt and Luna, 1994; Luna and Hitt, 1992; Rodriguez-Boulan and Nelson, 1989). Fully dissociated epithelial cells lack the epithelial trait of apico-basal polarity. Attachment to surfaces and to one another provides cues that discriminate apical membrane from basolateral, and that trigger remodeling of sub-cellular architecture (Nelson, 1991; Wang et al., 1990; Wollner et al., 1992; Wollner and Nelson, 1992).
One of the most extensively studied proteins used in earlier investigations of epithelial polarity was Na/K ATPase, the sodium pump. This ion transporter plays a central role in maintaining the sodium gradient responsible for the transport of virtually all nutrients and metabolites (Jaitovich and Bertorello, 2006). In epithelial monolayers Na/K ATPase is enriched to a cell-type-specific domain as required to mediate vectorial transport across the tissue. The mechanisms governing pump polarization were studied most intensively in the canine kidney-derived cell line MDCK where the pump is basolateral. Pump polarity is achieved by a complex sequence of events involving both targeting to and selective retention at the baso-lateral cell surface (Gottardi and Caplan, 1993; Hammerton et al., 1991; Mays et al., 1995; McNeill et al., 1990; Nelson, 1991; Nelson and Veshnock, 1987; Wollner and Nelson, 1992; Zurzolo and Rodriguez-Boulan, 1993).
In contrast to the monolayer epithelial cell models used to investigate Na/K ATPase polarity, the sodium pump is enriched in the opposite or apical membrane domain of RPE cells (Bok, 1982; Gundersen et al., 1991; Okami et al., 1990; Rizzolo, 1990; Steinberg and Miller, 1979). It was this ‘reverse polarity’ of the RPE that brought this cell type to the attention of the broader cell biology community and ushered in an era of high interest in epithelial morphogenesis of the RPE.
2.1. Human RPE cell cultures as a model for epithelial morphogenesis
Cells in culture are indispensable for investigating the mechanisms governing epithelial cell maturation, including protein polarization, but they have inherent weaknesses for modeling cellular behavior in situ. Cultures originating from the RPE have both benefits and disadvantages. The tissue is largely non-mitotic in situ, but the cells can be readily propagated in vitro even from aged human donors. However, RPE cultures appear to have a limited capacity to recapitulate epithelialization to achieve a high state of phenotypic maturation.
Despite their limited epithelial maturation (or perhaps because of it), RPE cultures may nonetheless be well suited for investigating whether and how RPE cells undergo re-morphogenesis. Growing cells in culture from explanted tissues inevitably involves disruption of pre-formed cell layers. Even RPE cultures of fetal origin are propagated from tissues that had previously differentiated into pigment epithelium prior to tissue isolation. Therefore studying morphogenesis taking place in RPE cell cultures may be particularly useful for probing the competence of the RPE to re-establish tissue-level architecture after the normally quiescent epithelium is disturbed. The relevance of this issue is illustrated by considering what might happen to the RPE damaged by retinal detachment. The desired outcome of detachment repair is a restored monolayer of contiguous cells with their phenotype re-established. Unfortunately, the outcome can be proliferative vitreoretinopathy (Agrawal et al., 2007; Asaria and Charteris, 2006), a disorder in which the RPE is hyperplastic and migratory with a phenotype that is far from epithelioid.
2.2. Mechanisms of morphogenesis in other monolayer epithelial cells: central role of cell–cell adhesions
Epithelial phenotype development in vitro is initiated on cell–cell contact and proceeds as cultures become more tightly packed at confluency and post-confluency. The triggering events in morphogenesis were ascribed in the 1990's to the formation of the calcium-dependent cell–cell adherens junction (AJ) (Nathke et al., 1993; Takeichi, 1991, 1995). At this time, the major binding elements of the AJ were identified as members of the cadherin family of adhesion molecules (Geiger and Ayalon, 1992; Takeichi, 1995), especially E-cadherin in most epithelial cells (Rodriguez-Boulan and Nelson, 1989; Takeichi, 1991). Cell contact initiates the time-dependent formation of cadherin-mediated adhesions during which the cytoplasmic domain of the cadherin molecule forms a complex with proteins that mediate association with the actin cytoskeleton (Gumbiner and McCrea, 1993; Hinck et al., 1994; Nathke et al., 1993), with the major linkage function attributed to members of the catenin family (α-catenin, β-catenin, plakoglobin). When cell contacts first form, E-cadherin is readily solubilized in buffers containing Triton-X-100, but it later becomes resistant to detergent extraction and localized to junctional sites (Hinck et al., 1994; McNeill et al., 1993). As the formation of cadherin adhesions progresses, actin filaments organize into circumferential bundles to produce an adherens junction that is zonular in epithelial cells (Yonemura et al., 1995). E-cadherin junctions can induce remodeling of the cell surface membrane cytoskeleton and associated plasma membrane molecules (such as Na/K ATPase (McNeill et al., 1990) leading to the development of distinct apical, lateral and basal membrane domains (Nathke et al., 1993; Rodriguez-Boulan and Nelson, 1989).
This basic model of E-cadherin induction of an epithelial phenotype developed nearly two decades ago still stands, although it has been considerably updated recently. Two useful reviews summarize current evidence regarding how cadherins, catenins and their binding partners or regulatory molecules participate in multiple biological functions, including tissue morphogenesis (Halbleib and Nelson, 2006; Perez-Moreno and Fuchs, 2006). In forming complexes with catenins, the cytoplasmic domain of E-cadherin directly binds β-catenin and p120-catenin. β-Catenin is a key regulator of cadherin adhesive function (Daugherty and Gottardi, 2007) together with p120-catenin, which modifies the F-actin cytoskeleton and functions to stabilize cadherins in the membrane (Davis et al., 2003). In addition to binding cadherins, β-catenin also has a binding domain for α-catenin, which in turn binds cytoskeletal actin. Because of its binary binding function, α-catenin was believed for many years to directly interlink cadherins to the actin cytoskeleton. However, recently a more complicated picture has emerged in which α-catenin does not simultaneously bind both β-catenin and F-actin to provide a direct cadherin-to-actin linkage (Drees et al., 2005; Yamada et al., 2005). Rather α-catenin in the form of cytosolic homodimers regulates the local actin cytoskeleton at the forming AJ to promote reduced actin polymerization and the formation of unbranched actin cables oriented parallel to membranes. The combined actions of p120-catenin and α-catenin modulate multiple actin-binding partners or effectors including Arp2/3, Rho GTPases, afadin and formin-1 to produce cadherin clustering and actin rearrangements at nascent AJs (Perez-Moreno and Fuchs, 2006). Recruitment of additional actin-binding proteins including α-actinin, vinculin and myosin II to the maturing junction of monolayer epithelial cells culminates in the formation of the tension-generating circumferential actin microfilament bundle that characterizes an epithelial phenotype. Collectively these recent investigations on the cadherin–catenin system point to a central role of cytoskeletal rearrangements in epithelial junction formation, maturation and maintenance (Miyoshi and Takai, 2008).
The formation of cadherin-mediated adhesions, and thus phenotype development and retention, is also regulated by other aspects of cadherin accumulation, notably transport to membranes and protein turnover. In epithelial cells, E-cadherin transport to the cell surface requires binding to β-catenin (Chen et al., 1999) and retention at the cell surface is regulated by phosphorylation, notably of β-catenin and p120-catenin (Fukumoto et al., 2008; Maeda et al., 2006; Perez-Moreno and Fuchs, 2006; Wheelock and Johnson, 2003a, 2003b). Downregulation of cadherins from formed epithelial adhesions is accompanied by internalization then recycling or degradation of the cadherin, and by a disruption of cell phenotype.
Cadherins are not the only integral membrane proteins of AJs, nor is the AJ the only adhesion implicated in generating an epithelial phenotype. Upstream of the cadherin-based adhesion and responsible for the recruitment of cadherins to the AJ is the more recently identified nectin-based adhesion (Miyoshi and Takai, 2007; Ogita and Takai, 2006; Sakisaka et al., 2007). Downstream of the AJ and dependent upon the AJ for its establishment and maintenance is the occluding or tight junction (TJ) (Miyoshi and Takai, 2005).
Nectins are calcium independent adhesion proteins that in epithelial cells cooperate with components of both AJs and TJs to generate junctions that determine epithelial phenotype and polarity (Miyoshi and Takai, 2007; Ogita and Takai, 2006; Sakisaka et al., 2007). Nectins recruit E-cadherin to the forming AJ and interact directly or indirectly with many proteins enriched at junctional sites. An important nectin binding partner is the F-actin-binding protein afadin which mediates nectin linkage with the AJ protein α-catenin, and via α-catenin with additional components of the mature AJ (e.g., α-actinin and vinculin). Additionally, nectins regulate junction assembly by interacting with integrins, thereby mediating cross-talk between integrins and cadherins at adhesive sites (Ogita and Takai, 2008). Nectins also induce local actin rearrangements by activation of several actin effectors including cdc42, Rac, and afadin, the latter via activation of a key regulator of intercellular junctions, the small GTPase Rap1 (Kooistra et al., 2007; Sakamoto et al., 2006).
Nectins in epithelial cells also participate in the formation of tight junctions by a similar combination of mechanisms involving recruitment of TJ components through afadin, which binds the TJ adaptor protein ZO-1, and through regulation of actin dynamics by activation of cdc42. The TJ has three integral membrane proteins: claudins, occludins and junctional adhesion molecules (JAMs) (Miyoshi and Takai, 2005; Oliveira and Morgado-Diaz, 2007; Shin et al., 2006). The AJ and TJ form cooperatively but segregate into separate junctions in the mature apico-lateral adhesion complex of epithelial cells. TJs are particularly important for establishing epithelial cell polarity. Together with the AJ, the TJ comprises the structural barrier or fence that demarcates apical from baso-lateral membrane domains, an essential prerequisite for epithelial polarization. The TJ also acts as the regulated functional barrier that determines the paracellular permeability of an intact epithelial monolayer. Several other TJ-associated proteins that are involved in multiprotein complexes have been implicated in polarization of epithelial and other cell types including Crumbs, Pals1, PATJ, PAR-3, PAR-6 and aPKC.
Once established, the adhesions involved in epithelial morphogenesis are dynamic structures. It is their disassembly and re-assembly during tissue remodeling, repair and cancer that clarified the importance of adhesions for inducing and maintaining a mature epithelial phenotype. The loss of a once-formed epithelial phenotype, called an epithelium-to-mesenchyme transition (EMT), has been studied extensively especially in the contexts of normal development and tumor progression (Huber et al., 2005), and also in proliferative diseases of the eye involving the RPE (Saika et al., 2008). The hallmark of EMT is junction dysregulation, which leads to a loss of epithelial organization and the acquisition of a migratory state.
Literally thousands of studies have been performed to dissect the mechanisms of EMT, a complex process that has particular relevance for the topic of epithelial morphogenesis of the RPE. As indicated above, in culture models of other monolayer epithelial cell types E-cadherin plays a central role in inducing an epithelial phenotype. However, in RPE cultures, including from human donor tissue, the dominant cadherin is not E-cadherin. It is N-cadherin (Burke et al., 1999; Davis et al., 1995; McKay et al., 1997; Rak et al., 2006; Van Aken et al., 2003), a related type I classical cadherin that is not typically expressed by monolayer epithelial cells. Indeed, when expressed in epithelia N-cadherin is associated with EMT and loss of epithelial organization (Hazan et al., 2004; Islam et al., 1996; Maeda et al., 2005); the N-cadherin gene is included among the EMT signature group of genes which when expressed are implicated in promoting a mesenchymal phenotype in carcinoma cells (Alonso et al., 2007; Andarawewa et al., 2007). Given that N-cadherin is abundantly expressed by the RPE, a legitimate question to ask is: how much of what is known about E-cadherin-induced epithelial morphogenesis applies to the RPE?
Discussing this question, and the broader issue of how the RPE phenotype comes about, requires a brief summary of the markers that are used to gauge the maturation state of an RPE monolayer in vitro.
3. Epithelial morphogenesis in human RPE cultures
3.1. Assessing morphogenetic state and markers of maturation
The developmental state of RPE cells in vitro is evaluated by gross morphology, by the expression of tissue-specific proteins, by the appropriate polarization of proteins known to be asymmetrically enriched in apical or baso-lateral domains, and by the ability of the cultures to perform tissue-level polarized functions.
Gross RPE cell phenotype is typically assessed by phase contrast microscopy, which shows a morphology at confluency that is often described as “epithelioid” or “cobblestone”. Other microscopic methods such as scanning or transmission electron microscopy show morphologic markers of epithelial organization in cultured RPE monolayers including apical microvilli (Anderson et al., 1990; Heth et al., 1987) or apico-lateral cell–cell junctions with their actin microfilament undercoat (Davis et al., 1995; Heth et al., 1987). While these features distinguish RPE cultures from fibroblastic cell types, they provide little concrete information about the extent of epithelialization.
With regards to protein expression markers for RPE cultures, the most commonly used are cytokeratin intermediate filament proteins, especially CK 8 and 18. Cytokeratins are readily detected in virtually all RPE cultures, but their presence indicates only that the cells are derived from an epithelium. More useful for evaluating RPE developmental state is determining whether the cells express genes or proteins that are specific for or highly expressed by the RPE. Commonly used markers in this category are cellular retinaldehyde binding protein (CRALBP) and RPE65, proteins that are involved in retinoid processing required for regeneration of visual pigments by photoreceptors. A more recently added identifier of RPE maturation is expression of bestrophin-1, a protein that plays incompletely understood roles in chloride conductance and calcium signaling (Marmorstein and Kinnick, 2007). Also occasionally used as RPE maturation markers are enzymes involved in melanogenesis, e.g., tyrosinase or tyrosinase-related proteins (Abul-Hassan et al., 2000; Ohno-Matsui et al., 2005; Rak et al., 2006), or the accumulation of pigment itself (Campochiaro and Hackett, 1993; Dorey et al., 1990; Gamm et al., 2008; Maminishkis et al., 2006; Ohno-Matsui et al., 2005; Pfeffer et al., 1986; Rak et al., 2006; Song and Lui, 1990). RPE65, CRALBP and/or bestrophin gene expression has been shown in RPE cultures derived from mammalian RPE, including both fetal and adult human RPE cells (Gamm et al., 2008; Kanuga et al., 2002; Maminishkis et al., 2006; Ohno-Matsui et al., 2005; Rak et al., 2006). Detection of the protein, however, is more limited. CRALBP protein has been found in cultures of adult human RPE under limited culture conditions (Rak et al., 2006) and in the spontaneously immortalized human RPE cell line ARPE-19 (Dunn et al., 1996), as well as in fetal cells (Gamm et al., 2008; Maminishkis et al., 2006). RPE65 and bestrophin proteins appear to accumulate only in cells of fetal origin (Gamm et al., 2008; Hu and Bok, 2001; Maminishkis et al., 2006). Melanization also appears to occur more reliably in cultured fetal RPE cells (Gamm et al., 2008; Maminishkis et al., 2006) than in human RPE cultures from adult donors where pigment accumulation is unpredictable, may require specific culture handling, and even then requires extended periods at confluency.
Perhaps even more important than cell-type-specific protein expression for gauging the maturity of RPE phenotype in vitro is determining whether the cultures display the appropriate sub-cellular localization of polarized proteins. The most common protein localization markers are adhesion proteins, especially AJ proteins N-cadherin and catenins (Burke et al., 1999; McKay et al., 1997; Rak et al., 2006), the TJ protein ZO-1 (Gamm et al., 2008; Geisen et al., 2006; Mandell et al., 2007; Rajasekaran et al., 1996; Tugizov et al., 1996), and junction-associated circumferential actin. The distribution of junctional proteins is typically analyzed by fluorescence microscopy in confluent cultures where the extent of epithelialization is estimated by inspecting the universality and completeness of a zonular pattern. The appearance of zonularity can be achieved for junctional proteins in most human RPE cultures, although, again, extended periods at confluency may be required and protein staining patterns are often heterogeneous among cells within the same cultures (Burke et al., 1996, 1999; McKay et al., 1997; McKay and Burke, 1994).
More critical evidence for RPE culture maturation is the attainment of a polarized protein distribution shown by morphologic methods such as confocal microscopy, or by the biochemical method of domain-specific cell surface biotinylation using cultures on filter supports that permit access to apical and basal surfaces. RPE polarity has been the topic of several reviews (Burke, 1998; Marmorstein et al., 1998; Marmorstein, 2001) in which many proteins are discussed that are preferentially enriched in either the apical or baso-lateral domains of mammalian RPE cells. The term ‘enriched’ is used because the protein asymmetry is not absolute. Further, polarization can result from a true asymmetry of the protein per unit area of membrane, or from different amounts of total membrane in apical versus basal domains (Marmorstein et al., 1998). The best known apically polarized protein of the RPE is Na/K ATPase which, as indicated above, is the opposite polarity found in most monolayer epithelial cells. Other apically enriched proteins in the RPE that were discussed in a review by Marmorstein (2001) include N-CAM (a cell adhesion molecule), EMMPRIN (an inducer of metalloproteinase secretion), αvβ5 integrin (a phagocytosis receptor), MCT1 (a monocarboxylate transporter), p75NTR (neurotrophic receptor), CFTR (cystic fibrosis transmembrane conductance regulator), and the kir family of K+ channels. Additional apically polarized proteins in the RPE include the folate transport protein RFT-1 (Chancy et al., 2000), transport vesicle trafficking proteins syntaxin 1A and 1B (Low et al., 2002), and EBP50 (Bonilha and Rodriguez-Boulan, 2001), a binding partner of the actin-binding protein ezrin. Ezrin itself is also enriched in apical microvilli and used as an apical domain marker (Gamm et al., 2008; Kivela et al., 2000; Maminishkis et al., 2006). Some proteins shown to be baso-laterally enriched in the RPE are MCT3, the ezrin-binding protein SAP97, the folate receptor FRa, and bestrophin (Bonilha and Rodriguez-Boulan, 2001; Chancy et al., 2000; Marmorstein, 2001; Marmorstein and Kinnick, 2007).
Whether a specific polarized protein distribution in RPE cultures is considered ‘appropriate’ depends upon whether that distribution is known to occur in the tissue in situ. An apical polarity for Na/K ATPase has been demonstrated in the intact RPE of many mammalian (and non-mammalian) species (Bok, 1982; Burke et al., 2000; Gundersen et al., 1991; Rizzolo, 1990). However, for many of the proteins mentioned above, information on polarized distribution in mammals is limited to rodent RPE, especially rat. This species has been particularly useful for studies of protein polarization in the RPE because developmental stages in situ can be analyzed using embryos, and cultures are available for detailed analyses of polarization mechanisms, both primary RPE cultures and the immortalized rat line RPE-J (Nabi et al., 1993). There is a growing body of work using the rat model, much of it generated by Rodriguez-Boulan and his collaborators, devoted to identifying the mechanisms used by the RPE to sort proteins exhibiting a polarized distribution (Bonilha and Rodriguez-Boulan, 2001; Deora et al., 2004, 2005; Marmorstein et al., 1998; Marmorstein, 2001; Mora et al., 2006). However, relatively little is known about protein sorting mechanisms in human RPE, not only because the polarized distribution of few proteins has been analyzed in situ, but also because human RPE cells, especially derived from adult donors, have a variable capacity to generate cultures that are highly polarized. As discussed further below, proteins that polarize variably or late in morphogenesis and therefore serve as benchmarks for a high epithelial maturation state (such as apical Na/K ATPase or basal bestrophin), usually fail to polarize in RPE cells from adult human donors.
Another category of markers for RPE maturation is functional measures of epithelial polarity such as transepithelial ion and nutrient transport, and the polarized secretion of growth and trophic factors. Such functions have been studied extensively in a variety of RPE preparations and cultures from several species including humans (Strauss, 2005). Regardless of what is transported or secreted, however, the ability of the RPE monolayer to vectorially move molecules is dependent upon the establishment of an effective barrier to paracellular flux. Barrier function, which defines a mature epithelium expressing tissue-level function, requires well-developed tight junctions and is commonly evaluated in cultures by measuring transepithelial electrical resistance across monolayers on filter supports. TJ formation in the pigment epithelium has been studied in some detail, especially in embryonic chick RPE investigated by Rizzolo and his collaborators (Rizzolo, 2007). It is clear from this body of work that TJ development is a complex progressive process during which there is sequential expression of genes for TJ proteins, an increase in the complexity of the tight junctional strands, a requirement for products of the neural retina in situ for junction maturation, and a dynamic remodeling and regulation of the TJ that determines its ongoing barrier function.
Indeed, TJ formation and other aspects of epithelial phenotype development are step-wise, time-dependent processes, giving cultured RPE cells the potential to exhibit a co-mingling of mature and immature traits.
3.2. RPE ‘re-morphogenesis’ in vitro
3.2.1. Cadherin cell–cell adhesion and RPE re-morphogenesis
As indicated above, the proteins comprising the epithelial adherens junction, especially E-cadherin, have been implicated as critical inducers of epithelial morphogenesis. However, E-cadherin is not the dominant cadherin of the RPE AJ, which is unusual for a monolayer epithelial cell type. For many years the RPE was believed to lack E-cadherin entirely (Gundersen et al., 1993; Huotari et al., 1995; Marrs et al., 1995), expressing instead B-, R-, P- and N-cadherin at various times during development and in various species (Huotari et al., 1995; Lagunowich and Grunwald, 1989, 1991; Liu et al., 1997; Murphy-Erdosh et al., 1994; Xu et al., 2002). For RPE cells in culture, including RPE cells from human donors (Burke et al., 1999; McKay et al., 1997; Rak et al., 2006; Van Aken et al., 2003), the dominant cadherin is N-cadherin.
As it turns out, E-cadherin is not completely absent from the RPE although it has a limited expression and junctional distribution. E-cadherin can be detected in extracts of human RPE cells, both from freshly isolated tissues and in primary cultures (Burke et al., 1999; Van Aken et al., 2003). However, the relative amount of E-cadherin protein, as compared to N- or P-cadherin, varies among samples from different donors (Burke et al., 1999). Further, E-cadherin localizes to junctions only in scattered patches of cells both in situ and in vitro, and long post-confluent time courses are required in cultured cells for E-cadherin to accumulate at junctional sites. Given the cell–cell heterogeneity in cadherin protein expression in RPE cells and the known morphogenetic function of E-cadherin, an obvious question to ask is whether RPE cell phenotype is related to cadherin phenotype. This question is another way of asking what was asked above: how much of what is known about E-cadherin-induced epithelial morphogenesis applies to the RPE. Do patches of cells with junctional E-cadherin differ phenotypically from cells that lack it? What role (if any) does N-cadherin, the dominant and ubiquitous cadherin of cultured RPE, play in RPE epithelial morphogenesis in vitro?
3.2.1.1. E-cadherin and RPE re-morphogenesis
In studies demonstrating the patchy accumulation of E-cadherin in the RPE (Burke et al., 1999) it was apparent that cultured cells do not have a homogeneous phenotype. Earlier investigations had shown striking morphologic heterogeneity among cells within the same human RPE cultures (Burke et al., 1996; Burke and Hjelmeland, 2005; McKay and Burke, 1994). Phenotypically distinct RPE subpopulations in vitro are revealed over a long post-confluent time course during which the cell morphologies diverge and, once established, remain stable for at least months provided that the phenotypes are not disrupted by dissociation and re-plating (Burke et al., 1996). RPE subpopulations with highly divergent morphologies, distinctly fusiform or highly epithelioid, can be partially separated by methods involving selective trypsinization of calcium-dependent adhesions (McKay and Burke, 1994). The effectiveness of this technique suggests that the complement of adhesive proteins, especially proteins associated with the calcium-dependent AJ, is likely to differ between RPE cell morpho types. However, in subsequent studies no differences were found between epithelioid and fusiform RPE in expression levels of several adhesion proteins (N-cadherin, α5β1 integrin, NCAM, PECAM) or other AJ-associated proteins that complex with cadherins or with cadherin-associated circumferential actin (α-catenin, β-catenin, plakoglobin, p120-catenin, α-actinin, vinculin) (McKay et al., 1997). The RPE phenotypic variants do, however, differ in junctional protein localization. Epithelioid but not fusiform cells undergo a slow, time-dependent protein re-distribution after confluency to generate a zonular organization of AJ proteins.
When E-cadherin was shown to accumulate slowly after confluency in the junctions of some human RPE cells, it was obvious to ask whether this known epithelial phenotype inducer was responsible for inducing the morphology of RPE subpopulations that appeared highly epithelioid. Examination of the relationship between junctional E-cadherin and RPE culture phenotype, however, did not resolve this issue and instead produced a more complex picture (Burke et al., 1999). RPE cells with junctional E-cadherin are, indeed, prominently epithelioid, but most clusters of epithelioid RPE lack junctional E-cadherin indicating that E-cadherin is not required for RPE cells to adopt a grossly epithelioid appearance. Rather E-cadherin accumulates in a subset of epithelioid cells late in confluency after the junction is formed. It then co-distributes with (but does not replace) N-cadherin.
Although E-cadherin is not required to produce highly epithelioid RPE, E-cadherin may nonetheless modify some aspects of RPE phenotype as indicated by distributional analyses of Na/K ATPase. As indicated previously, in other epithelial cell types E-cadherin engagement induces a baso-lateral distribution of the sodium pump (Marrs et al., 1993; Nelson et al., 1990). In cultured RPE cells Na/K ATPase usually fails to polarize, but when rat RPE cells were transfected to express high levels of exogenous E-cadherin, a baso-lateral sodium pump distribution was induced (Marrs et al., 1995). Given this observation, where is Na/K ATPase in epithelioid human RPE cultures and does its distribution differ in cells with, versus without, endogenous junctional E-cadherin? The answer to the latter is a qualified ‘yes’. In some highly epithelioid RPE cells Na/K ATPase polarizes in late confluency to the tissue-specific apical domain (McKay et al., 1997) (demonstrating, coincidentally, that some RPE cells from adult human donors are competent to achieve this marker of a high maturational state), but in the subset of epithelioid cells with junctional E-cadherin, the pump is non-polar (Burke et al., 1999). This outcome could result from E-cadherin having no effect on pump polarity, or conversely, E-cadherin could interfere with the apical polarization that can occur in epithelioid cells. The ‘interference’ theory is supported by observations on Na/K ATPase distribution in situ in bovine RPE (Burke et al., 2000). In bovine RPE, most cells show prominently apical Na/K ATPase as expected for this tissue, but similar to post-confluent cultures of human RPE, there are patches of cells scattered throughout the monolayer that have junctional E-(and/or P-)cadherin and in these patches the sodium pump protein is non-polar. Loss of apical pump polarity in E-/P-cadherin-positive RPE cells in situ appears to result from a smaller apical microvillar domain, a taller lateral membrane domain, a basal enrichment of the ankyrin–fodrin membrane cytoskeleton with which the sodium pump associates in other epithelial cells, and more baso-lateral Na/K ATPase immunostaining. Although E-cadherin has not been expressly shown to cause this ‘basal-ified’ phenotype, it is consistent with E-cadherin induction shown in other epithelial cells.
If E-cadherin is responsible for the reduced apical pump polarity in E-cadherin-positive RPE cells in situ or in vitro, it is not clear why the baso-lateral induction is incomplete. One possibility that we previously suggested (Burke et al., 1999) is that E-cadherin's inductive capacity is reduced because the protein localizes to junctions late in their formation, after the N-cadherin adhesion is established. This possibility then raises the questions: why is junctional accumulation of E-cadherin delayed, and why does it localize to the junctions in only a subset of cells. These issues were considered in another investigation which showed that although the E-cadherin gene is constitutively expressed by human RPE cells, newly synthesized protein may be proteolytically processed to generate truncated peptides that transiently localize to organellar compartments rather than trafficking to membranes and inserting in junctions (Burke and Hong, 2006). Should small amounts of E-cadherin protein escape degradation, it could become enriched over time in the junctions of some cells accounting for the patchy junctional distribution of E-cadherin in post-confluent cultures.
Whether this theory proves to be accurate requires additional study. At this time it is not clear what function, if any, E-cadherin performs in RPE cells, either the truncated peptides found transiently in most cells or the junctional protein that accumulates in some epithelioid subpopulations. In situ, the patchy junctional E-cadherin in the RPE could simply be tolerated, an epi-phenomenon with little impact on tissue organization or function. Regardless, E-cadherin is largely absent from the RPE adherens junction in cultured cells in early confluency when the cells undergo morphogenesis. E-cadherin is therefore unlikely to play a major role in inducing RPE phenotype development.
3.2.1.2. N-cadherin and RPE re-morphogenesis
N-cadherin, not E-cadherin, is the most abundant cadherin expressed by RPE cells during re-morphogenesis in vitro. Given the pivotal role of cell–cell adhesion in epithelial morphogenesis, it is reasonable to ask whether N-cadherin participates in the induction of RPE epithelialization. Some years ago we examined the development of the zonular N-cadherin-based AJ in epithelioid RPE (McKay et al., 1997) and observed a process that resembled the process of E-cadherin junction formation in other monolayer epithelial cells. In both types of cells the cadherin undergoes a time-dependent localization to junctions, first distributing to puncta at cell–cell contact sites, then forming an incomplete or segmented adhesion that over time becomes progressively more zonular, more resistant to detergent extraction, and more tightly apposed to an increasingly compact circumferential actin band. However, there is a striking cell-type difference: the N-cadherin zonular junction requires weeks at confluency to develop in RPE cells, whereas the E-cadherin zonular adhesion develops in minutes to hours in other epithelial cell types. Coincident with the formation of the zonular AJ, both types of cells develop an epithelioid appearance, but while E-cadherin has been specifically implicated as an inducer of epithelial morphogenesis, N-cadherin has not.
The formed N-cadherin junction in RPE cultures is apparently competent to at least sustain epithelial morphology as indicated by the retention of an epithelial phenotype in post-confluent monolayers disrupted by wounding (Kaida et al., 2000). However, there is no explicit evidence to indicate that N-cadherin is required for RPE epithelial phenotype development, nor is there precedent to implicate N-cadherin as an epithelial morphoregulator. In fact quite the contrary, N-cadherin is typically found in non-epithelial cells. As indicated above (Section 2.2), when found in epithelial cells, N-cadherin is associated with disruption of an epithelial phenotype and induction of a mesenchymal, migratory state – at least in carcinoma cells, which are the source of most information on N-cadherin in cells of epithelial origin due to its absence from most normal epithelial cells. There is some evidence, however, to suggest that N-cadherin interferes with, rather than generates, an epithelial cell shape in normal epithelial cells as well. In the normal kidney epithelial cell line MDCK, a major model of E-cadherin-induced morphogenesis, epithelial phenotype is disrupted in subpopulations of cells that express low levels of endogenous N-cadherin even on a backdrop of continued E-cadherin expression (Youn et al., 2005). This result is similar to the observation in carcinoma cells in which N-cadherin promoted motility regardless of the presence of E-cadherin (Nieman et al., 1999). In the RPE, too, N-cadherin has been implicated in inducing a fibroblastic and migratory or invasive morphology in pathologic states or models of pathologic states (Chen and Ma, 2007; Van Aken et al., 2003).
Given the evidence that N-cadherin in epithelial cells is usually associated with a mesenchymal phenotype, we previously theorized that RPE cells may develop an epithelial phenotype in spite of N-cadherin expression, rather than because of it (Youn et al., 2006). This suggestion was based partly on a study of an MDCK sub-clone that was selected for having a cadherin expression pattern resembling RPE cells: high endogenous N-cadherin and low E-cadherin. The MDCK sub-clone formed a zonular N-cadherin junction and, coincident in time, developed an epithelial phenotype, similar to RPE cells. However, as compared to morphogenesis in the E-cadherin-dominant parental MDCK line, junction formation and morphogenesis were slow in the N-cadherin-dominant sub-clone, again similar to RPE cells. This observation of delayed morphogenesis when N-cadherin dominates raises the possibility that N-cadherin may be compatible with the development of a zonular AJ in normal epithelial cells, but it may not, like E-cadherin, facilitate it.
If N-cadherin does not drive the development of an epithelial-type AJ in normal epithelial cells, why do cultured RPE cells form an AJ that is zonular? In non-epithelial cells like neurons, cardiac muscle or fibroblasts where N-cadherin is the typical dominant cadherin (Hatta and Takeichi, 1986; Kaufmann et al., 1999; Matsuyoshi and Imamura, 1997; Pouliot et al., 1990), N-cadherin forms a variety of cell-type-specific adhesion plaques suggesting that N-cadherin AJ morphology is determined by cellular context. In normal epithelial cells like the RPE an epithelial cell shape may therefore arise by a mechanism that is not directly related to cadherin induction. One attractive possibility is that RPE morphogenesis results from actin re-organization driven by mechanisms that induce the dominant actin cytoskeleton to remodel from the linear arrays seen in subconfluent cells to the circumferential band that characterizes an epithelial phenotype at confluency (Pletjushkina et al., 1994; Yonemura et al., 1995). According to this view, a circumferential actin pattern would develop in normal epithelial cells regardless of cadherin type, although different cadherins could differentially affect the zonular actin bundle; E-cadherin may accelerate its formation, while N-cadherin may passively associate with it after it is formed. This possibility implies a role for actin rearrangements upstream of cadherin engagement in the process of epithelialization, which is consistent with data implicating actin remodeling as a key step in epithelial morphogenesis (Perez-Moreno and Fuchs, 2006).
There is currently no evidence implicating specific actin remodeling pathways in epithelial cells like the RPE that produce a zonular AJ containing N-cadherin, although there are some tantalizing observations to suggest that actin rearrangements could differ with cadherin type. At the forming E-cadherin AJ, p120-catenin plays an important role in orchestrating actin dynamics thereby integrating cytoskeletal rearrangements, junction formation and morphogenesis (Perez-Moreno and Fuchs, 2006). However, p120-catenin is known to interact differently with E- and N-cadherin, associating at different times during cadherin–catenin complex assembly (Davis et al., 2003; Miranda et al., 2003; Wahl et al., 2003), and performing different functions in cadherin trafficking and retention at surface membranes (Chen et al., 2003; Davis et al., 2003). When expressed within the same epithelial cells, p120-catenin also forms stoichiometrically different complexes with E- and N-cadherin (Youn et al., 2005).
Whether p120-catenin regulates actin dynamics during the development of a zonular N-cadherin AJ in RPE cells or mediates other aspects of RPE epithelial morphogenesis has not been investigated. In general, the molecular mechanisms of N-cadherin AJ formation are poorly understood, but generating a zonular organization of this structure is likely to be critical for morphogenesis of the RPE. The earliest events in the formation of an E-cadherin epithelial AJ in other cells, those associated with generating nectin-based adhesions and associated actin remodeling, are unexplored in the RPE. Investigations patterned on studies of the E-cadherin AJ, especially with regards to cytoskeletal dynamics, could be particularly revealing for the ascertaining the role of cadherins in RPE morphogenesis. Further, the impact of this approach might be much broader. From the point of view of cancer biology, the RPE can be viewed as having a cadherin expression pattern, with its high N-cadherin and low E-cadherin, that simulates a metastatic carcinoma. Yet the RPE is able to develop a non-motile epithelioid morphology in culture, and metastatic tumors of the RPE in situ are exceedingly rare (Loeffler et al., 1996). Understanding how RPE cells develop and sustain an epithelial phenotype with N-cadherin as their dominant AJ protein therefore could not only aid in the development of strategies to maximize RPE re-morphogenesis and therefore tissue repair, but also have widespread implications for understanding dysregulation of adhesions in cancer.
3.2.2. RPE re-morphogenesis and re-pigmentation
As indicated in the previous sections, a mature phenotype in monolayer epithelial cells is characterized by cytoarchitectural features determined in large part by the development of cell–cell adhesions; for the RPE another important indicator of phenotypic maturity is pigmentation.
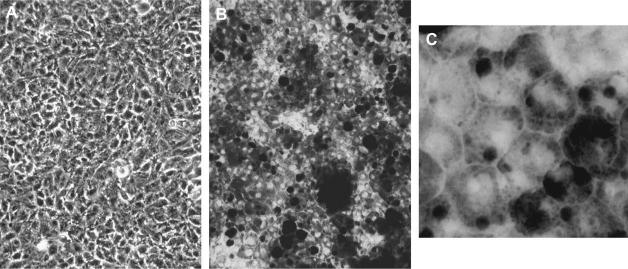
RPE cells synthesize melanin in situ during embryogenesis, after which pigment turnover is believed to be quite low (Boulton, 1998; Schraermeyer and Heimann, 1999). When RPE cells are propagated in culture they lose pigmentation as pigment granules (melanosomes) are exocytosed or diluted by cell division. RPE cells cultured from adult human donors typically remain amelanotic after pigmentation is lost because, despite expression of the genes required for pigment synthesis such as tyrosinase and tyrosinase-related proteins (Tyrps) (Abe et al., 1996, 1998; Abul-Hassan et al., 2000), the proteins apparently fail to accumulate. Nonetheless, several investigators have reported re-melanization of human RPE cultures, either ‘spontaneously’ or because of some combination of medium additives, culture substrate modification or culture handling (Campochiaro and Hackett, 1993; Dorey et al., 1990; Gamm et al., 2008; Maminishkis et al., 2006; Ohno-Matsui et al., 2005; Pfeffer et al., 1986; Rak et al., 2006; Song and Lui, 1990). Like epithelial phenotype development, melanin biosynthesis, when it occurs, is a protracted process, often requiring weeks at confluency for pigment to accumulate (Fig. 1).
Fig. 1.
Phase contrast micrographs of a human RPE culture from a post-natal human donor that spontaneously melanized after a long post-confluent interval. The culture is shown at two times post-confluency: 1 week (A) and 55 weeks (B). A higher magnification view of the late post-confluency culture is shown (C) to illustrate the epithelioid shape of the cells.
Cultures from fetal human donors appear more likely to re-pigment than RPE cells from other sources. These cells also achieve the highest overall morphogenetic state observed for cells propagated from the RPE. Highly developed fetal RPE cultures were described some years ago (Hu and Bok, 2001) and more recently protocols were developed that produced cultures in which prominent pigmentation was described as an explicit feature (Gamm et al., 2008; Maminishkis et al., 2006). In addition to pigmentation, the cultures exhibited an array of markers of high epithelial maturation including gene and protein expression of RPE65 and CRALBP, abundant apical ezrin, and (notably) expression and basal polarization of bestrophin (Gamm et al., 2008). It should be noted that fetal RPE are not typically passaged by enzyme treatment to dissociate cells, the protocol commonly used to propagate cultures from post-natal donors. As the discussion below implies, limiting degradation of cell adhesions could be a significant contributor to the higher level of morphogenesis attained by fetal cells.
An interrelationship between heavy pigmentation, a striking epithelial phenotype and cell–cell adhesion in RPE cultures was illustrated by the McKay laboratory (Rak et al., 2006). Using RPE cultures from adult human donors and a protocol that did not require specialized culture medium or coated substrates, this group was able to trigger a reversible shift in RPE culture phenotype from fusiform with no pigmentation to epithelioid with pigmentation. The method employed a calcium switch, which demonstrates an intriguing link between calcium-dependent adhesions and morphogenesis. According to the protocol, cells are initially plated at high density in culture medium containing calcium levels that are too low to support cadherin-dependent adhesion. Then days later, when cultures are switched to medium containing its normal high calcium content, adhesions rapidly form from cadherins pre-existing in membranes. The calcium switch protocol was developed several years ago to synchronize the timing of assembly of E-cadherin adhesions in MDCK epithelial cells (Nelson and Veshnock, 1987). In RPE cells, the switch is followed by N-cadherin adhesion formation and then days to weeks later by the expression of RPE maturation marker proteins (e.g., tyrosinase and CRALBP) and ultimately by the generation of phenotypically epithelioid and pigmented monolayers (Rak et al., 2006).
What underlying mechanisms could account for the effectiveness of the calcium switch protocol in triggering both RPE epithelialization and melanization? Given the long time interval between the trigger and the ultimate outcome, a complex cascade of mechanisms is likely induced that involves transcription factor networks and multiple signaling pathways. Sorting these out in RPE cells will be a challenge, but guidance might be provided by analyzing components of the Wnt/β-catenin signaling pathway.
4. Convergence of adhesion, morphogenesis and pigmentation: the Wnt/β-catenin pathway
The Wnt signaling pathway plays a pivotal role in regulating tissue differentiation not only during embryogenesis, but also post-natally where it functions in stem cell renewal, cancer and even aging (Clevers, 2006; Daugherty and Gottardi, 2007; Gavert and Ben-Ze'ev, 2007; Meshorer and Gruenbaum, 2008; Nelson and Nusse, 2004; Polakis, 2007). A central mediator of the canonical Wnt pathway is β-catenin, which, as indicated above, also binds the cytoplasmic domain of cadherins, indicating a point of convergence between the Wnt pathway and the cadherin adhesion system (Nelson and Nusse, 2004).
In the absence of Wnt signaling, β-catenin is complexed in the cytoplasm with other proteins including axin, adenomatous polyposis coli (APC) protein, and glycogen synthase kinase-3β (GSK-3β), which phosphorylates proteins in the complex and targets β-catenin for rapid degradation (Fig. 2). The Wnt pathway is activated by extracellular Wnt ligands that bind cell surface receptors (frizzleds) and co-receptors (lipoprotein receptor-related proteins 5/6 [Lrp5/6]) activating a signaling pathway that inhibits GSK-3β activity leading to the reduced degradation of β-catenin. β-Catenin then accumulates in the cytoplasm and translocates to the nucleus where it binds to the T cell factor (TCF)/lymphoid-enhancing factor (LEF) transcription factor machinery and promotes activation of target genes, notably proliferation regulators such as cyclin D1 and c-myc (Clevers, 2006; Gavert and Ben-Ze'ev, 2007; Thompson and Monga, 2007). β-Catenin pool size in the cytoplasm and thus its availability to participate in signaling is also regulated by cadherin binding (Nelson and Nusse, 2004).
Fig. 2.
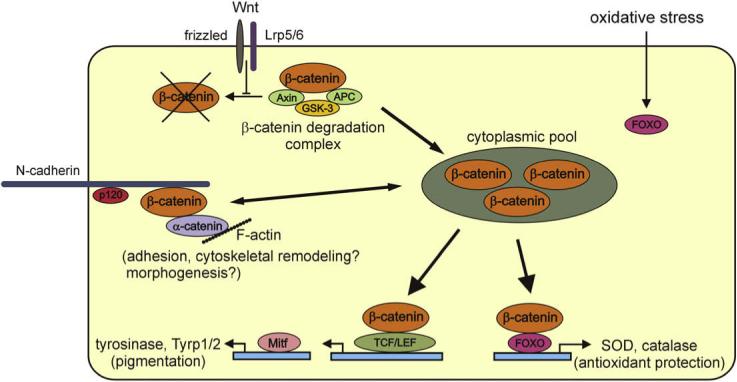
Highly simplified diagram of signaling pathways involving β-catenin that may be relevant for RPE cell morphogenesis and susceptibility to age-related oxidative stress. N-cadherin is specified as the cadherin adhesion protein since it is the major cadherin of cultured human RPE cells; its role in cytoskeletal remodeling and morphogenesis is unclear. For more detailed pathway maps, see the Science database of cell signaling, The Signal Transduction Knowledge Environment (STKE), at http://stke.sciencemag.org/cm/. Abbreviations: APC (adenomatous polyposis coli), FOXO (forkhead box O), GSK (glycogen synthase kinase), Lrp (lipoprotein receptor-related proteins), Mitf (microphthalmia-associated transcription factor); TCF/LEF (T cell factor/lymphoid-enhancing factor), Tyrp (tyrosinase-related proteins).
Consideration of a role for the Wnt/β-catenin pathway in RPE morphogenesis is motivated by several considerations. Some are related to the functions that have been identified for the pathway in affecting morphogenesis of other epithelial cells, and others relate specifically to the RPE.
From the point of view of epithelial morphogenesis, activation of the Wnt/β-catenin pathway is known to participate in the loss of epithelial phenotype that accompanies epithelial–mesenchymal transitions (EMT) during embryogenesis and cancer progression, or when induced experimentally in normal epithelial cell lines (Doble and Woodgett, 2007; Katoh and Katoh, 2006; Moustakas and Heldin, 2007). Central to EMT is changes in the phosphorylation state of the pivotal Wnt mediator β-catenin and, upstream of β-catenin, GSK-3β. GSK-3β is also located at the intersection of Wnt/β-catenin pathways and pathways induced by FGF and TGFβ, which change the expression or activation state of their transcriptional regulators Snail and Smads, thereby acting synergistically to affect EMT (Katoh and Katoh, 2006; Moustakas and Heldin, 2007; Nelson and Nusse, 2004).
Aside from its role in regulating phenotype in other epithelial cells, there are specific reasons to consider Wnt/β-catenin signaling as an important pathway in RPE morphogenesis. A direct target gene of the Wnt/β-catenin pathway is the Microphthalmia-associated transcription factor (Mitf), a key regulator of the formation of pigment cells, including the RPE (Deora et al., 2005; Larue and Delmas, 2006; Murisier and Beermann, 2006; Shibahara et al., 2000, 2001; Steingrimsson et al., 2004; Tachibana, 2000). Mitfs transactivate promoters of the tyrosinase gene family to induce expression of tyrosinase and tyrosinase-related proteins-1 and -2 thereby controlling pigment synthesis (Fig. 2). Mitf was also recently shown to positively regulate expression of the VMD2 gene that encodes bestrophin (Esumi et al., 2007), described above as a significant marker of RPE maturation. A role for Wnt signaling in RPE morphogenesis is further indicated by the observation that certain mutations in the APC gene produce a discrete RPE phenotype. As indicated above, the APC protein is a component of the degradation complex that regulates β-catenin availability for Wnt signaling. APC mutations produce familial adenomatous polyposis (FAP), a syndrome that predisposes to colorectal cancer (Ballhausen, 2000). Some variants of FAP result in congenital hypertrophy of the RPE (CHRPE), which is characterized by nodular RPE lesions that are often highly pigmented (Tiret and Parc, 1999).
Fragmentary evidence therefore suggests that investigating the Wnt/β-catenin pathway in RPE cells could provide a useful framework for probing mechanisms of RPE morphogenesis. However, determining whether/how this signaling network functions in the RPE is not trivial. Returning to the calcium switch protocol that induced both epithelialization and pigmentation in cultured human RPE (Rak et al., 2006), one potential result of the switch that implicates the Wnt pathway would be an abrupt shift in the balance of β-catenin available for signaling. As cadherin adhesions rapidly form in high calcium, β-catenin is theoretically stabilized at junctions reducing the signaling pool and down regulating the expression of Wnt target genes (Nelson and Nusse, 2004). A transient change in the activity of signaling pathways activated on cadherin engagement (including Wnt) can then have consequences that play out over long time frames, which appears to be the case for RPE morphogenesis triggered by the calcium switch. Changed gene expression patterns may be sustained and/or may induce a cascade of signaling events, perhaps including recurrent Wnt signaling episodes. The latter is suggested because the reduced expression of TCF/LEF target genes resulting from reduced Wnt signaling theoretically produces some outcomes that enhance and others that reduce RPE phenotype development. An example of the former might be reduced proliferation, which is permissive for epithelial maturation, and an example of the latter is decreased expression of Mitf, which is required for pigmentation and bestrophin expression.
A complex Wnt output is expected since Wnt signaling activates endogenous transcriptional cascades that vary with cell type and cell state. Cell proliferation, for example, is promoted under some conditions by Wnt signaling and terminal differentiation under others (Clevers, 2006). For the RPE, Wnt signaling may be required during embryogenesis for tissue specification and pigmentation, but if the RPE behaves like other epithelial cells, re-activation of Wnt signaling in the mature tissue may induce EMT. The ultimate effect of a change in the activity of the Wnt pathway is therefore determined by cellular ‘background’. An important component of that background is the expression of receptors and the availability of ligands that activate Wnt/β-catenin intersecting pathways. As implied above, several pathways converge on GSK-3β. Further, β-catenin itself is regulated by phosphorylation, not only in the degradation complex of the Wnt pathway but also by receptor and cytoplasmic tyrosine kinases in the cadherin–catenin complex at adhesion sites (Daugherty and Gottardi, 2007; Nelson and Nusse, 2004). The signaling background produces complex feedback loops that modify the output of the Wnt signal. For example, in addition to being a target gene of TCF/LEF transcription factors, the Mitf protein can directly bind β-catenin in a feedback mechanism that may regulate Wnt/β-catenin signaling (Schepsky et al., 2006). Further, Mitf in some pigmented cells is negatively regulated by FGF and positively regulated by TGF-β via their induced transcription factors such as SMADs (Bharti et al., 2006).
As implied above, the morphogenetic response of RPE cells to the calcium switch is apparently not due to a single event but rather due to the activation of a morphogenetic cascade. Further, cultures differ in their competence to respond to the switch, and in those cultures that do respond the re-pigmentation starts in scattered cells in the culture monolayer and subsequently spreads outward to neighboring cells (Rak et al., 2006). This intriguing observation suggests that at some critical stage in development, RPE cells may produce mediators that stimulate immediately adjacent cells, perhaps by gap junctional communication and/or by a paracrine mechanism that involves short-lived or low abundance mediators (perhaps Wnts?). Regardless of the mechanism, the observation that a subset of cells responds first and adjacent cells respond later implies that individual cells vary in their background active signaling networks, and that the triggers for morphogenesis are sequential.
Despite the complexity of the signaling networks that underlie RPE morphogenesis, sorting them out is essential to increase our understanding of RPE phenotype development and retention. There are currently few investigations of the Wnt pathway in cultured RPE cells that might inform us about the pathway's role in epithelialization. Inoue et al. (2007) recently analyzed the conditioned medium from rat RPE cultures using a reporter system with a TCF responsive element and identified an activity secreted by the RPE that suppresses Wnt signaling. This work, which followed studies implicating Wnt pathways in the rd6 mouse (Jones et al., 2000; Kameya et al., 2002; Mandal et al., 2006), demonstrated a potential for the RPE to produce Wnt effectors. Cells cultured from embryonic chick RPE have been used to implicate Wnt pathways in RPE cell differentiation/transdifferentiation, primarily via expression and function of Mitf (Iwakiri et al., 2005; Mochii et al., 1998). Of particular relevance to the issue of epithelial phenotype development in vitro, suppression of Mitf by small interfering RNA induced de-differentiation of chick RPE, which was indicated by a reduction in a pigmentation marker (melanosome glycoprotein 115) and by a loss of gross epithelial phenotype (Iwakiri et al., 2005). The latter manifested as a shift in the organization of the dominant actin cytoskeleton from a zonular band, typical of epithelial cells, to linear stress fibers, typical of mesenchymal cells.
For human RPE, there is only scattered evidence to indicate that known or potential effectors of the Wnt pathway are expressed and even less is known about Wnt signaling in cultured cells that could implicate a role for this pathway in morphogenesis. Expression of genes for the Wnt receptor FZD7 (Katoh and Katoh, 2007) and for a modulator of Wnt ligand binding SFRP 5 (secreted frizzled-related protein 5) (Chang et al., 1999) have been detected in human RPE (although the latter was no longer expressed when cells were cultured (Chang et al., 1999). Membrane frizzled-related protein (MFRP), which has a Wnt binding motif, was found in human RPE-choroid extracts (Mandal et al., 2006). (There is an ocular phenotype associated with MFRP mutations, which cause extreme hyperopia or nanophthalmos in humans (Sundin et al., 2005) and produce the progressive retinal degeneration found in the rd6 mouse (Kameya et al., 2002).) Explicit studies of Wnt pathways in human RPE cultures are few, with only one recent study probing the Wnt pathway in human RPE cells in the context morphogenesis (Krugluger et al., 2007). Even this investigation, which used the ARPE-19 cell line, was somewhat indirect, focusing not so much on Wnt signaling but rather on transcriptional regulation of Wnt proteins (β-catenin and GSK-3β) and markers of RPE differentiation (cytokeratin 18 and RPE65) by treatment of cells with ligands for receptor tyrosine kinases (e.g., EGF).
It is probably fair to say that despite the known role of Wnt signaling in morphogenesis of other epithelial cells and in RPE specification during embryogenesis, this pathway is virtually unexplored as a regulator of cell phenotype in cultures of the RPE.
5. RPE ‘de-morphogenesis’ and aging
5.1. RPE phenotype: pathology and aging
Research on RPE morphogenesis is partly driven by a basic interest in determining how cells acquire their cell-type-specific phenotype. Research is also strongly motivated by a desire to understand and ultimately control RPE phenotype in pathologic states. An area of current interest in RPE morphogenesis comes from the field of RPE transplantation. Transplantation is being explored as a mechanism to treat age-related macular degeneration (AMD) by replacing the aging, dysfunctional RPE with healthy tissue that is better able to support retinal photoreceptors. One strategy for generating a graft is to amplify RPE cells by culturing them. However, this approach has been stymied by the problems discussed above: RPE cells lose their phenotype on in vitro propagation, and then fail to fully re-epithelialize after confluency is attained. (The reader is referred to a recent review that thoroughly covers the topic of RPE transplantation in AMD (Binder et al., 2007). In that review, RPE phenotype is also discussed in some detail, including topics significant for RPE morphogenesis that are not discussed here. Notable among these are the effects of substrate and of humoral agents, including factors produced by the RPE itself, on cell phenotype.)
A presumption underlying efforts to transplant RPE to restore function to the aging monolayer is that RPE cells lose their normal, functionally optimal phenotype as they age. Although aging of the RPE has been the topic of many investigations, few relate specifically to whether aging affects the organizational state of the RPE as a polarized monolayer epithelium. Rather studies of aging of the RPE in situ have focused on changes in granule content, and on topographical differences between the macula and periphery that might explain why the macula is predisposed to age-related degeneration (Boulton and Dayhaw-Barker, 2001). The granule content of RPE cells clearly changes with age; unmodified melanin granules decline while lipofuscin and complex granules (melanolysosomes and melanolipofuscin) increase. There is also considerable evidence to indicate that macular and more peripheral RPE cells differ, both throughout life and as a consequence of age, in several fundamental properties including granule content, the expression of certain genes or proteins, and the activity of various enzymes (Burke and Hjelmeland, 2005; Kociok and Joussen, 2007; Mullins et al., 2007).
With regards to the specific issue of RPE cell phenotype and aging, the most relevant investigations have to do with age-related RPE cell loss, which would inevitably produce some organizational remodeling of remaining cells. RPE cell loss appears to occur with age, although the extent and topography are not entirely clear (Boulton and Dayhaw-Barker, 2001; Del Priore et al., 2002; Dorey et al., 1989; Feeney-Burns et al., 1990; Gao and Hollyfield, 1992; Watzke et al., 1993). One investigation with particular relevance for RPE phenotype was the work of Watzke et al. (1993) that demonstrated an age-related diminution in the hexagonality of foveal RPE. Decreased regularity in RPE cell shape can be viewed as a gross phenotypic manifestation of a reduced organizational state of the cells; however, whether and how aging might change the specific markers of epithelial maturation and polarity discussed above is undetermined.
Indeed, we have no standardized mechanism for defining alterations of RPE morphology in situ (Mecklenburg and Schraermeyer, 2007) and aging changes in RPE epithelial organization, should they occur, are likely to develop slowly and perhaps be quite subtle. Age-related structural changes may also be highly heterogeneous, not only across broad topographical regions (macula vs. periphery, for example), but also focally. The RPE monolayer is a mosaic of structurally variable cells regardless of age (Burke and Hjelmeland, 2005). Identifying aging changes in RPE cell phenotype on this variable background may therefore require detecting whether aging changes the frequency of cells that exhibit specific features of a mature polarized epithelium. Given donor variability, RPE cell–cell heterogeneity, and the complexity of aging plus its long time line, identifying phenotypic changes associated with normal aging could be a daunting task.
Although specific evidence for an effect of age on RPE phenotype in situ is limited, examination of cultured cells supports the notion that age affects cell organization. RPE cultures from adult human donors are typically used in early passage where they are presumed to display features most like the normal tissue. With repeated passage to induce senescence by forced replication, cultured RPE cells from both animal and human eyes become progressively more heteromorphic (Burke and McKay, 1993; Hjelmeland et al., 1999; Lee et al., 2001). Indeed, a morphology that deviates from the epithelioid – what could be called ‘de-morphogenesis’ – is a hallmark of aged RPE cultures.
5.2. Age-related RPE ‘de-morphogenesis’: oxidative stress and the Wnt/β-catenin pathway
How could aging in situ produce changes in the architecture of cells in a long-lived post-mitotic tissue like the RPE? One attractive possibility is that phenotype could be affected as a consequence of age-related oxidative stress.
The topic of oxidative stress and the RPE has a significant history and a growing recent interest. The focus in this field has been on the role of stress to the RPE as a contributing factor to AMD (Algvere et al., 2006; Bazan, 2008; Cai et al., 2000; Liang and Godley, 2003; Wu et al., 2006; Zarbin, 2004). The identification a few years ago of genes that confer AMD risk had a major impact on AMD research (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005), but how aging itself contributes to disease onset, progression and severity remain important questions, and oxidative stress has long been recognized as a major aging influence (Simm et al., 2008). AMD is believed to originate in the RPE where the earliest manifestations of the disease are seen, and oxidative stress has been implicated partly because the tissue is at unusually high risk for oxidative injury due to its location and function. The RPE resides in a highly oxygenated environment adjacent to the capillary bed of the choroid, and the tissue is exposed throughout life to light, including highly phototoxic blue light that produces cellular damage by oxidative mechanisms (Algvere et al., 2006; Wu et al., 2006). The RPE must also cope with a daily load of membranes that are rich in polyunsaturated fatty acids (Stinson et al., 1991) due to its function of phagocytizing shed photoreceptor outer segments. Although it is difficult to prove a direct link between oxidative stress and any slow-to-develop disease of aging, a role for stress in AMD is inferred from studies indicating that antioxidant supplementation has a protective effect (AREDS, 2001; Tan et al., 2008), and that life style factors with an oxidative stress component (such as cigarette smoking) confer disease risk (Klein et al., 2008; Zarbin, 2004).
There are many studies on the RPE using cultured cells that involve inducing oxidative stress with chemical oxidants or light irradiation. These treatments are followed by analyses of stress pathways, or by investigations of agents that exacerbate (prooxidants) or protect against oxidative damage (antioxidants and trophic factors) (Algvere et al., 2006; Bazan, 2008; Cai et al., 2000; Wong et al., 2007; Wu et al., 2006; Zarbin, 2004). The primary outcomes in most such studies are measures of cell death after induction of lethal stress, an experimental strategy that is based on the likelihood that oxidative stress contributes to AMD by inducing RPE cell loss via apoptosis (Cai et al., 2000; Del Priore et al., 2002; Dunaief et al., 2002).
However, if the issue is not the role of oxidative stress in RPE pathology but rather in normal aging, the relevant oxidative injury is not lethal but sub-lethal. Stress associated with aging results in a gradual loss in functional efficiency that only over time becomes sufficient to cause significant impairment or cell death. Considering the effects of sub-lethal stress on the RPE, there is strong rationale for theorizing that an important consequence would be ‘de-morphogenesis’ or a loss of cellular organization. Among the most highly susceptible biomolecules to oxidative modification are cytoskeletal proteins, notably actin and tubulin (Aslan et al., 2003; Banan et al., 2001, 2003, 2004; Dalle-Donne et al., 2001a, 2001b, 2002; Fratelli et al., 2003; Keshavarzian et al., 2003). These proteins comprise the interacting microfilament and microtubular networks that are important determinants of cell shape. As discussed above, regulated cytoskeletal rearrangements, especially actin dynamics, contribute to the generation and maintenance of an epithelial phenotype. However, cytoskeletal organization is sensitive to oxidative stress in many epithelial cell types, including in the RPE (Akeo et al., 2002; Bailey et al., 2004; Garg and Chang, 2003)(Fig. 3).
Fig. 3.
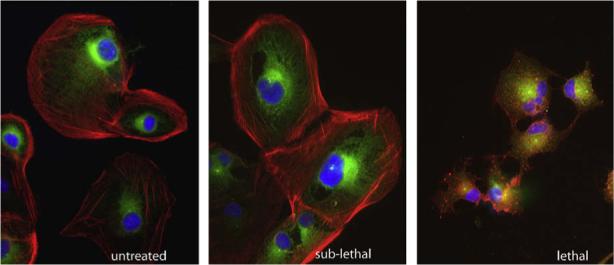
Fluorescence micrographs of ARPE-19 cultures showing the effects of blue light-induced stress on the cytoskeleton. Cells are stained with rhodamine-phalloidin to show filamentous actin (red), antibodies to β-tubulin to show microtubules (green) and Hoescht dye for nuclei (blue). The cultures were untreated, or irradiated with violet-blue light (400−410 nm) to produce sub-lethal (4 mW/mm2) or lethal (10.8 mW/mm2) injury. Lethality was determined by a dynamic measure of apoptotic blebbing as previously described (Zareba et al., 2007). Lethally damaged cells are less spread with a disrupted cytoskeletal network.
Indeed, in cultured RPE cells several markers of RPE epithelial maturation are susceptible to sub-lethal stress (Bailey et al., 2004; Ho et al., 2006). In addition to inducing re-organization of the actin cytoskeleton from circumferential to stress fiber-like, treatment of confluent ARPE-19 cells with the oxidant H2O2 also causes a decline in barrier function of the monolayers, and a loss of junctional integrity indicated by reduced association with junctional sites of both TJ (ZO-1; occludin) and AJ (N-cadherin and β-catenin) proteins. The latter changes occur in vivo as well as demonstrated in mice deficient in a major anti-oxidant system (SOD-1) where induction of oxidative stress produces a loss of junctional N-cadherin and β-catenin in the RPE (Imamura et al., 2006).
Accompanying the displacement of β-catenin from junctions by H2O2 treatment of ARPE-19 cells is an increase of β-catenin in the cytosol, which could increase its availability for signaling (Bailey et al., 2004). This observation is particularly interesting in the light of recent reports describing another important convergent pathway involving the Wnt mediator β-catenin. In addition to linking adhesion, morphogenesis and pigmentation via TCF/LEF transcriptional activation as discussed above, the Wnt/β-catenin pathway also links oxidative stress and aging (Bowerman, 2005; Essers et al., 2005; Liu et al., 2007; Manolagas and Almeida, 2007; White et al., 2007) (Fig. 2). β-Catenin can protect cells against oxidative stress by acting as a cofactor for another class of transcription factors, forkhead box O (FOXO) factors, which are induced by reactive oxygen species and which regulate expression of genes for antioxidant enzymes and for proteins involved in DNA repair (Calnan and Brunet, 2008). FOXOs not only protect against oxidative stress, but also regulate lifespan in animal models of longevity. FOXOs and TCF/LEF transcription factors may compete for β-catenin, thereby producing complex antagonisms between the two pathways. With the exception of the observation that homogenates of the RPE from donors with AMD showed upregulation of some FOXO target gene products (Decanini et al., 2007), little is known about whether the Wnt mediator β-catenin participates in pathways regulating the RPE response to oxidative stress or cell longevity.
The mechanisms whereby sub-lethal oxidative stress to the aging RPE could dysregulate cell phenotype are virtually unexplored. In other epithelial or endothelial cells where this question has been addressed, changes in turnover of polarized proteins (Grune et al., 2002) and in many signaling pathways, notably those regulating adhesions (Basuroy et al., 2003; Katsube et al., 2007; Krizbai et al., 2005; Rao et al., 2002; Saenz-Morales et al., 2006; Usatyuk et al., 2006), have been implicated. Cytoskeletal remodeling is a near universal observation in oxidatively stressed cells, but the exact mechanisms whereby this occurs remain to be determined. Alterations in the activity of actin effectors Rac1 and RhoA have been reported (Saenz-Morales et al., 2006), but undoubtedly multiple additional mechanisms are involved as well.
Whatever the mechanisms of stress-induced cytoskeletal alterations, widespread secondary effects on cell phenotype would likely result since the cytoskeleton is critical to overall cytoplasmic organization. There is a growing body of evidence to indicate that actin microfilaments, microtubules and their associated motors are required for the trafficking of membranes, organelles, and messenger RNAs to the cytoplasmic compartments where they function (Boldogh and Pon, 2007; Kloc and Bilinski, 2003; Lopez and Jansen, 2004; Stebbings, 2001; Tekotte and Davis, 2002). Even ‘soluble’ molecules in the cytoplasm are enriched in cellular sub-domains by the geometry of the cytoskeletal network (Leterrier, 2001). All cells translocate their organelles such as mitochondria (Boldogh and Pon, 2007) using cytoskeletal scaffolds, and for pigmented cells like the RPE, cytoskeletal networks and their motors are also essential for melanosome positioning (Barral and Seabra, 2004; Coudrier, 2007; Futter, 2006). Indeed, melanosomes have been an important model for studying actin-based and microtubule-based organelle motility (Barral and Seabra, 2004). Of specific relevance for epithelial phenotype, trafficking along cytoskeletal scaffolds, especially of endosomes, is required for epithelial cell polarity (Apodaca, 2001; Rodriguez-Boulan et al., 2005), including polarity of the RPE (Rodriguez-Boulan et al., 2005). If sub-lethal oxidative stress disrupts cytoskeletal organization in RPE cells as the available evidence suggests, inappropriate positioning of cellular components – a reasonable definition of loss of cell phenotype – seems unavoidable.
We are currently conducting investigations to determine whether sub-lethal oxidative stress impairs a trafficking function of cultured RPE cells, namely organelle translocation. Melanosomes are used for these studies not only because they are relevant for the RPE, but also because the granules can be effectively tracked by light microscopy in living cells. In these investigations melanosomes are isolated from porcine RPE then delivered to ARPE-19 cells for uptake by phagocytosis using low granule numbers to produce limited overlap of the trajectories of individual granules. Blue light illumination of the cells is used to induce oxidative stress (Algvere et al., 2006; Wu et al., 2006) and cells are imaged before and after light treatment to track granule motility (Fig. 4). Cells are also imaged for several additional hours after light exposure to confirm cell survival. In experiments to be reported in detail elsewhere, granule movement is observed to slow significantly when RPE cells are subjected to sub-lethal photic stress (Fig. 4). This observation is probably not specific for melanosomes but may apply to other membrane-bound organelles as well. Indeed the melanosomes used here should be more precisely considered phagosomes since the granules were internalized by phagocytosis; melanosomes and phagosomes move by similar mechanisms (Algvere et al., 2006; Barral and Seabra, 2004; Futter, 2006; Seabra and Coudrier, 2004). It appears from these studies that sub-lethal oxidative stress to the RPE can reduce the efficiency of organelle translocation. This raises the possibility that repeated or chronic low levels of stress associated with aging could affect RPE phenotype in situ by leading to a gradual mis-localization of organelles. Perhaps this mechanism accounts for the observation that melanosomes of the aging RPE are less apically restricted than in young cells (Feeney, 1978). Further, a stress-induced slowing of organelle movement may have ramifications for the efficiency of RPE cells to translocate phagosomes containing photoreceptor outer segments from the cell surface to the peri-nuclear region where they fuse with lysosomes to degrade phagosome contents. Stress-induced organelle slowing would not only affect organelle distribution, but also theoretically impact photoreceptor survival by impairing the essential process of photoreceptor turnover.
Fig. 4.
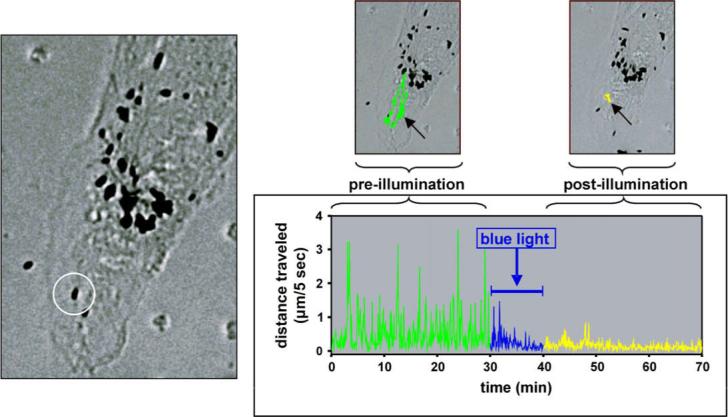
Effect of blue light treatment on the motility of phagocytized porcine melanosomes in ARPE-19 cells. Left: phase contrast micrograph showing a granule used for motility tracking (circled). Right: the granule's movement pre-illumination (green) and post-illumination (yellow) shown graphically and by colored traces superimposed on smaller micrographs. For the experiment, cultures were illuminated for 10 min with violet-blue light (400−410 nm at 4 mW/mm2) delivered via the epi-illumination system of the microscope using a protocol empirically determined to induce sub-lethal photic stress. To track granule movement, images were captured at 5 s intervals; image acquisition and data analysis were performed using Premier MetaMorph software. The blue light effect is quantified by comparing the total distance traveled over 30 min pre-illumination (188 μm) versus post-illumination (45 μm). For the granule illustrated here, motility decreased 76% on blue light treatment.
There is no specific evidence at this time to indicate that the stress-induced organelle slowing we have observed results from oxidative modification of the RPE cytoskeleton per se. Indeed, the sub-lethal stress levels that are used do not overtly disrupt the gross actin or microtubule networks within cells that are detectable by light microscopy (see sub-lethal photic treatment in Fig. 3). Many other mechanisms can be envisioned that could affect motility, ranging from dysregulation of motor complexes to insufficient energy. However, it seems safe to say that sub-lethal oxidative stress to the RPE over time has the potential to produce widespread derangements in sub-cellular organization. Effects of these changes on cell morphology with aging may be subtle, but RPE cell ‘phenotype’ is more than the superficial microscopic picture of the tissue. It is the cell's ‘molecular phenotype’ that ultimately determines the functional efficiency of the RPE, but our understanding of this level of cellular organization is still in its infancy.
6. Summary and future directions
RPE cells present an interesting challenge for students of epithelial morphogenesis. In the tissue in situ, RPE cells are growth quiescent with a highly regular cell shape. The cells can remain in this state in the human eye for decades, and yet retain the capacity to de-epithelialize and to grow when explanted into cell cultures. However, for reasons that remain elusive, after propagation in vitro, the cells show an incomplete and variable ability to re-develop an epithelial phenotype. In recent years cultured RPE cells have proved quite useful for exploring cell-type-specific pathways to establish protein polarity, pathways that are central to epithelial morphogenesis. However, initial events in RPE phenotype development, events that first demarcate the apical and basal membrane domains and that are a prerequisite for polarization are still poorly understood.
In other epithelial cells, formation of an E-cadherin intercellular adhesion plays an important early inductive role in morphogenesis. However, this protein is largely absent from RPE cells during in vitro phenotype development and it is not clear whether or how the dominant RPE cadherin, N-cadherin, participates in morphogenesis. Recent studies of the E-cadherin-containing adherens junction in other epithelial cells point to a critical role in phenotype development for actin cytoskeletal remodeling at the AJ. Actin rearrangements also accompany the formation of nectin-based adhesions that precede AJ assembly and the development of the tight junctions that follow. The ultimate outcome is an actin organization that is dominantly zonular, a fundamental epithelial characteristic. In RPE cells, analyses of actin dynamics during the maturation of N-cadherin adhesions, patterned on the recent studies of E-cadherin in other epithelial cells, may be a fruitful strategy for determining how RPE cells generate an epithelial-type zonular AJ. Regarding actin dynamics, analysis of the functions of the cadherin-binding regulatory protein p120-catenin may be a useful place to start. It is probably also time to ask whether RPE cells express additional newly identified or minor cadherins that could participate in junction assembly or phenotype induction.
Aside from failure to regenerate a highly epithelioid cell shape, another phenotypic deficiency of cultured human RPE is the absence of pigmentation. An argument is made here that phenotype development and melanization are linked, and that both might be regulated by a common signaling pathway: the Wnt/β-catenin pathway. Currently, evidence implicating this widespread and highly complex pathway in RPE morphogenesis is suggestive at best. Nonetheless, the pathway could provide a useful framework for teasing out the steps in RPE morphogenesis, which appears to be a slow, step-wise process with many points of regulation where cell maturation can cease, or perhaps be diverted. A judicious and methodical series of investigations of the Wnt/β-catenin pathway in RPE cells will not be a simple proposition, but it seems inevitable that useful information about RPE phenotype development and retention will result. Comparing β-catenin signaling in fetal versus adult human RPE cells might be a useful approach since the former produce more highly pigmented and epithelioid monolayers than the latter.
Cytoskeletal rearrangements and the activity of the Wnt/β-catenin pathway may both be related to the important question of whether RPE phenotype is affected by aging. Ultimately one of our major reasons for interest in RPE morphogenesis is the belief that normal phenotype is essential for optimal cell function, and that loss of RPE phenotype contributes to pathology, especially age-related retinal disease. Here the concept is developed that aging may produce disruptions of RPE phenotype by mechanisms involving oxidative stress (a well established aging influence) as a disrupter of the organization or integrity of the cytoskeleton (a well established determinant of phenotype). Age-related modifications are presumed to result from stress that is sub-lethal and which produces structural modifications that are subtle, affecting what might be called the ‘molecular phenotype’ rather than the gross morphology of aging cells. Analyzing sub-lethal stress that is revealed as declines in functional efficiency rather than overt cell death is a useful future direction for understanding the effects of age on RPE structure and function. Further, β-catenin participates in a Wnt-related pathway that helps regulate the expression of genes that protect against oxidative stress. Exploration of the Wnt/β-catenin pathway recommended above may therefore aid our understanding not only of re-morphogenesis and re-pigmentation, but also of RPE stress in aging. Activity of the Wnt/β-catenin pathway could provide a unifying theory helping to explain many of the most vexing problems associated with understanding RPE cell structure, function, and contribution to age-related pathology.
Acknowledgements
The author thanks Dr. Brian McKay, University of Arizona, for his helpful suggestions on the manuscript and Dr. Mariusz Zareba for assistance with the studies of organelle motility and with preparation of the figures. Support for research from the author's laboratory was provided by NIH grants R01 EY015284, R01 EY013722 and P30 EY01931, by an unrestricted grant from Research to Prevent Blindness, Inc., and by Milwaukee area donors and foundations supporting macular degeneration research.
References
- Abe T, Durlu YK, Tamai M. The properties of retinal pigment epithelial cells in proliferative vitreoretinopathy compared with cultured retinal pigment epithelial cells. Exp. Eye Res. 1996;63:201–210. doi: 10.1006/exer.1996.0109. [DOI] [PubMed] [Google Scholar]
- Abe T, Sato M, Tamai M. Dedifferentiation of the retinal pigment epithelium compared to the proliferative membranes of proliferative vitreoretinopathy. Curr. Eye Res. 1998;17:1103–1109. doi: 10.1076/ceyr.17.12.1103.5126. [DOI] [PubMed] [Google Scholar]
- Abul-Hassan K, Walmsley R, Tombran-Tink J, Boulton M. Regulation of tyrosinase expression and activity in cultured human retinal pigment epithelial cells. Pigment Cell Res. 2000;13:436–441. doi: 10.1034/j.1600-0749.2000.130605.x. [DOI] [PubMed] [Google Scholar]
- Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR. In vivo models of proliferative vitreoretinopathy. Nat.Protoc. 2007;2:67–77. doi: 10.1038/nprot.2007.4. [DOI] [PubMed] [Google Scholar]
- Akeo K, Hiramitsu T, Yorifuji H, Okisaka S. Membranes of retinal pigment epithelial cells in vitro are damaged in the phagocytotic process of the photo-receptor outer segment discs peroxidized by ferrous ions. Pigment Cell Res. 2002;15:341–347. doi: 10.1034/j.1600-0749.2002.02054.x. [DOI] [PubMed] [Google Scholar]
- Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol. Scand. 2006;84:4–15. doi: 10.1111/j.1600-0420.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- Alonso SR, Tracey L, Ortiz P, Perez-Gomez B, Palacios J, Pollan M, Linares J, Serrano S, Saez-Castillo AI, Sanchez L, Pajares R, Sanchez-Aguilera A, Artiga MJ, Piris MA, Rodriguez-Peralto JL. A high-throughput study in melanoma identifies epithelial–mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007;67:3450–3460. doi: 10.1158/0008-5472.CAN-06-3481. [DOI] [PubMed] [Google Scholar]
- Andarawewa KL, Erickson AC, Chou WS, Costes SV, Gascard P, Mott JD, Bissell MJ, Barcellos-Hoff MH. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor beta induced epithelial to mesenchymal transition. Cancer Res. 2007;67:8662–8670. doi: 10.1158/0008-5472.CAN-07-1294. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Guerin CJ, Matsumoto B, Pfeffer BA. Identification and localization of a beta-1 receptor from the integrin family in mammalian retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1990;31:81–93. [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- AREDS A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaria RH, Charteris DG. Proliferative vitreoretinopathy: developments in pathogenesis and treatment. Compr. Ophthalmol. Update. 2006;7:179–185. [PubMed] [Google Scholar]
- Aslan M, Ryan TM, Townes TM, Coward L, Kirk MC, Barnes S, Alexander CB, Rosenfeld SS, Freeman BA. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J. Biol. Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- Ballhausen WG. Genetic testing for familial adenomatous polyposis. Ann. N.Y. Acad. Sci. 2000;910:36–47. doi: 10.1111/j.1749-6632.2000.tb06699.x. [DOI] [PubMed] [Google Scholar]
- Banan A, Farhadi A, Fields JZ, Zhang LJ, Shaikh M, Keshavarzian A. The delta-isoform of protein kinase C causes inducible nitric-oxide synthase and nitric oxide up-regulation: key mechanism for oxidant-induced carbonylation, nitration, and disassembly of the microtubule cytoskeleton and hyper-permeability of barrier of intestinal epithelia. J. Pharmacol. Exp. Ther. 2003;305:482–494. doi: 10.1124/jpet.102.047308. [DOI] [PubMed] [Google Scholar]
- Banan A, Fields JZ, Zhang Y, Keshavarzian A. iNOS upregulation mediates oxidant-induced disruption of F-actin and barrier of intestinal monolayers. Am. J. Physiol Gastrointest. Liver Physiol. 2001;280:G1234–G1246. doi: 10.1152/ajpgi.2001.280.6.G1234. [DOI] [PubMed] [Google Scholar]
- Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Novel effect of NF-kappaB activation: carbonylation and nitration injury to cytoskeleton and disruption of monolayer barrier in intestinal epithelium. Am. J. Physiol. Cell Physiol. 2004;287:C1139–C1151. doi: 10.1152/ajpcell.00146.2004. [DOI] [PubMed] [Google Scholar]
- Barral DC, Seabra MC. The melanosome as a model to study organelle motility in mammals. Pigment Cell Res. 2004;17:111–118. doi: 10.1111/j.1600-0749.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neurotrophins induce neuroprotective signaling in the retinal pigment epithelial cell by activating the synthesis of the anti-inflammatory and anti-apoptotic neuroprotectin D1. Adv. Exp. Med. Biol. 2008;613:39–44. doi: 10.1007/978-0-387-74904-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006;19:380–394. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Bok D. Autoradiographic studies on the polarity of plasma membrane receptors in RPE cells. In: Field H, editor. The Structure of the Eye. Elsevier North Holland; New York: 1982. pp. 247–256. [Google Scholar]
- Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Bonilha VL, Rodriguez-Boulan E. Polarity and developmental regulation of two PDZ proteins in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2001;42:3274–3282. [PubMed] [Google Scholar]
- Boulton M. Melanin and the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford University Press; New York: 1998. pp. 68–85. [Google Scholar]
- Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye. 2001;15:384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- Bowerman B. Cell biology. Oxidative stress and cancer: a beta-catenin convergence. Science. 2005;308:1119–1120. doi: 10.1126/science.1113356. [DOI] [PubMed] [Google Scholar]
- Burke JM. Determinants of retinal pigment epithelial cell phenotype and polarity. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford University Press; New York: 1998. pp. 86–102. [Google Scholar]
- Burke JM, Cao F, Irving PE. High levels of E-/P-cadherin: correlation with decreased apical polarity of Na/K ATPase in bovine RPE cells in situ. Investig. Ophthalmol. Vis. Sci. 2000;41:1945–1952. [PubMed] [Google Scholar]
- Burke JM, Cao F, Irving PE, Skumatz CM. Expression of E-cadherin by human retinal pigment epithelium: delayed expression in vitro. Investig. Ophthalmol. Vis. Sci. 1999;40:2963–2970. [PubMed] [Google Scholar]
- Burke JM, Hjelmeland LM. Mosaicism of the retinal pigment epithelium: seeing the small picture. Mol. Interv. 2005;5:241–249. doi: 10.1124/mi.5.4.7. [DOI] [PubMed] [Google Scholar]
- Burke JM, Hong J. Fate of E-cadherin in early RPE cultures: transient accumulation of truncated peptides at nonjunctional sites. Investig. Ophthalmol. Vis. Sci. 2006;47:3635–3643. doi: 10.1167/iovs.06-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, McKay BS. In vitro aging of bovine and human retinal pigment epithelium: number and activity of the Na/K ATPase pump. Exp. Eye Res. 1993;57:51–57. doi: 10.1006/exer.1993.1098. [DOI] [PubMed] [Google Scholar]
- Burke JM, Skumatz CM, Irving PE, McKay BS. Phenotypic heterogeneity of retinal pigment epithelial cells in vitro and in situ. Exp. Eye Res. 1996;62:63–73. doi: 10.1006/exer.1996.0008. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr., Jones DP. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Hackett SF. Corneal endothelial cell matrix promotes expression of differentiated features of retinal pigmented epithelial cells: implication of laminin and basic fibroblast growth factor as active components. Exp. Eye Res. 1993;57:539–547. doi: 10.1006/exer.1993.1158. [DOI] [PubMed] [Google Scholar]
- Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J. Biol. Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- Chang JT, Esumi N, Moore K, Li Y, Zhang S, Chew C, Goodman B, Rattner A, Moody S, Stetten G, Campochiaro PA, Zack DJ. Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum. Mol. Genet. 1999;8:575–583. doi: 10.1093/hmg/8.4.575. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Ma ZZ. N-cadherin expression in a rat model of retinal detachment and reattachment. Investig. Ophthalmol. Vis. Sci. 2007;48:1832–1838. doi: 10.1167/iovs.06-0928. [DOI] [PubMed] [Google Scholar]
- Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J. Cell Biol. 2003;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J. Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Coudrier E. Myosins in melanocytes: to move or not to move? Pigment Cell Res. 2007;20:153–160. doi: 10.1111/j.1600-0749.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Gagliano N, Di SP, Colombo R, Milzani A. Methionine oxidation as a major cause of the functional impairment of oxidized actin. Free Radic. Biol. Med. 2002;32:927–937. doi: 10.1016/s0891-5849(02)00799-2. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Gagliano N, Lusini L, Milzani A, Di SP, Colombo R. Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic. Biol. Med. 2001a;31:1075–1083. doi: 10.1016/s0891-5849(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Milzani A, Di SP, Colombo R. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic. Biol. Med. 2001b;31:1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- Daugherty RL, Gottardi CJ. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AA, Bernstein PS, Bok D, Turner J, Nachtigal M, Hunt RC. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Investig. Ophthalmol. Vis. Sci. 1995;36:955–964. [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am. J. Ophthalmol. 2007;143:607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Priore LV, Kuo YH, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Investig. Ophthalmol. Vis. Sci. 2002;43:3312–3318. [PubMed] [Google Scholar]
- Deora AA, Gravotta D, Kreitzer G, Hu J, Bok D, Rodriguez-Boulan E. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol. Biol. Cell. 2004;15:4148–4165. doi: 10.1091/mbc.E04-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16245–16250. doi: 10.1073/pnas.0504419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues. Organs. 2007;185:73–84. doi: 10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- Dorey CK, Torres X, Swart T. Evidence of melanogenesis in porcine retinal pigment epithelial cells in vitro. Exp. Eye Res. 1990;50:1–10. doi: 10.1016/0014-4835(90)90003-d. [DOI] [PubMed] [Google Scholar]
- Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Investig. Ophthalmol. Vis. Sci. 1989;30:1691–1699. [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- Esumi N, Kachi S, Campochiaro PA, Zack DJ. VMD2 promoter requires two proximal E-box sites for its activity in vivo and is regulated by the MITF-TFE family. J. Biol. Chem. 2007;282:1838–1850. doi: 10.1074/jbc.M609517200. [DOI] [PubMed] [Google Scholar]
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Investig. Ophthalmol. Vis. Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- Feeney-Burns L, Burns RP, Gao CL. Age-related macular changes in humans over 90 years old. Am. J. Ophthalmol. 1990;109:265–278. doi: 10.1016/s0002-9394(14)74549-0. [DOI] [PubMed] [Google Scholar]
- Fratelli M, Demol H, Puype M, Casagrande S, Villa P, Eberini I, Vandekerckhove J, Gianazza E, Ghezzi P. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics. 2003;3:1154–1161. doi: 10.1002/pmic.200300436. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Shintani Y, Reynolds AB, Johnson KR, Wheelock MJ. The regulatory or phosphorylation domain of p120 catenin controls E-cadherin dynamics at the plasma membrane. Exp. Cell Res. 2008;314:52–67. doi: 10.1016/j.yexcr.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE. The molecular regulation of organelle transport in mammalian retinal pigment epithelial cells. Pigment Cell Res. 2006;19:104–111. doi: 10.1111/j.1600-0749.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Gamm DM, Melvan JN, Shearer RL, Pinilla I, Sabat G, Svendsen CN, Wright LS. A novel serum-free method for culturing human prenatal retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2008;49:788–799. doi: 10.1167/iovs.07-0777. [DOI] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- Garg TK, Chang JY. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: prevention of cell death by AG126 and 15-deoxy-delta 12, 14-PGJ2. BMC. Ophthalmol. 2003;3:5. doi: 10.1186/1471-2415-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A. beta-Catenin signaling in biological control and cancer. J. Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- Geiger B, Ayalon O. Cadherins. Annu. Rev. Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Geisen P, McColm JR, King BM, Hartnett ME. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr. Eye Res. 2006;31:739–748. doi: 10.1080/02713680600837408. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Caplan MJ. Delivery of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1993;260:552–554. doi: 10.1126/science.8386395. [DOI] [PubMed] [Google Scholar]
- Grune T, Reinheckel T, North JA, Li R, Bescos PB, Shringarpure R, Davies KJ. Ezrin turnover and cell shape changes catalyzed by proteasome in oxidatively stressed cells. FASEB J. 2002;16:1602–1610. doi: 10.1096/fj.02-0015com. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, McCrea PD. Catenins as mediators of the cytoplasmic functions of cadherins. J. Cell Sci. 1993;(Suppl 17):155–158. doi: 10.1242/jcs.1993.supplement_17.22. [DOI] [PubMed] [Google Scholar]
- Gundersen D, Orlowski J, Rodriguez-Boulan E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin sub-membrane cytoskeleton. J. Cell Biol. 1991;112:863–872. doi: 10.1083/jcb.112.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen D, Powell SK, Rodriguez-Boulan E. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J. Cell Biol. 1993;121:335–343. doi: 10.1083/jcb.121.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Hammerton RW, Krzeminski KA, Mays RW, Ryan TA, Wollner DA, Nelson WJ. Mechanism for regulating cell surface distribution of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1991;254:847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann. N.Y. Acad. Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- Heth CA, Yankauckas MA, Adamian M, Edwards RB. Characterization of retinal pigment epithelial cells cultured on microporous filters. Curr. Eye Res. 1987;6:1007–1019. doi: 10.3109/02713688709034872. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J. Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt AL, Luna EJ. Membrane interactions with the actin cytoskeleton. Curr. Opin. Cell Biol. 1994;6:120–130. doi: 10.1016/0955-0674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Hjelmeland LM, Cristofolo VJ, Funk W, Rakoczy E, Katz ML. Senescence of the retinal pigment epithelium. Mol.Vis. 1999;5:33. [PubMed] [Google Scholar]
- Ho TC, Yang YC, Cheng HC, Wu AC, Chen SL, Tsao YP. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem. Biophys. Res. Commun. 2006;342:372–378. doi: 10.1016/j.bbrc.2006.01.164. [DOI] [PubMed] [Google Scholar]
- Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Huotari V, Sormunen R, Lehto VP, Eskelinen S. The polarity of the membrane skeleton in retinal pigment epithelial cells of developing chicken embryos and in primary culture. Differentiation. 1995;58:205–215. doi: 10.1046/j.1432-0436.1995.5830205.x. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kawaji T, Inoue-Mochita M, Taga T, Tanihara H. Media conditioned by retinal pigment epithelial cells suppress the canonical Wnt pathway. Neurosci. Lett. 2007;424:190–193. doi: 10.1016/j.neulet.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell–cell adhesion. J. Cell Biol. 1996;135:1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri R, Kobayashi K, Okinami S, Kobayashi H. Suppression of Mitf by small interfering RNA induces dedifferentiation of chick embryonic retinal pigment epithelium. Exp. Eye Res. 2005;81:15–21. doi: 10.1016/j.exer.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Jaitovich AA, Bertorello AM. Na+, K+-ATPase: an indispensable ion pumping-signaling mechanism across mammalian cell membranes. Semin. Nephrol. 2006;26:386–392. doi: 10.1016/j.semnephrol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Altered expression of secreted frizzled-related protein-2 in retinitis pigmentosa retinas. Investig. Ophthalmol. Vis. Sci. 2000;41:1297–1301. [PubMed] [Google Scholar]
- Kaida M, Cao F, Skumatz CM, Irving PE, Burke JM. Time at confluence for human RPE cells: effects on the adherens junction and in vitro wound closure. Investig. Ophthalmol. Vis. Sci. 2000;41:3215–3224. [PubMed] [Google Scholar]
- Kameya S, Hawes NL, Chang B, Heckenlively JR, Naggert JK, Nishina PM. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum. Mol. Genet. 2002;11:1879–1886. doi: 10.1093/hmg/11.16.1879. [DOI] [PubMed] [Google Scholar]
- Kanuga N, Winton HL, Beauchene L, Koman A, Zerbib A, Halford S, Couraud PO, Keegan D, Coffey P, Lund RD, Adamson P, Greenwood J. Characterization of genetically modified human retinal pigment epithelial cells developed for in vitro and transplantation studies. Investig. Ophthalmol. Vis. Sci. 2002;43:546–555. [PubMed] [Google Scholar]
- Katoh M, Katoh M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol. Ther. 2006;5:1059–1064. doi: 10.4161/cbt.5.9.3151. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Comparative integromics on FZD7 orthologs: conserved binding sites for PU.1, SP1, CCAAT-box and TCF/LEF/SOX transcription factors within 50-promoter region of mammalian FZD7 orthologs. Int. J. Mol. Med. 2007;19:529–533. [PubMed] [Google Scholar]
- Katsube T, Tsuji H, Onoda M. Nitric oxide attenuates hydrogen peroxide-induced barrier disruption and protein tyrosine phosphorylation in monolayers of intestinal epithelial cell. Biochim. Biophys. Acta. 2007;1773:794–803. doi: 10.1016/j.bbamcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Kaufmann U, Martin B, Link D, Witt K, Zeitler R, Reinhard S, Starzinski-Powitz A. M-cadherin and its sisters in development of striated muscle. Cell Tissue Res. 1999;296:191–198. doi: 10.1007/s004410051280. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y, Fields JZ. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–728. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela T, Jaaskelainen J, Vaheri A, Carpen O. Ezrin, a membrane-organizing protein, as a polarization marker of the retinal pigment epithelium in vertebrates. Cell Tissue Res. 2000;301:217–223. doi: 10.1007/s004410000225. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch. Ophthalmol. 2008;126:115–121. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M, Bilinski SM. RNA localization and its role in the spatially restricted protein synthesis. Folia Histochem. Cytobiol. 2003;41:3–11. [PubMed] [Google Scholar]
- Kociok N, Joussen AM. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:101–113. doi: 10.1007/s00417-006-0266-x. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J. Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmari E, Traweger A, Wejksza K, Bauer HC. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol. Neurobiol. 2005;25:129–139. doi: 10.1007/s10571-004-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugluger W, Seidel S, Steindl K, Binder S. Epidermal growth factor inhibits glycogen synthase kinase-3 (GSK-3) and beta-catenin transcription in cultured ARPE-19 cells. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:1543–1548. doi: 10.1007/s00417-007-0635-0. [DOI] [PubMed] [Google Scholar]
- Lagunowich LA, Grunwald GB. Expression of calcium-dependent cell adhesion during ocular development: a biochemical, histochemical and functional analysis. Dev. Biol. 1989;135:158–171. doi: 10.1016/0012-1606(89)90166-8. [DOI] [PubMed] [Google Scholar]
- Lagunowich LA, Grunwald GB. Tissue and age-specificity of post-translational modifications of N-cadherin during chick embryo development. Differentiation. 1991;47:19–27. doi: 10.1111/j.1432-0436.1991.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Lee SC, Kwon OW, Seong GJ, Kim SH, Ahn JE, Kay ED. Epitheliomesenchymal transdifferentiation of cultured RPE cells. Ophthalmic Res. 2001;33:80–86. doi: 10.1159/000055648. [DOI] [PubMed] [Google Scholar]
- Leterrier JF. Water and the cytoskeleton. Cell Mol. Biol. (Noisy.-le-grand) 2001;47:901–923. [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Liu X, Mizoguchi A, Takeichi M, Honda Y, Ide C. Developmental changes in the subcellular localization of R-cadherin in chick retinal pigment epithelium. Histochem. Cell Biol. 1997;108:35–43. doi: 10.1007/s004180050144. [DOI] [PubMed] [Google Scholar]
- Loeffler KU, Kivela T, Borgmann H, Witschel H. Malignant tumor of the retinal pigment epithelium with extraocular extension in a phthisical eye. Graefes Arch. Clin. Exp. Ophthalmol. 1996;234(Suppl 1):S70–S75. doi: 10.1007/BF02343051. [DOI] [PubMed] [Google Scholar]
- Lopez de HM, Jansen RP. mRNA localization and the cytoskeleton. Curr. Opin. Cell Biol. 2004;16:80–85. doi: 10.1016/j.ceb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Low SH, Marmorstein LY, Miura M, Li X, Kudo N, Marmorstein AD, Weimbs T. Retinal pigment epithelial cells exhibit unique expression and localization of plasma membrane syntaxins which may contribute to their trafficking phenotype. J. Cell Sci. 2002;115:4545–4553. doi: 10.1242/jcs.00116. [DOI] [PubMed] [Google Scholar]
- Luna EJ, Hitt AL. Cytoskeleton–plasma membrane interactions. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Maeda M, Johnson E, Mandal SH, Lawson KR, Keim SA, Svoboda RA, Caplan S, Wahl JK, III, Wheelock MJ, Johnson KR. Expression of inappropriate cadherins by epithelial tumor cells promotes endocytosis and degradation of E-cadherin via competition for p120(ctn). Oncogene. 2006;25:4595–4604. doi: 10.1038/sj.onc.1209396. [DOI] [PubMed] [Google Scholar]
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J. Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Investig. Ophthalmol. Vis. Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Vasireddy V, Jablonski MM, Wang X, Heckenlively JR, Hughes BA, Reddy GB, Ayyagari R. Spatial and temporal expression of MFRP and its interaction with CTRP5. Investig. Ophthalmol. Vis. Sci. 2006;47:5514–5521. doi: 10.1167/iovs.06-0449. [DOI] [PubMed] [Google Scholar]
- Mandell KJ, Berglin L, Severson EA, Edelhauser HF, Parkos CA. Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function. Investig. Ophthalmol. Vis. Sci. 2007;48:3928–3936. doi: 10.1167/iovs.06-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007;21:2605–2614. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Finnemann SC, Bonilha VL, Rodriguez-Boulan E. Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases. Ann. N.Y. Acad. Sci. 1998;857:1–12. doi: 10.1111/j.1749-6632.1998.tb10102.x. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Kinnick TR. Focus on molecules: bestrophin (best-1). Exp. Eye Res. 2007;85:423–424. doi: 10.1016/j.exer.2006.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K(+)-ATPase distributions in polarized epithelia. J. Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs JA, ndersson-Fisone C, Jeong MC, Cohen-Gould L, Zurzolo C, Nabi IR, Rodriguez-Boulan E, Nelson WJ. Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J. Cell Biol. 1995;129:507–519. doi: 10.1083/jcb.129.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. Bioessays. 2004;26:766–777. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- Matsuyoshi N, Imamura S. Multiple cadherins are expressed in human fibroblasts. Biochem. Biophys. Res. Commun. 1997;235:355–358. doi: 10.1006/bbrc.1997.6707. [DOI] [PubMed] [Google Scholar]
- Mays RW, Siemers KA, Fritz BA, Lowe AW, van MG, Nelson WJ. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J. Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BS, Burke JM. Separation of phenotypically distinct subpopulations of cultured human retinal pigment epithelial cells. Exp. Cell Res. 1994;213:85–92. doi: 10.1006/excr.1994.1176. [DOI] [PubMed] [Google Scholar]
- McKay BS, Irving PE, Skumatz CM, Burke JM. Cell–cell adhesion molecules and the development of an epithelial phenotype in cultured human retinal pigment epithelial cells. Exp. Eye Res. 1997;65:661–671. doi: 10.1006/exer.1997.0374. [DOI] [PubMed] [Google Scholar]
- McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- McNeill H, Ryan TA, Smith SJ, Nelson WJ. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J. Cell Biol. 1993;120:1217–1226. doi: 10.1083/jcb.120.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenburg L, Schraermeyer U. An overview on the toxic morphological changes in the retinal pigment epithelium after systemic compound administration. Toxicol. Pathol. 2007;35:252–267. doi: 10.1080/01926230601178199. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Gruenbaum Y. Gone with the Wnt/Notch: stem cells in laminopathies, progeria, and aging. J. Cell Biol. 2008;181:9–13. doi: 10.1083/jcb.200802155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda KC, Joseph SR, Yap AS, Teasdale RD, Stow JL. Contextual binding of p120ctn to E-cadherin at the basolateral plasma membrane in polarized epithelia. J. Biol. Chem. 2003;278:43480–43488. doi: 10.1074/jbc.M305525200. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug Deliv. Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Nectin and nectin-like molecules: biology and pathology. Am. J. Nephrol. 2007;27:590–604. doi: 10.1159/000108103. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim. Biophys. Acta. 2008;1778:670–691. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Mochii M, Mazaki Y, Mizuno N, Hayashi H, Eguchi G. Role of Mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev. Biol. 1998;193:47–62. doi: 10.1006/dbio.1997.8800. [DOI] [PubMed] [Google Scholar]
- Mora RC, Bonilha VL, Shin BC, Hu J, Cohen-Gould L, Bok D, Rodriguez-Boulan E. Bipolar assembly of caveolae in retinal pigment epithelium. Am. J. Physiol. Cell Physiol. 2006;290:C832–C843. doi: 10.1152/ajpcell.00405.2005. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Kuehn MH, Faidley EA, Syed NA, Stone EM. Differential macular and peripheral expression of bestrophin in human eyes and its implication for best disease. Investig. Ophthalmol. Vis. Sci. 2007;48:3372–3380. doi: 10.1167/iovs.06-0868. [DOI] [PubMed] [Google Scholar]
- Murisier F, Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histol. Histopathol. 2006;21:567–578. doi: 10.14670/HH-21.567. [DOI] [PubMed] [Google Scholar]
- Murphy-Erdosh C, Napolitano EW, Reichardt LF. The expression of B-cadherin during embryonic chick development. Dev. Biol. 1994;161:107–125. doi: 10.1006/dbio.1994.1013. [DOI] [PubMed] [Google Scholar]
- Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci. 1993;104(Pt 1):37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Hinck LE, Nelson WJ. Epithelial cell adhesion and development of cell surface polarity: possible mechanisms for modulation of cadherin function, organization and distribution. J. Cell Sci. 1993;(Suppl 17):139–145. doi: 10.1242/jcs.1993.supplement_17.20. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Cytoskeleton functions in membrane traffic in polarized epithelial cells. Semin. Cell Biol. 1991;2:375–385. [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 1990;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita H, Takai Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement, and proliferation. IUBMB. Life. 2006;58:334–343. doi: 10.1080/15216540600719622. [DOI] [PubMed] [Google Scholar]
- Ogita H, Takai Y. Cross-talk among integrin, cadherin, and growth factor receptor: roles of nectin and nectin-like molecule. Int. Rev. Cytol. 2008;265:1–54. doi: 10.1016/S0074-7696(07)65001-3. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Ichinose S, Nakahama K, Yoshida T, Kojima A, Mochizuki M, Morita I. The effects of amniotic membrane on retinal pigment epithelial cell differentiation. Mol. Vis. 2005;11:1–10. [PubMed] [Google Scholar]
- Okami T, Yamamoto A, Omori K, Takada T, Uyama M, Tashiro Y. Immunocytochemical localization of Na+,K(+)-ATPase in rat retinal pigment epithelial cells. J. Histochem. Cytochem. 1990;38:1267–1275. doi: 10.1177/38.9.2167328. [DOI] [PubMed] [Google Scholar]
- Oliveira SS, Morgado-Diaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol. Life Sci. 2007;64:17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev. Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer BA, Clark VM, Flannery JG, Bok D. Membrane receptors for retinol-binding protein in cultured human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1986;27:1031–1040. [PubMed] [Google Scholar]
- Pletjushkina OJ, Ivanova OJ, Kaverina IN, Vasiliev JM. Taxol-treated fibroblasts acquire an epithelioid shape and a circular pattern of actin bundles. Exp. Cell Res. 1994;212:201–208. doi: 10.1006/excr.1994.1135. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Pouliot Y, Holland PC, Blaschuk OW. Developmental regulation of a cadherin during the differentiation of skeletal myoblasts. Dev. Biol. 1990;141:292–298. doi: 10.1016/0012-1606(90)90385-v. [DOI] [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J. Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak DJ, Hardy KM, Jaffe GJ, McKay BS. Ca++-switch induction of RPE differentiation. Exp. Eye Res. 2006;82:648–656. doi: 10.1016/j.exer.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr., Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo LJ. The distribution of Na+,K(+)-ATPase in the retinal pigmented epithelium from chicken embryo is polarized in vivo but not in primary cell culture. Exp. Eye Res. 1990;51:435–446. doi: 10.1016/0014-4835(90)90156-o. [DOI] [PubMed] [Google Scholar]
- Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int. Rev. Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Saenz-Morales D, Escribese MM, Stamatakis K, Garcia-Martos M, Alegre L, Conde E, Perez-Sala D, Mampaso F, Garcia-Bermejo ML. Requirements for proximal tubule epithelial cell detachment in response to ischemia: role of oxidative stress. Exp. Cell Res. 2006;312:3711–3727. doi: 10.1016/j.yexcr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Flanders KC, Okada Y, Miyamoto T, Sumioka T, Shirai K, Kitano A, Miyazaki K, Tanaka S, Ikeda K. Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr. Metab Immune.Disord. Drug Targets. 2008;8:69–76. doi: 10.2174/187153008783928343. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Ogita H, Hirota T, Kawakatsu T, Fukuyama T, Yasumi M, Kanzaki N, Ozaki M, Takai Y. Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J. Biol. Chem. 2006;281:19631–19644. doi: 10.1074/jbc.M600301200. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr. Opin. Cell Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR, Steingrimsson E, Hecht A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol. Cell Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999;12:219–236. doi: 10.1111/j.1600-0749.1999.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S, Takeda K, Yasumoto K, Udono T, Watanabe K, Saito H, Takahashi K. Microphthalmia-associated transcription factor (MITF): multiplicity in structure, function, and regulation. J. Investig. Dermatol. Symp. Proc. 2001;6:99–104. doi: 10.1046/j.0022-202x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S, Yasumoto K, Amae S, Udono T, Watanabe K, Saito H, Takeda K. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Res. 2000;13(Suppl 8):98–102. doi: 10.1034/j.1600-0749.13.s8.18.x. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Simm A, Nass N, Bartling B, Hofmann B, Silber RE, Navarrete SA. Potential biomarkers of ageing. Biol. Chem. 2008;389:257–265. doi: 10.1515/BC.2008.034. [DOI] [PubMed] [Google Scholar]
- Song MK, Lui GM. Propagation of fetal human RPE cells: preservation of original culture morphology after serial passage. J. Cell Physiol. 1990;143:196–203. doi: 10.1002/jcp.1041430127. [DOI] [PubMed] [Google Scholar]
- Stebbings H. Cytoskeleton-dependent transport and localization of mRNA. Int. Rev. Cytol. 2001;211:1–31. doi: 10.1016/s0074-7696(01)11016-8. [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Miller SS. Transport and membrane properties of the retinal pigment epithelium. In: Zinn KM, Marmor MF, editors. The Retinal Pigment Epithelium. Harvard University Press; Cambridge: 1979. pp. 205–225. [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- Stinson AM, Wiegand RD, Anderson RE. Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes. Exp. Eye Res. 1991;52:213–218. doi: 10.1016/0014-4835(91)90261-c. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Sundin OH, Leppert GS, Silva ED, Yang JM, Dharmaraj S, Maumenee IH, Santos LC, Parsa CF, Traboulsi EI, Broman KW, Dibernardo C, Sunness JS, Toy J, Weinberg EM. Extreme hyperopia is the result of null mutations in MFRP, which encodes a Frizzled-related protein. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9553–9558. doi: 10.1073/pnas.0501451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. MITF: a stream flowing for pigment cells. Pigment Cell Res. 2000;13:230–240. doi: 10.1034/j.1600-0749.2000.130404.x. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr.Opin. Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115:334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Tekotte H, Davis I. Intracellular mRNA localization: motors move messages. Trends Genet. 2002;18:636–642. doi: 10.1016/s0168-9525(02)02819-6. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- Tiret A, Parc C. Fundus lesions of adenomatous polyposis. Curr. Opin. Ophthalmol. 1999;10:168–172. doi: 10.1097/00055735-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J. Gen. Virol. 1996;77(Pt 1):61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J. Biol. Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- Van Aken EH, De WO, Van HL, Bruyneel E, De Laey JJ, Mareel MM. Invasion of retinal pigment epithelial cells: N-cadherin, hepatocyte growth factor, and focal adhesion kinase. Investig. Ophthalmol. Vis. Sci. 2003;44:463–472. doi: 10.1167/iovs.01-1096. [DOI] [PubMed] [Google Scholar]
- Wahl JK, III, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin–catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J. Biol. Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci. 1990;95(Pt 1):137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- Watzke RC, Soldevilla JD, Trune DR. Morphometric analysis of human retinal pigment epithelium: correlation with age and location. Curr. Eye Res. 1993;12:133–142. doi: 10.3109/02713689308999481. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr. Opin. Cell Biol. 2003a;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003b;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- White BD, Nguyen NK, Moon RT. Wnt signaling: it gets more humorous with age. Curr. Biol. 2007;17:R923–R925. doi: 10.1016/j.cub.2007.08.062. [DOI] [PubMed] [Google Scholar]
- Wolfensberger TJ. The historical discovery of the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford University Press; New York: 1998. pp. 13–22. [Google Scholar]
- Wollner DA, Krzeminski KA, Nelson WJ. Remodeling the cell surface distribution of membrane proteins during the development of epithelial cell polarity. J. Cell Biol. 1992;116:889–899. doi: 10.1083/jcb.116.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Nelson WJ. Establishing and maintaining epithelial cell polarity. Roles of protein sorting, delivery and retention. J. Cell Sci. 1992;102(Pt 2):185–190. doi: 10.1242/jcs.102.2.185. [DOI] [PubMed] [Google Scholar]
- Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina. 2007;27:997–1003. doi: 10.1097/IAE.0b013e318074c290. [DOI] [PubMed] [Google Scholar]
- Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv. Ophthalmol. 2006;51:461–481. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci. 1995;108(Pt 1):127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- Youn YH, Hong J, Burke JM. Endogenous N-cadherin in a subpopulation of MDCK cells: distribution and catenin complex composition. Exp. Cell Res. 2005;303:275–286. doi: 10.1016/j.yexcr.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Youn YH, Hong J, Burke JM. Cell phenotype in normal epithelial cell lines with high endogenous N-cadherin: comparison of RPE to an MDCK subclone. Investig. Ophthalmol. Vis. Sci. 2006;47:2675–2685. doi: 10.1167/iovs.05-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- Zareba M, Sarna T, Szewczyk G, Burke JM. Photobleaching of melanosomes from retinal pigment epithelium: II. Effects on the response of living cells to photic stress. Photochem. Photobiol. 2007;83:925–930. doi: 10.1111/j.1751-1097.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- Zurzolo C, Rodriguez-Boulan E. Delivery of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1993;260:550–552. doi: 10.1126/science.8386394. [DOI] [PubMed] [Google Scholar]