Abstract
OBJECTIVE—Antioxidized LDL (anti-oxLDL) antibodies have recently been suggested to be protective against the development of diabetes. We measured the changes in anti-oxLDL antibody levels in the inverse situation of improvement in carbohydrate metabolism.
RESEARCH DESIGN AND METHODS—The study was undertaken in 73 morbidly obese individuals, 21 of whom had type 2 diabetes, before and 7 months after they underwent bariatric surgery and in 11 healthy, nonobese individuals. Measurements were made of the area under the curve of glucose (AUCGlu) by an intravenous glucose tolerance test and of oxidized LDL (oxLDL) and IgG and IgM anti-oxLDL antibodies.
RESULTS—The morbidly obese patients with diabetes had significantly higher levels of oxLDL compared with the morbidly obese patients with normal fasting glucose and the control subjects and significantly lower levels of IgM anti-oxLDL antibodies. An inverse correlation was found between the levels of oxLDL and IgM anti-oxLDL antibodies (r = −0.352, P = 0.012). Although the levels of IgG and IgM anti-oxLDL antibodies rose after surgery, this increase was only significant in the diabetic patients, who experienced an improvement in their metabolic profile. Different multiple linear regression models showed that the AUCGlu was the main factor explaining the behavior of the levels of oxLDL and anti-oxLDL antibodies.
CONCLUSIONS—We found a close association between carbohydrate metabolism and IgM anti-oxLDL antibodies, which were significantly reduced in the morbidly obese patients with diabetes. The improvement in carbohydrate metabolism after bariatric surgery led to a significant increase in the levels of IgG and IgM anti-oxLDL antibodies.
The high prevalence of obesity is one of the main reasons for the increase in several diseases, such as atherosclerosis, diabetes, and hypertension. Underlying all these diseases is the phenomenon of oxidative stress (1). The increase in systemic oxidative stress seems to be an important mechanism leading to the increase in lipid peroxidation and oxidative modification of LDL. An increase in oxidized LDL (oxLDL) has been associated with a greater risk for atherosclerosis (2). Slight changes in LDL make it highly immunogenic. Antibodies exist against malonyldialdehyde (MDA) and CuSO2-oxLDL, as well as against LDL modified by glycation. Initially, these antibodies were detected mostly in patients with advanced atherosclerotic lesions, with several studies showing high levels in patients with coronary artery atherosclerosis (3). Nowadays, however, the clinical value of these anti-oxidized LDL (anti-oxLDL) antibodies is under question. Recent studies have failed to find any association between levels of anti-oxLDL antibodies and coronary artery disease (4). Indeed, others have found an inverse association between anti-oxLDL antibodies and carotid artery atherosclerosis (5), diabetes (6), and plasma cholesterol concentrations in the general population (7). Even more contradictory is the report that anti-oxLDL antibodies are lower in insulin-dependent diabetic patients with microvascular complications than in those without these complications (8). Several experimental animal models have shown that immunization with oxLDL induces high levels of anti-oxLDL antibodies and reduces the degree of atherosclerosis (9).
We also know that oxidative stress plays an important role in the genesis of type 2 diabetes. Carantoni et al. (10) found that the steady-state plasma glucose concentration and plasma glucose and insulin responses to oral glucose remained significantly correlated with oxLDL. However, the association between anti-oxLDL antibodies and insulin resistance and β-cell function remains unknown. Nevertheless, our group has shown in a prospective study undertaken in the general population that the levels of anti-oxLDL antibodies appear to predict the development of diabetes, with low levels of these antibodies determining an increased risk for the development of type 2 diabetes at 7 years (6). In the present study, we compared the changes produced in the levels of anti-oxLDL antibodies in the opposite situation, i.e., an improvement in insulin resistance and diabetes as a result of bariatric surgery in a group of patients with morbid obesity.
RESEARCH DESIGN AND METHODS
The study was undertaken in 73 morbidly obese individuals and in 11 healthy, nonobese persons (BMI <30 kg/m2) with no alterations in lipid or glucose metabolism. The morbidly obese individuals were classified in three groups, according to their fasting glucose levels before bariatric surgery: morbidly obese with normal fasting glucose (NFG) (glucose <5.6 mmol/l) (n = 21), morbidly obese with impaired fasting glucose (IFG) (glucose ≥5.6 and <7.0 mmol/l) (n = 31), and morbidly obese with type 2 diabetes (glucose ≥7.0 mmol/l) (n = 21). None of the morbidly obese individuals with type 2 diabetes were receiving insulin therapy. All the patients underwent bariatric surgery with mixed techniques, combining gastric reduction with an intestinal bypass, biliopancreatic diversion (n = 50), or gastric bypass (n = 23). All of the participants gave their informed consent, and the study was reviewed and approved by the ethics and research committees of Carlos Haya Regional University Hospital and the Virgen de la Victoria Clinical University Hospital (Malaga, Spain).
Intravenous glucose tolerance test
An intravenous glucose tolerance test (IVGTT) was performed in the morbidly obese individuals before bariatric surgery and 7 months afterward and in the control subjects (11). The insulin sensitivity index (SI) and the acute insulin response to glucose (AIRg) were calculated after introduction of the results for glucose and insulin obtained during the IVGTT into the MINMOD program. The elimination of glucose during the IVGTT, or the glucose tolerance index, was expressed as KG (12). The glucose increase above baseline (above fasting) was expressed as the area under the curve (AUCGlu) and calculated using the trapezoidal method.
Laboratory measurements
Serum biochemical parameters were measured in duplicate. Serum glucose, triglycerides, cholesterol, and HDL cholesterol (Randox Laboratories, Antrium, U.K.) were measured by standard enzymatic methods. LDL cholesterol was calculated from the Friedenwald equation. Insulin was analyzed by an immunoradiometric assay (BioSource International, Camarillo, CA). High-sensitivity C-reactive protein was analyzed by enzyme immunoassay (ELISA) kits (BLK Diagnostics, Barcelona, Spain). The concentration of oxLDL was analyzed by a solid-phase two-site ELISA (Mercodia, Uppsala, Sweden). The intra- and inter-assay coefficients of variation (CVs) were 6.4 and 4.7%, respectively. The sensitivity of the technique was <1 mU/l.
Anti-oxLDL antibodies
Anti-oxLDL antibodies were measured in duplicate, as has been previously described (13). In brief, LDL was isolated from fasting plasma from human blood donors by density gradient ultracentrifugation. OxLDL was prepared by incubating this native LDL with MDA (MDA-LDL). Microtiter plates for determination of IgM and IgG anti–MDA-LDL antibodies were coated with either native LDL or MDA-LDL and the serum of each patient. The binding to native LDL was considered as nonspecific binding. The absorbance was read, and the binding of antibodies to MDA-LDL (anti-oxLDL antibodies) was calculated by subtracting the binding of native LDL from the binding of MDA-LDL. The results were expressed as an optical density. The intra- and inter-assay CVs were 5.0 and 10.1%, respectively.
Statistical analysis.
The statistical analysis was done with SPSS (version 11.5 for Windows; SPSS, Chicago, IL). Results of the different groups were compared with one-way ANOVA, and the post hoc analysis was done with Duncan's multiple range test. The differences in the various study variables within the same group before and after bariatric surgery were compared with Student's t test for paired samples. Pearson's correlation coefficients were calculated to estimate the linear correlations between variables. Multiple regression analysis was used to study which variables were associated with the variability of the anti-oxLDL antibodies. Values were considered to be statistically significant when P ≤ 0.05. The results are given as means ± SD.
RESULTS
Table 1 summarizes the anthropometric and biochemical characteristics of the three groups of morbidly obese patients, before and after bariatric surgery. No significant differences were found between any of the anthropometric variables studied in the different groups of morbidly obese patients.
Table 1.
Anthropometric and biochemical variables of the morbidly obese patients before and after bariatric surgery
| Control | Morbidly obese: preoperative |
Morbidly obese: postoperative |
|||||
|---|---|---|---|---|---|---|---|
| NFG | IFG | Diabetes | NFG | IFG | Diabetes | ||
| n (men/women) | 11 (3/8) | 21 (7/14) | 31 (10/21) | 21 (7/14) | |||
| Age (years) | 39.9 ± 6.5 | 39.5 ± 11.0 | 44.1 ± 10.6 | 44.5 ± 7.4 | |||
| Weight (kg) | 67.6 ± 10.2b | 145.2 ± 33.1a | 150.9 ± 24.5a | 145.0 ± 22.4a | 95.9 ± 17.5‡ | 104.5 ± 17.8‡ | 100.0 ± 11.0‡ |
| BMI (kg/m2) | 24.1 ± 2.2b | 52.2 ± 8.1a | 55.6 ± 6.5a | 54.0 ± 7.1a | 34.5 ± 4.6‡ | 38.7 ± 5.9‡ | 37.4 ± 4.7‡ |
| Waist circumference (cm) | 84.8 ± 8.7b | 139.9 ± 21.6a | 143.3 ± 18.1a | 141.9 ± 13.9a | 104.9 ± 13.6‡ | 111.5 ± 14.1‡ | 114.1 ± 10.9‡ |
| Systolic blood pressure (mmHg) | 121.0 ± 3.7b | 136.0 ± 15.1a | 134.5 ± 18.1a,b | 147.4 ± 21.8a | 131.1 ± 17.5 | 128.8 ± 12.8 | 137.0 ± 25.7 |
| Diastolic blood pressure (mmHg) | 69.3 ± 6.0b | 82.7 ± 10.1a | 81.8 ± 13.0a | 82.6 ± 10.7a | 77.7 ± 11.1 | 78.6 ± 12.7 | 83.4 ± 12.5 |
| Glucose (mmol/l) | 4.66 ± 0.33c | 5.13 ± 0.29c | 5.94 ± 0.39b | 9.57 ± 2.46a | 4.72 ± 0.45 | 4.80 ± 0.50 | 4.90 ± 0.68‡ |
| Insulin (pmol/l) | 55.0 ± 19.5b | 171.6 ± 118.0a | 188.3 ± 89.8a | 220.1 ± 112.2a | 61.5 ± 24.3† | 74.4 ± 29.7‡ | 87.6 ± 41.3‡ |
| Cholesterol (mmol/l) | 4.88 ± 0.98b | 4.76 ± 0.85b | 5.15 ± 0.83a,b | 5.41 ± 0.59a | 3.55 ± 0.77† | 3.68 ± 0.96‡ | 3.93 ± 1.06* |
| LDL cholesterol (mmol/l) | 2.85 ± 0.96 | 2.98 ± 0.75 | 3.29 ± 0.83 | 3.26 ± 0.59 | 2.07 ± 0.59* | 2.02 ± 0.85‡ | 2.25 ± 0.88* |
| HDL cholesterol (mmol/l) | 1.55 ± 0.44a | 1.16 ± 0.36b | 1.24 ± 0.34b | 1.06 ± 0.21b | 1.06 ± 0.34* | 1.11 ± 0.33 | 1.01 ± 0.26 |
| Triglycerides (mmol/l) | 0.99 ± 0.71b | 1.23 ± 0.71b | 1.43 ± 0.80b | 2.51 ± 2.04a | 0.91 ± 0.30 | 1.20 ± 0.43 | 1.43 ± 0.51‡ |
| C-reactive protein (mg/l) | 1.06 ± 0.44b | 2.41 ± 1.04a | 2.92 ± 0.92a | 2.90 ± 0.713a | 1.42 ± 1.21 | 1.54 ± 0.95* | 1.23 ± 1.08‡ |
| OxLDL (units/l) | 69.8 ± 19.1b | 67.0 ± 16.6b | 83.4 ± 45.1a,b | 100.0 ± 38.1a | 47.7 ± 12.9† | 51.7 ± 25.2‡ | 64.8 ± 24.0† |
| OxLDL-to-LDL ratio (units/mmol) | 22.7 ± 5.0b | 27.4 ± 6.9b | 25.8 ± 13.5a,b | 33.22 ± 10.4a | 25.1 ± 6.2 | 26.2 ± 12.3 | 30.8 ± 17.7 |
| IgG anti-oxLDL (OD) | 0.136 ± 0.056 | 0.144 ± 0.072 | 0.160 ± 0.064 | 0.155 ± 0.052 | 0.154 ± 0.076 | 0.161 ± 0.073 | 0.167 ± 0.067* |
| IgM anti-oxLDL (OD) | 0.128 ± 0.060a | 0.121 ± 0.046a | 0.127 ± 0.050a | 0.089 ± 0.034b | 0.138 ± 0.051 | 0.142 ± 0.053 | 0.116 ± 0.056† |
| AUCGlu (mmol · l−1 · h−1) | 12.9 ± 2.89b | 16.6 ± 4.4b | 16.5 ± 3.3b | 21.2 ± 6.2a | 11.6 ± 4.7 | 11.9 ± 3.9‡ | 16.2 ± 5.1 |
| AIRg (pmol · ml−1 · min−1) | 4.68 ± 3.40a | 4.67 ± 4.44a | 2.79 ± 2.43a,b | 0.34 ± 0.42b | 2.66 ± 1.34 | 3.75 ± 2.57* | 2.62 ± 1.50† |
| KG (%/min) | 1.65 ± 0.26a | 1.28 ± 0.43b | 0.83 ± 0.19c | 0.51 ± 0.27d | 1.60 ± 0.48 | 1.23 ± 0.41 | 1.21 ± 0.51 |
| SI (10−4 · min−1/[μU/ml]) | 7.57 ± 5.51a | 3.05 ± 3.86b | 1.41 ± 1.33b,c | 0.28 ± 0.51c | 7.92 ± 5.75* | 4.08 ± 2.81† | 5.45 ± 3.27† |
Data are means ± SD. Different letters indicate significant differences between the means of the different groups of preoperative morbidly obese patients and the control subjects (P < 0.05).
P < 0.05;
P < 0.01;
P < 0.001, significant differences within the same group of obese persons, before and after bariatric surgery. OD, optical density.
Bariatric surgery improves insulin sensitivity and secretion in morbidly obese patients
Table 1 shows the different variables related with glucose metabolism obtained from the IVGTT. Before surgery, the morbidly obese patients with type 2 diabetes had a significantly higher AUCGlu and a significantly lower AIRg and SI than the control subjects and the morbidly obese patients with IFG or NFG. Bariatric surgery was associated with a significant improvement in SI, AIRg, KG, and AUCGlu (Table 1), with the previous significant differences between the morbidly obese patients disappearing.
OxLDL levels are increased in diabetes and fall after bariatric surgery
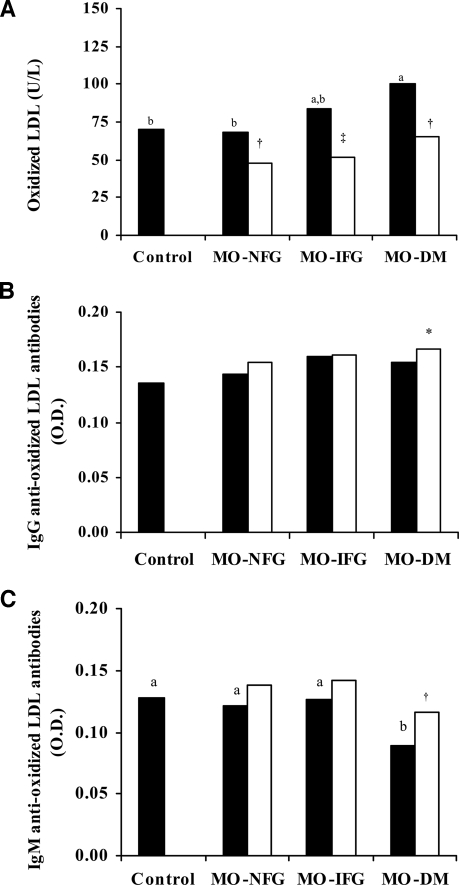
No significant differences were found in the levels of oxLDL according to sex (data not shown). Before bariatric surgery the levels of oxLDL and the oxLDL-to-LDL ratio were significantly higher in the morbidly obese patients with diabetes (P < 0.05) (Table 1, Fig. 1A). After the surgery, the levels of oxLDL fell significantly (Table 1, Fig. 1A), with the previous differences between the three groups of morbidly obese patients disappearing. The oxLDL-to-LDL ratio fell, but not significantly so, only in the morbidly obese individuals who had diabetes (Table 1), with the previous differences between the three groups of morbidly obese patients disappearing. This reduction was independent of the type of bariatric surgery undergone (data not shown).
Figure 1.
A: Levels of oxLDL in the control subjects and the morbidly obese patients before (▪) and after (□) surgery. IgG anti-oxLDL antibodies (B) and IgM anti-oxLDL antibodies (C) in the control subjects and the morbidly obese patients before (▪) and after (□) surgery. Different letters indicate significant differences between the means of the different groups of preoperative morbidly obese patients and the control subjects (P < 0.05). *P < 0.05; †P < 0.01; ‡P < 0.001, significant differences within the same group of obese persons, before and after bariatric surgery. DM, diabetes mellitus; MO, morbid obesity; O.D., optical density.
Levels of IgM anti-oxLDL antibodies are reduced in diabetes and rise after bariatric surgery
No significant differences were found in the levels of IgG or IgM anti-oxLDL antibodies according to sex (data not shown). Before surgery, no significant differences were seen in the levels of IgG anti-oxLDL antibodies between the three groups of morbidly obese patients and the control subjects (Table 1, Fig. 1B). However, the levels of IgM anti-oxLDL antibodies were significantly lower in the morbidly obese patients with type 2 diabetes (P < 0.05) (Table 1, Fig. 1C). After bariatric surgery, the levels of IgG anti-oxLDL antibodies rose significantly just in the group of morbidly obese patients who were diabetic before the surgery (P = 0.044) (Table 1, Fig. 1B). Although the levels of IgM anti-oxLDL antibodies rose in the three groups of morbidly obese patients, this increase was again only significant in those who had diabetes (P = 0.003) (Table 1, Fig. 1C). In no case were significant differences found according to the type of bariatric surgery undergone (data not shown).
Association between levels of oxidized LDL and study variables
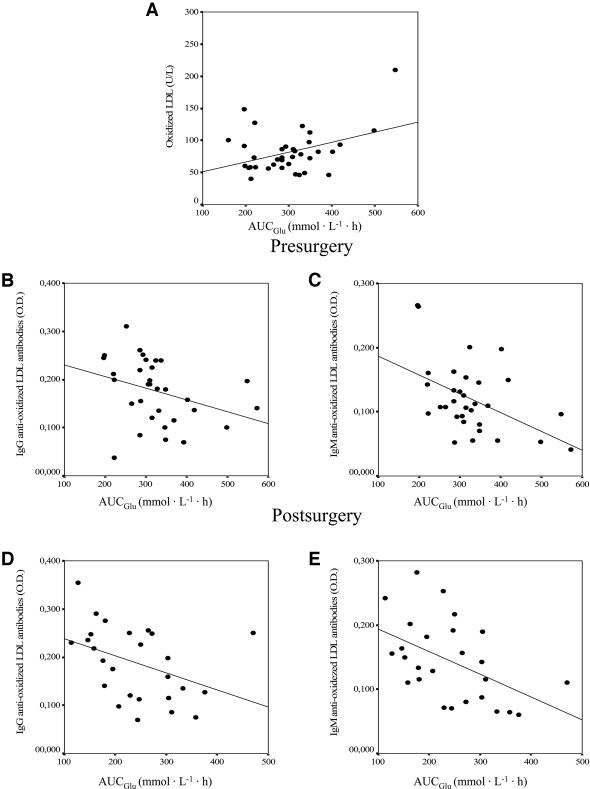
Before surgery, the levels of oxLDL correlated significantly and positively with glucose (r = 0.400, P = 0.004), cholesterol (r = 0.433, P = 0.005), LDL cholesterol (r = 0.286, P = 0.046), and AUCGlu (r = 0.401, P = 0.038) (Fig. 2A) and negatively with the SI (r = −0.432, P = 0.017) and the levels of IgM anti-oxLDL antibodies (r = −0.352, P = 0.012). The variable that best explained the levels of oxLDL before surgery in a multiple linear regression model was the AUCGlu (P = 0.038; r2 = 0.161). This association remained after adjustment of the model for age, weight, BMI, cholesterol, HDL cholesterol, LDL cholesterol, glucose, insulin, AIRg, KG, SI, and the levels of IgG and IgM anti-oxLDL antibodies. After the surgery, the levels of oxLDL correlated significantly with cholesterol (r = 0.407, P = 0.010), LDL cholesterol (r = 0.294, P = 0.047), and glucose (r = 0.352, P = 0.026). None of the study variables explained the variance in the levels of oxLDL after surgery.
Figure 2.
A: Association between presurgery levels of oxLDL and the AUCGlu (r = 0.401, P = 0.038). B: Association between the presurgery levels of IgG anti-oxLDL antibodies and the AUCGlu (r = −0.328, P = 0.047). C: Association between the presurgery levels of IgM anti-oxLDL antibodies and the AUCGlu (r = −0.475, P = 0.006). D: Association between the postsurgery levels of IgG anti-oxLDL antibodies and the AUCGlu (r = −0.403, P = 0.041). E: Association between the postsurgery levels of IgM anti-oxLDL antibodies and the AUCGlu (r = −0.487, P = 0.012). O.D., optical density.
Levels of anti-oxidized LDL antibodies are mainly associated with glucose metabolism
Before the surgery, the levels of IgG anti-oxLDL antibodies correlated positively with the waist circumference (r = 0.321, P = 0.010) and negatively with AUCGlu (r = −0.328, P = 0.047) (Fig. 2B). The variable that best explained the levels of IgG anti-oxLDL antibodies before surgery in a multiple linear regression model was insulin (P = 0.026; r2 = 0.134). This association remained after adjustment of the model for age, weight, BMI, cholesterol, LDL cholesterol, HDL cholesterol, glucose, AUCGlu, AIRg, KG, SI, and oxLDL.
Before surgery, the levels of IgM anti-oxLDL antibodies correlated negatively with glucose (r = −0.335, P = 0.004), oxLDL (r = −0.352, P = 0.012), and AUCGlu (r = −0.475, P = 0.006) (Fig. 2C) and positively with KG (r = 0.485, P = 0.005) and the SI (r = 0.508, P = 0.001). The variables that best explained the levels of IgM anti-oxLDL antibodies in a multiple linear regression model were SI (P = 0.027) and the AUCGlu (P = 0.048) (r2 = 0.376). This association remained after adjustment of the model for age, weight, BMI, cholesterol, LDL cholesterol, HDL cholesterol, glucose, insulin, AIRG, KG, and oxLDL.
After surgery, the levels of IgG anti-oxLDL antibodies correlated with AUCGlu (r = −0.403, P = 0.041) (Fig. 2D) and positively with KG (r = 0.385, P = 0.042) and SI (r = 0.367, P = 0.045). The variables that best explained the levels of IgG anti-oxLDL antibodies in a multiple linear regression model were age (P = 0.005) and AUCGlu (r2 = 0.516, P = 0.011). This association remained after adjustment of the model for weight, BMI, cholesterol, LDL cholesterol, HDL cholesterol, glucose, insulin, AIRg, KG, SI, and oxLDL.
After surgery, the levels of IgM anti-oxLDL antibodies correlated significantly with AUCGlu (r = −0.487, P = 0.012) (Fig. 2E) and KG (r = 0.455, P = 0.019). The variable that best explained the levels of IgM anti-oxLDL antibodies in a multiple linear regression model was the AUCGlu (r2 = 0.196, P = 0.035). This association remained after adjustment of the model for age, weight, BMI, cholesterol, LDL cholesterol, HDL cholesterol, glucose, insulin, AIRg, KG, SI, and oxLDL.
CONCLUSIONS
In this study we found that the levels of IgM anti-oxLDL antibodies were significantly reduced in morbidly obese patients with diabetes and that they were inversely associated with the levels of oxLDL. However, the main finding was the improvement noted in carbohydrate metabolism as a result of bariatric surgery, accompanied by an increase in the levels of IgG and IgM anti-oxLDL antibodies and a reduction in the levels of oxLDL, especially in those morbidly obese patients who had diabetes.
Reports of LDL oxidizability in obesity already exist. However, whereas some studies found a direct relation between LDL oxidation and BMI (14), in our study, as in others, no association was found between LDL oxidation and obesity (15). Indeed, the morbidly obese patients with normal fasting glucose or IFG had oxLDL levels similar to those of the nonobese, healthy individuals. However, the morbidly obese patients who had diabetes had significantly higher levels of oxLDL. These results indicate that morbid obesity per se does not lead to increased levels of oxLDL. Nevertheless, recent studies have shown an association between obesity and LDL oxidation (14).
Oxidative stress is generally accepted to be a common biological feature in the various metabolic alterations occurring in diabetes. We have previously shown a direct association between increased insulin resistance and increased oxidative stress in patients with morbid obesity (16). Among the various molecular targets probably affected by lipid peroxidation during oxidative stress in diabetes, LDL is one of the most important. Our results thus highlight the fact that when serum glucose concentrations increase, the levels of oxLDL and the oxLDL-to-LDL ratio also increase, significantly so in morbidly obese patients with type 2 diabetes, even though they have a concentration of LDL similar to that of the other morbidly obese patients.
As far as we are aware, this is the only study showing that levels of oxLDL are significantly reduced in morbidly obese patients 7 months after bariatric surgery with restrictive techniques, irrespective of whether the patients had diabetes or not. Data on the effects of weight reduction on LDL oxidation in vivo are scare (15,17). However, we should recall that the reduction in oxLDL noted in our study was the consequence of the important and significant fall in levels of LDL after bariatric surgery. Even so, the reductions in oxLDL and the oxLDL-to-LDL ratio were greater in the morbidly obese patients with diabetes, such that the earlier differences present between the three groups disappeared after the surgery. Others reported a similar finding (17). These findings indicate that the improvement in carbohydrate metabolism after bariatric surgery contributes to a reduction in LDL oxidation. This idea is reinforced by other studies showing that bariatric surgery may have a positive effect on oxidative stress, reducing the concentration of oxidized lipids (15).
Measurement of anti-oxLDL antibodies has been proposed as an indirect marker of LDL oxidation in vivo. Nevertheless, not all authors agree (6,8,18). In addition, some studies have shown that anti-oxLDL antibodies are lower in individuals with insulin-dependent diabetes with microvascular complications than in those without these complications (8). Our results in patients with morbid obesity are in line with those of a prospective study undertaken in the general population, which showed that low levels of anti-oxLDL antibodies were predictive of the development of type 2 diabetes (6). In this study we showed that IgM anti-oxLDL antibodies are significantly reduced in morbidly obese patients with diabetes, with no significant differences between the control subjects and the morbidly obese patients who were not diabetic, as has been reported in other studies (18).
Very few studies have examined the evolution of anti-oxLDL antibodies over time in different disorders (6). Although the association between diabetes and anti-oxLDL antibodies is becoming clearer, this is the first study to show the evolution of these antibodies in the opposite situation, i.e., an improvement in carbohydrate metabolism. We show clearly that after bariatric surgery the levels of IgG and IgM anti-oxLDL antibodies are raised, particularly and significantly so in morbidly obese diabetic patients in whom diabetes disappears. In fact, the original differences between the various study groups disappeared. These results confirm that an improvement in glucose metabolism plays an important role in the reduction in oxLDL levels and in the increase in anti-oxLDL antibody levels.
The mechanism explaining this inverse relation between diabetes and anti-oxLDL antibody levels is unknown. One suggestion is that patients with diabetes experience greater plasma clearance of these antibodies owing to different mechanisms. One of these might be the binding of these antibodies to their antigens (oxLDL) and the formation of immune complexes. Previous studies have shown the presence of an inverse association between levels of anti-oxLDL antibodies and immune complexes (8). This possible mechanism is reinforced by our results, which show an inverse association between levels of anti-oxLDL antibodies and oxLDL, as was also seen by others (19). Another possible mechanism may be a greater subendothelial retention. Several studies have suggested that these antibodies may bind to macrophages, thereby inhibiting oxLDL binding (20). After the surgery, the diabetic patients experienced a normalization of their carbohydrate metabolism and a reduction in oxLDL levels. These results, together with a possible reduction in the subendothelial retention of those anti-oxLDL antibodies, which inhibit the binding of the oxLDL to the macrophages (20), could increase the plasma levels of these antibodies. This mechanism, though, still needs to be demonstrated in other experimental studies.
Insulin resistance is an important component of the metabolic abnormalities associated with diabetes (11). It seemed to be of great interest to determine whether there were any associations between insulin resistance and the oxidative states of circulating LDL and the levels of anti-oxLDL antibodies. In a state of insulin resistance, LDL is more easily oxidized (10). However, Ho et al. (21) found that SI is only weakly associated with oxLDL. Another study showed that in vitro LDL oxidizability is only increased in patients with noninsulin-dependent diabetes, not in healthy individuals or in individuals with impaired glucose tolerance (17). The authors suggested that hyperglycemia, rather than insulin resistance, is associated with LDL oxidizability. As we have found, the main factor responsible for the variation in levels of oxLDL and anti-oxLDL antibodies is AUCGlu. Other studies have reported similar observations, showing a significant association between the plasma glucose response to oral glucose and oxLDL (10). Others have shown that postprandial hyperglycemia is associated with the generation of oxidative stress, which is strictly dependent on the level of glycemia reached (22).
A limitation of this study concerns the lack of a dietary questionnaire before and after the bariatric surgery. The variable intake of fatty acids affects the level of anti-oxLDL antibodies (23). However, even though the participants did not complete a dietary questionnaire, they were all prescribed the same low-calorie diet both before and after the surgery.
In summary, we show that type 2 diabetes in morbidly obese patients is associated with a reduction in levels of IgM anti-oxLDL antibodies and an increase in levels of oxLDL, with the two variables being inversely related. As far as we know, this is the only study showing that improvement in carbohydrate metabolism after bariatric surgery results in increased levels of IgG and IgM anti-oxLDL antibodies. This increase coincides with the reduction in levels of oxLDL. We have thus shown that postprandial hyperglycemia, measured here by an IVGTT, is responsible for the variation in levels of anti-oxLDL antibodies and oxLDL. This result would imply a beneficial effect of control of hyperglycemia on oxidative stress in patients with morbid obesity.
Acknowledgments
This work was supported in part by a grant from the Instituto de Salud Carlos III (CP04/00133) and Servicio Andaluz de Salud (0438/2006). CIBER Fisiopatología de la Obesidad y Nutrición (CB06/03) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) are ISCIII projects.
We thank Ian Johnstone for the English language version of the text.
Published ahead of print at http://care.diabetesjournals.org on 3 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ: Framingham Study: Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23:434–439, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Regnström J, Nilsson J, Tornvall P, Landou C, Hamsten A: Susceptibility to low-density lipoprotein oxidation and coronary atherosclerosis in man. Lancet 339:1183–1186, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Lehtimäki T, Lehtinen S, Solakivi T, Nikkilä M, Jaakkola O, Jokela H, Ylä-Herttuala S, Luoma JS, Koivula T, Nikkari T: Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease. Aterioscler Thromb Vasc Biol 19:23–27, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Rossi GP, Cesari M, De Toni R, Zanchetta M, Maiolino G, Pedon L, Ganzaroli C, Maiolino P, Pessina AC: Antibodies to oxidized low-density lipoproteins and angiographically assessed coronary artery disease in white patients. Circulation 108:2467–2472, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S: Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 108:2107–2112, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Garrido-Sánchez L, Cardona F, García-Fuentes E, Rojo-Martínez G, Gómez-Zumaquero JM, Picón MJ, Soriguer F, Tinahones FJ: Anti-oxidized low-density lipoprotein antibody levels are associated with the development of type 2 diabetes mellitus. Eur J Clin Invest 38:615–621, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Tinahones FJ, Gómez-Zumaquero JM, Rojo-Martínez G, Cardona F, Esteva de Antonio IE, Ruiz de Adana MS, Soriguer FJ: Increased levels of anti-oxidized low-density lipoprotein antibodies are associated with reduced levels of cholesterol in the general population. Metabolism 51:429–431, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Festa A, Kopp HP, Schernthaner G, Menzel EJ: Autoantibodies to oxidised low density lipoproteins in IDDM are inversely related to metabolic control and microvascular complications. Diabetologia 41:350–356, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK: LDL immunization induces T-cell–dependent antibody formation and protection against atherosclerosis. Aterioscler Thromb Vasc Biol 21:108–114, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Carantoni M, Abbasi F, Warmerdam F, Klebanov M, Wang PW, Chen YD, Azhar S, Reaven GM: Relationship between insulin resistance and partially oxidized LDL particles in healthy, nondiabetic volunteers. Arterioscler Thromb Vasc Biol 18:762–767, 1998 [DOI] [PubMed] [Google Scholar]
- 11.García-Fuentes E, García-Almeida JM, García-Arnés J, Rivas-Marín J, Gallego-Perales JL, González-Jiménez B, Cardona I, García-Serrano S, Garriga MJ, Gonzalo M, Ruiz de Adana MS, Soriguer F: Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg 16:1179–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bergman RN: Lilly Lecture: Toward physiological understanding of glucose tolerance: minimal-model approach. Diabetes 38:1512–1527, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Tinahones FJ, Gómez-Zumaquero JM, Garrido-Sánchez L, García-Fuentes E, Rojo-Martínez G, Esteva I, Ruiz de Adana MS, Cardona F, Soriguer F: Influence of age and sex on levels of anti-oxidized LDL antibodies and anti-LDL immune complexes in the general population. J Lipid Res 46:452–457, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Weinbrenner T, Schröder H, Escurriol V, Fito M, Elosua R, Vila J, Marrugat J, Covas MI: Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr 83:30–35, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Uzun H, Zengin K, Taskin M, Aydin S, Simsek G, Dariyerli N: Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes Surg 14:659–665, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Tinahones FJ, Murri-Pierri M, Garrido-Sánchez L, García-Almeida JM, García-Serrano S, García-Arnés J, García-Fuentes E: Oxidative stress in morbidly obese persons is greater in those with insulin resistance. Obesity. In press [DOI] [PubMed]
- 17.Linna MS, Borg P, Kukkonen-Harjula K, Fogelholm M, Nenonen A, Ahotupa M, Vasankari TJ: Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int J Obes (Lond) 31:245–253, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Mironova MA, Klein RL, Virella GT, Lopes-Virella MF: Anti-modified LDL antibodies, LDL-containing immune complexes, and susceptibility of LDL to in vitro oxidation in patients with type 2 diabetes. Diabetes 49:1033–1041, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Shoji T, Nishizawa Y, Fukumoto M, Shimamura K, Kimura J, Kanda H, Emoto M, Kawagishi T, Morii H: Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis 148:171–177, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL: Human derived anti oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol 21:1333–1339, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ho RC, Davy K, Davy B, Melby CL: Whole-body insulin sensitivity, low-density lipoprotein (LDL) particle size, and oxidized LDL in overweight, nondiabetic men. Metabolism 51:1478–1483, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Ceriello A, Quagliaro L, Catone B, Pascon R, Piazzola M, Bais B, Marra G, Tonutti L, Taboga C, Motz E: Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care 25:1439–1443, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Garrido-Sánchez L, García-Fuentes E, Rojo-Martínez G, Cardona F, Soriguer F, Tinahones FJ: Inverse relation between levels of anti-oxidized-LDL antibodies and eicosapentanoic acid (EPA). Br J Nutr 6:1–5, 2008 [DOI] [PubMed] [Google Scholar]