Abstract
OBJECTIVE—Bedtime administration of 5.0 mg of the β2-adrenergic agonist terbutaline prevents nocturnal hypoglycemia but causes morning hyperglycemia in type 1 diabetes. We tested the hypothesis that 2.5 mg terbutaline prevents nocturnal hypoglycemia without causing morning hyperglycemia.
RESEARCH DESIGN AND METHODS—This was a randomized double-blind crossover pilot study (placebo, 2.5 mg terbutaline, and 5.0 mg terbutaline) in 15 patients with type 1 diabetes.
RESULTS—Mean ± SE nadir nocturnal plasma glucose concentrations were 87 ± 14 mg/dl following placebo, 100 ± 14 mg/dl following 2.5 mg terbutaline, and 122 ± 13 mg/dl following 5.0 mg terbutaline (P < 0.05 vs. placebo). Nadir levels were <50 mg/dl in 5, 2, and 0 patients (P < 0.05 vs. placebo), respectively. Morning levels were 113 ± 18, 127 ± 17, and 183 ± 19 mg/dl (P < 0.02 vs. placebo), respectively.
CONCLUSIONS—Terbutaline may be shown to be effective and safe in the prevention of nocturnal hypoglycemia in type 1 diabetes in a suitably powered randomized controlled trial.
Iatrogenic hypoglycemia is the limiting factor in the glycemic management of diabetes (1). Most episodes of hypoglycemia occur at night, specifically during sleep, in type 1 diabetes—a finding in the Diabetes Control and Complications Trial (2) that continues to be documented (3,4). Sympathoadrenal responses to hypoglycemia are reduced further during sleep (5,6), and, probably because of their markedly reduced sympathoadrenal responses, patients with type 1 diabetes are substantially less likely to be awakened by hypoglycemia than nondiabetic individuals (6,7).
Among the approaches to the prevention of nocturnal hypoglycemia in type 1 diabetes, we found bedtime administration of a conventional snack, uncooked cornstarch, or an α-glucosidase inhibitor to be ineffective (3). In contrast, bedtime administration of the epinephrine-simulating β2-adrenergic agonist terbutaline in a dose of 5.0 mg prevented nocturnal hypoglycemia (3). However, it also caused hyperglycemia the following morning. Therefore, we used a randomized double-blind crossover design (placebo, 2.5 mg terbutaline, and 5.0 mg terbutaline) in a pilot study to test the hypothesis that bedtime administration of 2.5 mg terbutaline prevents nocturnal hypoglycemia without causing morning hypoglycemia in patients with aggressively treated type 1 diabetes.
RESEARCH DESIGN AND METHODS
Fifteen patients (seven women) with type 1 diabetes gave their written consent to participate in this study, which was approved by the Washington University Human Research Protection Office and conducted at the institution's General Clinical Research Center. Mean ± SD age was 28.6 ± 7.5 years, BMI 29.3 ± 5.6 kg/m2, duration of type 1 diabetes 14.9 ± 7.0 years, and A1C 7.1 ± 0.5%. Subjects were selected for an A1C ≤8.0% and the absence of diabetes complications or use of a potentially interfering medication. Nine subjects were using continuous subcutaneous insulin infusion with insulin analogs, and six were using multiple daily injection with insulin analogs (aside from one using basal NPH insulin and one using prandial regular insulin).
As in our earlier study (3), the patients pursued their usual activities and used their individual treatment regimens with guidance from their individual caregivers throughout the study. They were admitted to the Washington University General Clinical Research Center early in the evening on three occasions. Venous blood samples for plasma glucose measurements (YSI Glucose Analyzer; Yellow Springs Instruments, Yellow Springs, OH) were drawn at 15-min intervals from 2200 h through 0700 h. Glucose levels <40 mg/dl were treated with small doses of intravenous glucose (3).
One of three oral bedtime treatments was administered, in random sequence and in double-blind fashion, at 2200 h. These included placebo, 2.5 mg terbutaline (Brethine; Novartis Pharmaceuticals, East Hanover, NJ), and 5.0 mg terbutaline.
Statistical methods
Data are expressed as means ± SE except where SD is specified. Time- and condition-related data were analyzed by mixed-model repeated-measures ANOVA. Contrasts of interest were assessed with a t test. P values < 0.05 were considered to indicate statistically significant differences.
RESULTS
Bedtime administration of 5.0 mg terbutaline, but not 2.5 mg, raised mean plasma glucose concentrations during the night (ANOVA P < 0.01) (data not shown). Nadir nocturnal plasma glucose concentrations were <70 mg/dl in seven patients (47%), <60 mg/dl in six (40%), <50 mg/dl in five (33%), and <40 mg/dl in two (13%) following bedtime placebo. Corresponding nadir nocturnal concentrations were seen in seven, six, two, and zero patients, respectively, following administration of 2.5 mg terbutaline and in three, zero (P < 0.02 vs. placebo), zero (P < 0.05 vs. placebo), and zero patients following administration of 5.0 mg terbutaline.
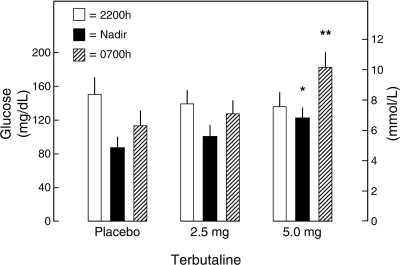
Mean nadir plasma glucose concentrations were 87 ± 14 mg/dl following placebo, 100 ± 14 mg/dl following 2.5 mg terbutaline, and 122 ± 13 mg/dl following 5.0 mg terbutaline (P < 0.05 vs. placebo) (Fig. 1). Mean 0700 h glucose levels were 113 ± 18, 127 ± 17, and 183 ± 19 mg/dl (P < 0.02 vs. placebo), respectively (Fig. 1). Mean 0700 h heart rates were 78 ± 5, 82 ± 4, and 88 ± 5 bpm (P < 0.02 vs. placebo, respectively). Terbutaline was seemingly well tolerated.
Fig. 1.
Mean ± SE bedtime (2200 h), nocturnal nadir, and morning (0700 h) plasma glucose concentrations following bedtime oral administration of placebo, 2.5 mg terbutaline, or 5.0 mg terbutaline in 15 patients with type 1 diabetes. *P < 0.05 vs. placebo. **P < 0.02 vs. placebo.
CONCLUSIONS
These data confirm a high frequency of nocturnal hypoglycemia in patients with aggressively treated type 1 diabetes (1–4). In the absence of an active bedtime treatment (placebo administration), nadir nocturnal plasma glucose concentrations were <70 mg/dl (3.9 mmol/l), the alert value recommended by the American Diabetes Association Workgroup on Hypoglycemia (8), in 7 of 15 patients (47%). They were <60 mg/dl (3.3 mmol/l) in six patients (40%), <50 mg/dl (2.8 mmol/l) in five patients (33%), and <40 mg/dl (2.2 mmol/l) in two patients (13%).
These data also confirm that bedtime administration of 5.0 mg of the epinephrine-simulating β2-adrenergic agonist terbutaline effectively prevents nocturnal hypoglycemia in patients with aggressively treated type 1 diabetes (3) and that dose of terbutaline increased plasma glucose concentrations throughout the night, raised the nocturnal nadir plasma glucose concentration significantly, and eliminated nocturnal plasma glucose concentrations <60 mg/dl. However, as in our earlier study (3), it caused hyperglycemia the following morning.
Here, we tested the hypothesis that bedtime administration of 2.5 mg terbutaline prevents nocturnal hypoglycemia without causing hyperglycemia the following morning. That hypothesis was not confirmed statistically in this small sample. However, the key efficacy end points, the number of patients with nocturnal plasma glucose concentrations <50 mg/dl and the mean nadir nocturnal plasma glucose concentration, were intermediate between those taking placebo at bedtime and those taking 5.0 mg terbutaline at bedtime. Documentation of the efficacy and safety of bedtime administration of terbutaline in the prevention of nocturnal hypoglycemia in patients with type 1 diabetes will require a suitably powered randomized controlled trial of relatively long-term terbutaline administration.
Acknowledgments
This study was supported, in part, by National Institutes of Health grants R37 DK27085, MO1 RR00036, and P60 DK20579 and a fellowship award from the American Diabetes Association.
The authors acknowledge the skilled assistance of the staff of the Washington University General Clinical Research Center and the assistance of Janet Dedeke in the preparation of this manuscript.
Published ahead of print at http://care.diabetesjournals.org on 9 September 2008.
P.E.C. has consulted for Merck & Co., Marcadia Biotech, Novo Nordisk, Johnson & Johnson, MannKind, Medtronic MiniMed, Takeda, and TolerRx in recent years.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Cryer PE: Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 350:2272–2279, 2004 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group: Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 46:271–286, 1997 [PubMed] [Google Scholar]
- 3.Raju B, Arbelaez AM, Breckenridge SM, Cryer PE: Nocturnal hypoglycemia in type 1 diabetes: an assessment of preventive bedtime treatments. J Clin Endocrinol Metab 91:2087–2092, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Wentholt IME, Maran A, Masurel N, Heine RJ, Hoekstra JBL, DeVries JH: Nocturnal hypoglycaemia in type 1 diabetic patients, assessed with continuous glucose monitoring: frequency, duration and associations. Diabet Med 24:527–532, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Jones TW, Porter P, Sherwin RS, Davis EA, O'Leary P, Frazer F, Byrne G, Stick S, Tamborlane WV: Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 338:1657–1662, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Banarer S, Cryer PE: Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes 52:1195–1203, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Schultes B, Jauch-Chara K, Gais S, Hallschmid M, Reiprich E, Kern W, Oltmanns KM, Peters A, Fehm HL, Born J: Defective awakening response to nocturnal hypoglycemia in patients with type 1 diabetes mellitus. PLoS Medicine 4:e69, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association Workgroup on Hypoglycemia: Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 28:1245–1249, 2005 [DOI] [PubMed] [Google Scholar]