Abstract
OBJECTIVE—Gestational diabetes mellitus (GDM) is an increasingly prevalent risk factor for the development of type 2 diabetes in the mother and is responsible for morbidity in the child. To better identify women at risk of developing GDM we examined sociodemographic correlates and changes in the prevalence of GDM among all births between 1995 and 2005 in Australia's largest state.
RESEARCH DESIGN AND METHODS—A computerized database of all births (n = 956,738) between 1995 and 2005 in New South Wales, Australia, was used in a multivariate logistic regression that examined the association between sociodemographic characteristics and the occurrence of GDM.
RESULTS—Between 1995 and 2005, the prevalence of GDM increased by 45%, from 3.0 to 4.4%. Women born in South Asia had the highest adjusted odds ratio (OR) of any region (4.33 [95% CI 4.12–4.55]) relative to women born in Australia. Women living in the three lowest socioeconomic quartiles had higher adjusted ORs for GDM relative to women in the highest quartile (1.54 [1.50–1.59], 1.74 [1.69–1.8], and 1.65 [1.60–1.70] for decreasing socioeconomic status quartiles). Increasing age was strongly associated with GDM, with women aged >40 years having an adjusted OR of 6.13 (95% CI 5.79–6.49) relative to women in their early 20s. Parity was associated with a small reduced risk. There was no association between smoking and GDM.
CONCLUSIONS—Maternal age, socioeconomic position, and ethnicity are important correlates of GDM. Future culturally specific interventions should target prevention of GDM in these high-risk groups.
Type 2 diabetes affects an estimated 246 million individuals worldwide—a figure that is predicted to increase to 380 million by 2025, with a disproportionate number of affected individuals living in lower- and middle-income countries of the Asia-Pacific region (1). Diabetes is a major cardiovascular risk factor, more than doubling the risk of having a stroke or heart attack. Moreover, diabetes appears to be particularly hazardous in women, as there is a 50% greater risk of dying from coronary heart disease compared with that of men with the same condition (2).
Gestational diabetes mellitus (GDM), defined as glucose intolerance first detected during pregnancy, is a strong predictor of type 2 diabetes. Women with GDM are up to six times more likely to develop type 2 diabetes than women with normal glucose tolerance in pregnancy (3). The incidence of GDM varies among populations, similar to the variation of type 2 diabetes, with recent prevalence estimates ranging from 2.8% of pregnant women in Washington, DC, to 18.9% in India and 22% in Sardinia, Italy (4). The risk for GDM increases with age, and incidence rates vary by ethnicity within a population, again similar to the risk for type 2 diabetes (4,5). There is also evidence that obesity, parity, smoking, and family history are risk factors for GDM (5). However, less is known regarding the sociodemographic distribution of GDM. Given the strong link between GDM and the subsequent risk of diabetes for the mother and the perinatal morbidity for mother and child—an association recently updated with findings of a continuous association of maternal glucose levels and adverse perinatal outcomes by the Hyperglycemia and Adverse Pregnancy Outcomes Study Cooperative Research Group (6)—a better understanding of the sociodemographic determinants of GDM may provide novel opportunities to reduce the incidence and to prevent the onset of type 2 diabetes in later life.
Most studies that have examined the etiology of GDM have been hospital based or have been based on samples of births in a particular region (4,5). There are currently no large, comprehensive population-wide urban and rural datasets that have been collected in an attempt to examine multiple risk factors for GDM over a number of years and no population-based studies outside the U.S. The New South Wales (NSW) Midwives Dataset has information on nearly 1 million births in the state of NSW during the period from 1995 to 2005 in a health system in which there is almost universal screening for GDM. This dataset was used to study the current and changing population rates of GDM and its associated sociodemographic risk factors in a large, ethnically diverse population of women.
RESEARCH DESIGN AND METHODS
This study was undertaken using data from the NSW Department of Health Midwives Data Collection (MDC) dataset collected between 1995 and 2005 (inclusive). The dataset records information collected at all births of >20 weeks’ gestation in NSW by attending midwives at public and private hospitals and home births.
Study design and assessment of GDM
Information on infant's date of birth, birth weight, maternal age, maternal country of origin, number of previous pregnancies of >20 weeks, maternal postcode, maternal smoking status (including average number of cigarettes smoked per day), presence of previously existing type 1 or type 2 diabetes, and presence of GDM was available. GDM status was assigned according to information recorded on the NSW MDC form that was collected at the time of birth. In Australia, the Australasian Diabetes in Pregnancy Society criteria for the diagnosis of GDM are usually applied. A 75-g oral glucose tolerance test is performed, with a fasting plasma glucose level of >5.5 mmol/l or a 2-h level >8.0 mmol/l being diagnostic for GDM. Most centers practice universal screening for GDM with an initial nonfasting 50-g glucose challenge at 26–28 weeks. If results of this test are positive (1-h glucose >7.8 mmol/l) or if there is strong clinical suspicion, an oral glucose tolerance test is performed (7). Maternal country of birth was categorized into regions according to the Health Outcomes and Information Statistical Toolkit, which is a collection of databases maintained by the Epidemiology and Surveillance Branch of the NSW Department of Health (8). The regions were as follows: Australia and New Zealand, Europe and North America, Northeast and Southeast Asia, South Asia, Middle East and North Africa, the Pacific, the rest of Africa, and the Caribbean and Central and South America. Socioeconomic status was assigned according to maternal postcode, using the index of advantage/disadvantage from the Australian Bureau of Statistics Socio-Economic Indexes For Areas (SEIFA) (9). Socioeconomic status was categorized into quartiles, ranked from highest to lowest. Maternal age was categorized as <20, 20–24, 25–29, 30–34, 35–39, and ≥40 years. The NSW MDC has been validated by comparison with hospital medical records in 1993 and 1998. In 1993, it was found that reporting of GDM could be improved (10) and the MDC form was subsequently redesigned and first used in 1998. A further validation study was undertaken in 1998, at which time there were excellent levels of agreement between MDC data and information obtained directly from medical records (99%), with high specificity and sensitivity (11).
Statistical analysis
Variables were compared using Student's t test for means and χ2 tests for proportions between women who had GDM and the whole population and between those who had GDM and those who did not have GDM. Women with identified preexisting type 1 or type 2 diabetes were excluded from the analysis. The relationship between each of the potential explanatory variables with GDM was tested using binary logistic regression, and the odds ratios (ORs) and 95% CIs were reported. Variables that were significantly associated with GDM were subsequently tested in multivariate logistic regression models. Hosmer-Lemeshow goodness-of fit tests and residual and influence analyses were performed. The explanatory variables tested were socioeconomic status, maternal age-group, smoking status, parity, and region of maternal country of birth. The reference group for each variable was of the highest socioeconomic status, aged 20–24 years, nonsmoking in the third trimester, and born in Australia or New Zealand and had no previous pregnancies >20 weeks. Analyses were carried out using SAS (version 9.1; SAS Institute, Cary, NC).
RESULTS
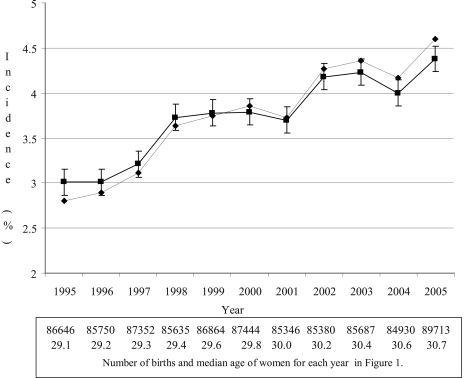
In the years 1995–2005 there were 957,982 births in NSW. Women with type 1 or type 2 diabetes detected before pregnancy (0.47%) and women with no information on age (0.4%), smoking status (0.1%), or parity (0.02%) were excluded from the analysis. Hence, the analyses were based on 950,747 births. Annual crude and adjusted incidences of GDM are shown in Fig. 1. For each year, women with GDM were on average older, had a lower prevalence of cigarette smoking, and were more likely to have had a previous pregnancy compared with women without GDM (P < 0.001). Overall, the age- and ethnicity-adjusted incidence of GDM increased by 45% from 3.0 to 4.4% between 1995 and 2005 (Fig. 1).
Figure 1.
Annual crude and adjusted incidence of GDM, number of births, and annual median age of women. ♦, crude incidence; ▪, age- and ethnicity-adjusted incidence and 95% CI.
Risk factors for GDM
The associations of different risk factors with GDM adjusted for all other recorded risk factors are shown in Table 1. Age was strongly and positively associated with risk of GDM and increased with each successive age-group. Compared with women aged 20–24 years, women aged 35–39 years had an approximately four times higher risk of GDM. In women aged >40 years, the risk of GDM was more than six times that of those aged 20–24 years.
Table 1.
Total number of births, mean maternal age, prevalence of smoking, crude prevalence of GDM, and multivariate logistic regression analysis OR for GDM, by risk factors, for all births 1995–2005
| Risk factor | n (% all births) | Mean age (years) | % smoking | Crude prevalence GDM (%) | Adjusted OR for GDM (95% CI)* |
|---|---|---|---|---|---|
| Age-group | |||||
| <20 years | 42,752 (4.5) | 18.8 | 43.2 | 1.2 | 0.60 (0.54–0.67) |
| 20–24 years† | 152,150 (16.0) | 23.0 | 29.5 | 1.8 | 1.00 |
| 25–29 years | 289,635 (30.5) | 27.8 | 17.4 | 2.9 | 1.68 (1.61–1.76) |
| 30–34 years | 299,743 (31.5) | 32.3 | 12.4 | 4.2 | 2.53 (2.42–2.64) |
| 35–39 years | 139,582 (14.7) | 36.8 | 11.7 | 6.4 | 3.97 (3.80–4.16) |
| >40 years | 26,875 (2.8) | 41.3 | 11.1 | 9.8 | 6.13 (5.79–6.49) |
| Region of country of birth | |||||
| Australia and New Zealand† | 715,200 (75.2) | 29.4 | 21.7 | 2.7 | 1.00 |
| Northeast and Southeast Asia | 84,497 (8.9) | 31.6 | 2.0 | 9.4 | 3.24 (3.16–3.34) |
| Europe and North America | 62,924 (6.6) | 32.5 | 11.5 | 3.7 | 1.21 (1.16–1.26) |
| Middle East and North Africa | 38,005 (4.0) | 29.0 | 8.9 | 6.4 | 2.40 (2.30–2.51) |
| South Asia | 17,266 (1.8) | 30.0 | 0.9 | 10.5 | 4.22 (4.01–4.44) |
| Pacific | 16,763 (1.8) | 30.1 | 9.9 | 8.2 | 2.94 (2.78–3.11) |
| Other Africa | 8,281 (0.9) | 31.6 | 5.6 | 4.4 | 1.62 (1.46–1.80) |
| Caribbean, Central and South America | 7,811 (0.8) | 31.0 | 6.2 | 5.3 | 1.82 (1.65–2.01) |
| Quartiles of socioeconomic status | |||||
| 1 (highest)† | 237,488 (25.0) | 32.2 | 7.5 | 3.1 | 1.00 |
| 2 | 230,825 (24.3) | 29.9 | 15.8 | 4.1 | 1.54 (1.50–1.59) |
| 3 | 240,633 (25.3) | 28.7 | 22.4 | 4.0 | 1.74 (1.69–1.80) |
| 4 (lowest) | 241,801 (25.4) | 28.2 | 25.7 | 3.9 | 1.65 (1.60–1.70) |
| Previous pregnancies >20 weeks | |||||
| None† | 391,766 (41.2) | 28.1 | 15.3 | 3.4 | 1.00 |
| 1 | 321,035 (33.8) | 30.3 | 15.9 | 3.7 | 0.90 (0.88–0.92) |
| ≥2 | 237,408 (25.0) | 32.1 | 24.8 | 4.5 | 0.95 (0.93–0.98) |
Adjusted for all other variables in table.
Reference category.
Region of country of birth, as a proxy for ethnicity, was associated with an increased risk for GDM for all regions compared with women born in Australia and New Zealand (Table 1). Women born in South Asia had the greatest risk, with odds of developing GDM >4 times greater than that of those born in Australia and New Zealand.
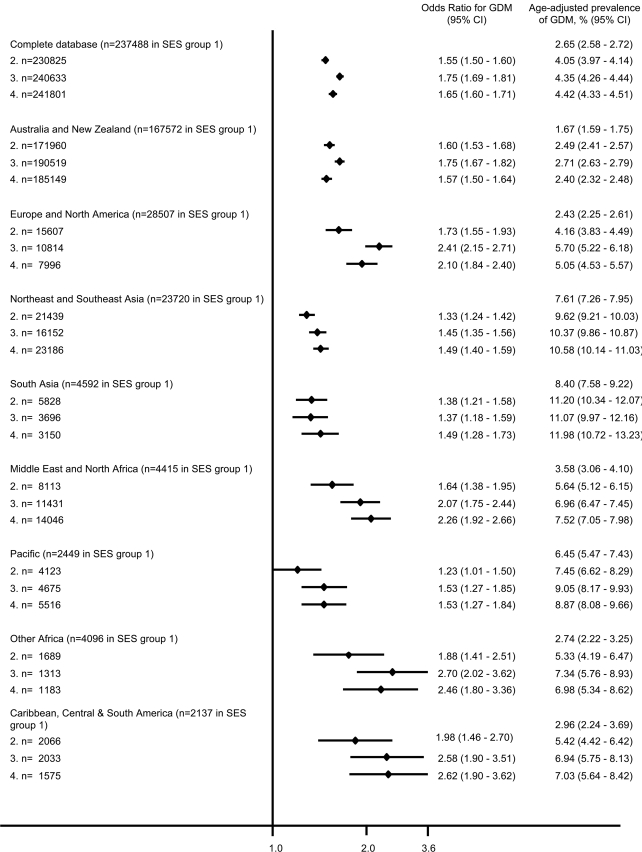
Socioeconomic status was inversely associated with risk of GDM. The risk of GDM was approximately two-thirds higher in women living in the lowest socioeconomic postcodes compared with women in the highest group. The inverse relationship between socioeconomic status and risk of GDM was apparent across all ethnic groups when data were stratified by maternal region of birth, with women in the bottom half of SEIFA postcodes having at least a 30% higher risk of GDM relative to that for the highest quartile (Fig. 2). Women in the lowest socioeconomic group aged >40 years had a risk of 10.26 (95% CI 8.75–12.03) compared with that of women aged 20–24 years residing the highest quartile of SEIFA postcodes.
Figure 2.
OR for risk of GDM by socioeconomic status (SES) for each region of birth (determined by postcode). Reference group is SES 1 (highest SES quartile; OR 1.0). 2, women living in areas in the second highest SES quartile; 3, women living in areas in the second lowest SES quartile; 4, women living in areas in the lowest SES quartile.
Women who had reported a previous pregnancy of >20 weeks’ gestation had a small but significantly reduced risk of GDM in subsequent pregnancies. There was nearly a 10% reduction in risk in women who had a previous pregnancy compared with that in women having their first pregnancy. A similar small protective effect was also apparent among women who had two or more previous pregnancies (Table 1).
CONCLUSIONS
In this large, multiethnic, population-based study of nearly 1 million births over 11 years, we observed that the incidence of GDM increased and the prevalence was strongly correlated with socioeconomic status, ethnicity, and maternal age. There was no relationship with smoking and a small, but significant, inverse relationship with parity. This is the first large population study to show a strong correlation between GDM and socioeconomic status. The relationships between the risk of GDM with maternal region of country of birth and maternal age are in agreement with other studies (5). Age and ethnic or racial background have also been shown to be strongly related to risk of type 2 diabetes in previous studies (12). Smoking, parity, maternal body weight, and family history of diabetes have also been reported to be associated with GDM (5). Maternal body weight and family history were not available in this dataset.
Socioeconomic status, although well established as a risk for obesity and type 2 diabetes, has been less well correlated with GDM (13–15). The reported increasing incidence of GDM, independent of ethnicity, socioeconomic status, or maternal age, has important public health implications in terms of short-term adverse pregnancy outcomes and long-term future risk of type 2 diabetes and its associated morbidities in these women and potential long-term morbidity in the children.
Australia, like the U.S. and U.K., has a large, diverse, and increasing immigrant population. Previous descriptive studies have shown an increased association between GDM and many ethnicities, with women from Asian, African, and Hispanic backgrounds being most at risk (5). Our study supports these findings, and the size of the immigrant population in NSW allowed us to reliably compare the risks of GDM for women born all over the world. In the current study, women from across Asia had an increased risk of GDM compared with women born in Australia and New Zealand. Asian Australians are also found to have an increased risk for type 2 diabetes relative to Caucasian Australians (12). Approximately 6% of Australia's population is composed of people born in Asia, from where an increasing proportion of new migrants will continue to arrive, as well as from other regions where our study shows women have a greater risk of GDM, such as the Middle East and Africa. However, it is likely our study has underestimated the association of ethnicity and GDM, as women born in Australia of non-Caucasian backgrounds could not be identified in our study. This group includes indigenous Australians, who are also a group with a high risk for developing GDM (16).
The current study identified a strong inverse association between socioeconomic status and GDM. Lower socioeconomic status is well recognized as a risk for chronic disease in developed and developing countries (17). The association between GDM and socioeconomic status is less well established, with conflicting results seen in previous studies. These studies cannot easily be compared because of different definitions of socioeconomic status, but three studies have used indexes of relative deprivation of the area of residence of the women as in the present study. Two found no association (14,18), and one showed that living in an area of deprivation was positively associated with GDM (19). Other factors used to determine socioeconomic status were public or private health care sector (public sector increased association with GDM) (15), income, health insurance, residential zip code (no association) (20), education and current employment (inverse relationship, as in the present study) (13). The strength of our study is the number of births over rural and urban sectors. Interestingly, the strong correlation was maintained when the database was stratified by region of country of birth of the women, with each group showing a very similar relationship between GDM and socioeconomic status. For those women born in regions with the highest odds for GDM (all of Asia and the Pacific), the socioeconomic status correlation was not as evident as that for other women, with only a 23–38% increased risk for lower socioeconomic status (compared with a risk of at least 55% in all other women).
The large difference in adjusted ORs between the highest and all lower socioeconomic status quartiles but small difference between the three lower quartiles may be due to bias created by using the SEIFA of the postcode of residence as our socioeconomic indicator. It is unlikely that women of low socioeconomic status can afford to live in high socioeconomic areas, whereas women of high income and educational status may live in areas with a lower SEIFA. Hence, the methodology we used may have clouded the relationship with GDM risk among the lower socioeconomic quartiles. Obesity may also be a factor. The Australian Institute for Health and Welfare estimates that women in the most disadvantaged socioeconomic group have double the rates of obesity of those in the most advantaged group (21), and obesity is a recognized risk factor for GDM. The lack of data on pregestational weight or BMI of women is a limitation of this study but not unique to studies reporting the association of socioeconomic status with GDM (14,18,20). However, in those studies in which an adjustment for maternal weight was made, the inverse association with socioeconomic status was still evident (13,15,19). Family history of diabetes has also been reported to be associated with GDM, though we were not able to include this factor in the present study.
In clinical studies, parity has been described as a risk factor for GDM (5). However, although epidemiological studies have also demonstrated this relationship, it may not persist after adjustment for other risk factors such as age and obesity (15). Our analysis showed a positive association with parity in univariate analysis that reversed to be a slight inverse association with adjustment for age. This finding suggests that there are additional factors that influence the relationship between parity and GDM. One possibility is that there is a large subgroup of women who have fewer children but have a high risk of GDM. Women with polycystic ovary syndrome might be in this group, as they comprise up to 10% of women of reproductive age, are subfertile, and are likely to develop GDM. There are indirect data to support this hypothesis. Some studies have shown that nulliparous women are more likely to develop diabetes than multiparous women, and, indeed, it has been postulated that polycystic ovary syndrome contributes to this effect (22).
During the years examined in the current study, the crude incidence of GDM increased each subsequent year. The median age of mothers also increased each year, and after adjustment for age and country of birth, the incidence of GDM still increased by 45% over the 11-year period. This increase may be caused by other risk factors that we could not account for or may be partially due to variations in screening and reporting over the 11 years. Obesity is a major risk factor for GDM but was not recorded in this dataset and thus could not be included in our analyses (5). There is an increasing prevalence of overweight and obesity in Australia, as there is elsewhere in the world, which is likely to contribute to the increase in incidence of GDM. In the U.S., studies have detailed the increasing prevalence of GDM by up to 68% in a similar period with no significant increase in screening (23). Over the 11 years, there would also have been an increase in Australian-born women of non-Caucasian ethnicities whose parents were born elsewhere, and these could not be adjusted for when our incidence was adjusted for country of birth.
Universal screening for GDM is recommended in NSW, but there are no published data on the actual screening rates. There have been minor improvements in screening over time. The greatest increases in GDM incidence observed in this study occurred between 1997 and 1998 and between 2001 and 2002. Possible contributors to the former may be the publication of the Australasian Diabetes in Pregnancy Society guidelines for GDM in 1998, which strongly recommended universal screening and may have improved screening incidence (7). Reporting became more accurate after the MDC form redesign in 1998 (11), and a subsequent study showed that the MDC underestimated the incidence of GDM in one area of NSW by ∼1% (24). In 2002, the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) study was published, detailing the increasing prevalence of type 2 diabetes in Australia (25). This study raised awareness of diabetes and may also have stimulated some increase in screening and reporting of GDM in Australia around that time.
The recent report on the outcomes of hyperglycemia during pregnancy highlights that adverse outcomes occur where there is suboptimal glucose control below the cutoff for GDM (6). Therefore, we are possibly underestimating the burden of poor glycemic control. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study did not use socioeconomic status as a possible confounder for poor pregnancy outcome. Clearly, a large-scale study is required to determine the combined impact of socioeconomic status and BMI, as well as age, ethnicity, family history, and parity, on glycemic control during pregnancy.
Globally, the increase in GDM parallels the increase in type 2 diabetes in the general population. In Australia, as for many other developed countries, the median age of women having their first pregnancy is now >32 years, which equates to the majority of pregnancies being at higher risk of GDM. These women, as well as those from lower socioeconomic areas and from all immigrant and indigenous populations, must be targeted for primary (where possible) and secondary prevention of GDM to reduce its morbidity and to reduce the prevalence or delay the onset of type 2 diabetes.
Acknowledgments
We thank Lee Taylor from the Centre for Epidemiology and Research of the New South Wales Department of Health for providing and assisting us with access to the Midwives Data Collection database. We also thank Federica Barzi from the George Institute for International Health for her advice regarding statistics.
Published ahead of print at http://care.diabetesjournals.org on 22 September 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.International Diabetes Federation: Diabetes Atlas 2006. 3rd ed. Brussels, International Diabetes Federation, 2006
- 2.Huxley R, Barzi F, Woodward M: Excess risk of fatal coronary heart diseases associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332:73–78, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung NW, Byth K: Population health significance of gestational diabetes. Diabetes Care 26:2005–2009, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Hunt KJ, Schuller KL: The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am 34:173–199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Haroush A, Yogev Y, Hod M: Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med 21:103–113, 2004 [DOI] [PubMed] [Google Scholar]
- 6.HAPO Study Cooperative Research Group: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991–2002, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Hoffman L, Nolan C, Wilson JD, Oats JJN, Simmons D: Gestational diabetes mellitus—management guidelines: the Australasian Diabetes in Pregnancy Society. Med J Aust 169:93–97, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Muscatello D, Travis S: Using the International Classification of Diseases with HOIST. NSW Pub Health Bull 12:289–293, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Australian Bureau of Statistics: Socioeconomic Indexes for Areas. Canberra, Australian Bureau of Statistics, 2001
- 10.Epidemiology and Health Services Evaluation Branch: Public Health Bulletin Suppl. no. 8. Sydney, NSW Department of Health, 1993
- 11.Taylor L, Pym M, Bajuk B, Sutton L, Travis S, Banks C: Part 8: Validation Study of NSW Midwives Data Collection 1998 [article online]. 2000. Available from http://www.health.nsw.gov.au/pubs/2000/mdc98.html. Accessed 28 November 2007
- 12.Thow AM, Waters A-M: Diabetes in Culturally and Linguistically Diverse Australians: Identification of Communities at High Risk. Canberra, Australian Institute of Health and Welfare, 2005
- 13.Bo S, Menato G, Bardelli C, Lezo A, Signorile A, Repetti E, Massobrio M, and Pagano G: Low socioeconomic status as a risk factor for gestational diabetes. Diabetes Metab 28:139–140, 2002 [PubMed] [Google Scholar]
- 14.Janghorbani M, Stenhouse EA, Jones RB, Millward BA: Is neighbourhood deprivation a risk factor for gestational diabetes mellitus? Diabet Med 23:313–317, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz GS, Lapinski RH, Wein R, Lee D: Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol 135:965–973, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Ishak M, Petocz P: Gestational diabetes among Aboriginal Australians: prevalence, time trend, and comparisons with non-Aboriginal Australians. Ethn Dis 13:55–60, 2003 [PubMed] [Google Scholar]
- 17.Popkin BM, Gordon-Larsen P: The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes 28:S2–S9, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Jaamaa G, Kaiser M, Hills E, Soim A, Zhu M, Shcherbatykh IY, Samelson R, Bell E, Zdeb M, McNutt LA: Racial disparity in hypertensive disorders of pregnancy in New York state: a 10-year longitudinal population-based study. Am J Public Health 97:163–170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clausen T, Øyen N, Henriksen T: Pregnancy complications by overweight and residential area: a prospective study of an urban Norwegian cohort. Acta Obstet Gynecol Scand 85:526–533, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Shen JJ, Tymkow C, MacMullen N: Disparities in maternal outcomes among four ethnic populations. Ethn Dis 15:492–497, 2005 [PubMed] [Google Scholar]
- 21.Australian Institute of Health and Welfare: Australia's Health 2006. Canberra, Australian Institute of Health and Welfare, 2006
- 22.Hanley AJG, McKeown-Eyssen G, Harris SB, Hegele RA, Wolever T, Kwan J, Zinman B: Association of parity with risk of type 2 diabetes and related metabolic disorders. Diabetes Care 25:690–695, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Ferrara A: Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 30 (Suppl 2):S141–S146, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Moses RG, Webb AJ, Comber CD, Moses RG, Webb AJ, Comber CD: Gestational diabetes mellitus: accuracy of Midwives Data Collection. Med J Aust 179:218–219, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Dunstan DW, Zimmet PZ, Welborn TA, de Courten MP, Cameron AJ, Sicree RA, Dwyer T, Colagiuri S, Jolley D, Knuiman M, Atkins R, Shaw JE: The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 25:829–834, 2002 [DOI] [PubMed] [Google Scholar]