Abstract
OBJECTIVE—Progressive nephropathy represents a substantial source of morbidity and mortality in type 1 diabetes. Increasing albuminuria is a strong predictor of progressive renal dysfunction and heightened cardiovascular risk. Early albuminuria probably reflects vascular endothelial dysfunction, which may be mediated in part by chronic inflammation.
RESEARCH DESIGN AND METHODS—We measured baseline levels of four inflammatory biomarkers (high-sensitivity C-reactive protein, soluble intercellular adhesion molecule-1 [sICAM-1], soluble vascular cell adhesion molecule-1, and soluble tumor necrosis factor-α receptor-1) in stored blood samples from the 1,441 participants of the Diabetes Control and Complication Trial (DCCT). We used mixed-effects regression models to determine the average annual change in urinary albumin excretion rate (AER) by tertiles of each biomarker. We also used Cox proportional hazards models to estimate the relative risk of incident sustained microalbuminuria according to levels of each biomarker.
RESULTS—After adjustment for baseline age, sex, duration of diabetes, A1C, and randomized treatment assignment, we observed a significantly higher 5.9 μg · min−1 · year−1 increase in AER among those in the highest compared with the lowest tertile of baseline sICAM-1 (P = 0.04). Those in the highest tertile of sICAM-1 had an adjusted relative risk of 1.67 (95% CI 0.96–2.92) of developing incident sustained microalbuminuria (Ptrend = 0.03).
CONCLUSIONS—Higher baseline sICAM-1 levels predicted an increased risk of progressive nephropathy in type 1 diabetes and may represent an early risk marker that reflects the important role of vascular endothelial dysfunction in this long-term complication.
Morbidity and mortality from kidney disease and cardiovascular disease (CVD) are important complications of type 1 diabetes. Development of microalbuminuria is a signal of relatively early nephropathy in diabetes and is an independent risk factor for CVD and mortality in epidemiologic studies and secondary analyses of cardiovascular trials. With the recognition of the central role played by inflammatory processes in both CVD (1) and type 1 (2) as well as type 2 diabetes (3), the association between potentially modifiable inflammatory biomarkers and clinical outcomes such as nephropathy and CVD in diabetes is of interest. Moreover, although diabetic nephropathy has traditionally not been considered an inflammatory nephritis, more recent evidence that macrophage accumulation is characteristic of diabetic glomerulosclerosis has challenged this perception (4).
A few cross-sectional studies of individuals with type 1 diabetes have reported associations between increased AER and elevated levels of inflammatory markers including high-sensitivity (hs) C-reactive protein (CRP) (5), soluble (s) tumor necrosis factor-α receptor-1 (TNFR-1) (6), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) (7), but whether the elevation in these markers of inflammation precedes nephropathy progression is not well described. If inflammation contributes to the development of nephropathy, it would represent a potential therapeutic target for slowing the microvascular and macrovascular complications of diabetes. We therefore undertook a prospective analysis of inflammatory biomarkers and change in urinary albumin excretion over time in 1,441 individuals with type 1 diabetes participating in the Diabetes Control and Complications Trial (DCCT).
RESEARCH DESIGN AND METHODS
The DCCT was a multicenter randomized trial of intensive versus conventional diabetes therapy for type 1 diabetes in the development and progression of microvascular diabetes complications. The 1,441 DCCT participants were recruited at 29 centers from 1983 through 1989. All participants were between the ages of 13 and 39 years and had baseline A1C levels greater than 3 SDs above the mean for individuals without diabetes.
There were two strata in the DCCT, defined by the absence versus presence of early clinical microvascular complications: 1) a primary prevention cohort with no retinopathy, diabetes duration of 1–5 years, and no evidence of microalbuminuria at baseline (albuminuria <28 μg/min [<40 mg/24 h]), and 2) a secondary intervention cohort with minimal to moderate nonproliferative retinopathy, diabetes duration between 1 and 15 years, and ≤140 μg/min (200 mg/24 h) of albuminuria. After a mean follow-up of 6.5 years (range 3–9 years), the DCCT reported clinically and statistically highly significant reductions (range of 35–>70%) in microvascular end points in the intensive compared with the conventional therapy group. Follow-up in the DCCT was nearly complete, with subjects attending 99% of all scheduled follow-up visits.
Laboratory studies
Fasting serum samples were obtained from DCCT participants at baseline and at each annual visit. Samples were maintained at −70°C at the DCCT Central Biochemistry Laboratory, Department of Laboratory Medicine and Pathology, University of Minnesota until preparation for the present study. Baseline serum samples were thawed and assayed for levels of sICAM-1, sVCAM-1, and sTNFR-1 by enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN) in the Children's Hospital Laboratory in Boston. Serum levels of hsCRP were determined by a latex-enhanced immunonephelometric assay on a BN II analyzer (Dade Behring, Newark, DE). The day-to-day variability of each biomarker assay was <10%. A1C levels were determined from whole blood at the time of collection using high-performance liquid chromatography in the DCCT Central A1C Laboratory.
Creatinine clearance and urinary albumin excretion rate (AER) were assessed annually in the DCCT, using 4-h timed urine collections. Serum albumin and serum creatinine were also measured annually. As in prior analyses from the DCCT, we defined incident sustained microalbuminuria as the first development of urinary AER of >28 μg/min (>40 mg/24 h) among those without microalbuminuria at baseline that was sustained for at least 1 year.
Statistical analysis
We initially examined the relationships between tertiles of inflammatory biomarkers and baseline characteristics of the study population. Spearman correlation coefficients were used to test for associations between continuous variables. We then performed mixed-effects regression modeling using time as a continuous variable and assumed randomly varying intercepts and slopes for each participant to determine the average change in AER over time. We tested for differences across tertiles of the inflammatory biomarkers by modeling time × biomarker tertile interaction terms to examine whether the slope of change of AER (dependent variable) was significantly different between tertiles of biomarkers (independent variable). Therefore, the magnitude of association between tertiles of biomarkers and AER is represented as a difference in change in AER per year in tertiles 2 and 3 compared with the lowest tertile (referent group) when averaged over the 9-year study period.
In addition, we used proportional hazards regression to estimate the rate ratios and 95% CI for the relationships of each inflammatory biomarker with the development of sustained microalbuminuria. We considered several possible confounders including age, sex, duration of diabetes, A1C, treatment assignment, baseline AER, smoking status (never, past, or current), baseline systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (difference between SBP and DBP at baseline), and BMI. SAS (version 8.2, SAS Institute, Cary NC) was used for all analyses.
This study was approved by the Partners’ Healthcare Brigham and Women's Hospital Human Research Committee Institutional Review Board.
RESULTS
The DCCT comprised a young cohort (median age 27 years) with almost equal numbers of predominantly white male and female participants (Table 1). The median duration of type 1 diabetes was 51 months. The majority of this population was normotensive at enrollment (only 24 had SBP >140 mmHg and 26 had DBP >90 mmHg at baseline) and had normal plasma creatinine and directly measured creatinine clearance. Less than 7% of the study population had missing data for one or more biomarkers; median and range values within tertiles of each biomarker are given in Table 1. In looking at the distribution of the data, we noted that only 5% of the participants had baseline hsCRP >7.8 mg/l.
Table 1.
Baseline characteristics of participants in DCCT trial
| All participants | |
|---|---|
| Age (years) | 27 (22–32) |
| Sex (% men/% women) | 761 (53)/680 (47) |
| White | 1,391 (96.5) |
| BMI (kg/m2) | 23.3 (21.3–25.2) |
| Ever smoked | 516 (36) |
| Duration of diabetes (months) | 51 (21–109) |
| SBP (mmHg) | 114 (106–122) |
| DBP (mmHg) | 72 (68–80) |
| A1C (%) | 8.7 (7.8–9.9) |
| Plasma creatinine (mg/dl) | 0.8 (0.7–0.9) |
| Creatinine clearance (ml/min) | 126 (110–143) |
| Baseline AER (μg/min) | 8.0 (5–13) |
| hsCRP (mg/l) | |
| Tertile 1 (n = 450) | 0.28 (0.4–0.54) |
| Tertile 2 (n = 451) | 0.88 (0.55–1.42) |
| Tertile 3 (n = 449) | 3.09 (1.43–45.2) |
| sTNFR-1 (pg/ml) | |
| Tertile 1 (n = 463) | 833 (512–925.7) |
| Tertile 2 (n = 464) | 1011 (926.1–1122.1) |
| Tertile 3 (n = 463) | 1261 (1122.5–2910.8) |
| sICAM-1 (ng/ml) | |
| Tertile 1 (n = 465) | 235 (52.2–258.1) |
| Tertile 2 (n = 466) | 282 (258.2–306.2) |
| Tertile 3 (n = 465) | 346 (306.3–847.1) |
| sVCAM (ng/ml) | |
| Tertile 1 (n = 465) | 366 (125–408.8) |
| Tertile 2 (n = 465 | 448 (409.0–492.7) |
| Tertile 3 (n = 465) | 558 (492.9–1180.7) |
Data are median (interquartile range) or number (%). n = 1,441.
At baseline, cross-sectional measures of sICAM-1 and sVCAM-1 were associated with (r = 0.34, P < 0.001) and sTNFR-1 was also significantly correlated with sICAM-1 (r = 0.32, P < 0.001) and sVCAM-1 (r = 0.31, P < 0.001). hsCRP was associated with sTNFR-1 (r = 0.12, P < 0.001), and sICAM (r = 0.17, P < 0.001) but not sVCAM-1 (r = 0.04, P = 0.17). By Spearman correlation, A1C was significantly associated with hsCRP (r = 0.11, P < 0.001) and sICAM-1 (r = 0.18, P < 0.001), and diabetes duration were significantly associated with hsCRP (r = 0.10, P < 0.001), sTNFR-1 (r = 0.13, P < 0.001), and sICAM-1 (r = 0.05, P = 0.04) at baseline. We additionally observed a statistically significant direct cross-sectional correlation between baseline AER and sICAM tertiles (median AER 7.0 vs. 7.5 vs. 8.0 μg/min, P = 0.01) but not between AER and other biomarkers.
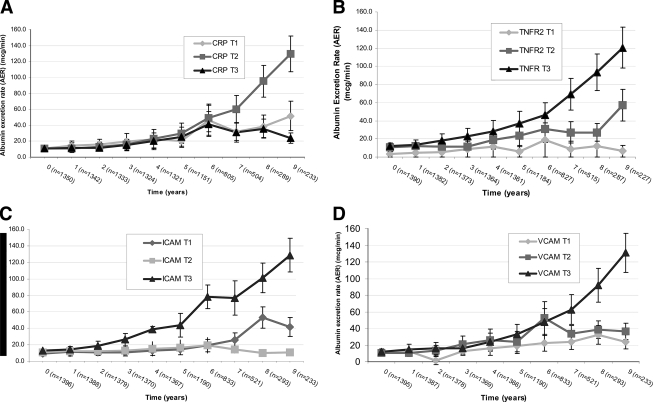
Evaluation of the mean change in AER over time by hsCRP tertiles demonstrated no difference in AER excretion until after year 7, and no consistent direct relationship was seen between higher hsCRP and change in AER (Fig. 1A). In examining the mean change in AER over time stratified by tertiles of baseline sTNFR-1, sICAM-1, and sVCAM-1 (Figs. 1B–D), we noted a steeper increase in AER over time for those in the highest tertile of each of these markers; however, this difference did not become notable until year 7 of follow-up for sTNFR-1 and sVCAM-1. In contrast, those in the highest tertile of sICAM-1 experienced a steeper increase in AER beginning after year 3 that persisted throughout follow-up.
Figure 1.
A: Mean change over time of AER stratified by tertile (T) of baseline hsCRP. B: Mean change over time of AER stratified by tertile of baseline sTNFR-1. C: Mean change over time of AER stratified by tertile of baseline sICAM-1. D: Mean change in AER over time stratified by tertile of baseline sVCAM-1. Vertical bars represent SEM. Note that sample sizes for each biomarker vary slightly at each time point because of missing data of individual baseline markers.
Unadjusted mixed-effects regression showed that subjects in the highest tertile of baseline sICAM-1 had a significant 8.1 μg/min greater increase in AER per year compared with that of subjects in the lowest tertile of baseline sICAM-1 (P = 0.002). In contrast, there were no significant effects of hsCRP (P = 0.54), sTNFR-1 (P = 0.45), or sVCAM-1 (P = 0.15) on change in AER over time (supplemental Table A2, available in an online appendix at http://dx.doi.org/10.2337/dc08-0277). In multivariable models adjusted for age, sex, duration of diabetes, baseline A1C, and treatment assignment, we continued to observe a significant 5.9 μg/min increase in AER per year (P = 0.04) among those in the highest versus lowest tertiles of baseline sICAM-1 (supplemental Table A3, available in an online appendix at http://dx.doi.org/10.2337/dc08-0277). We did not observe significant associations between smoking (−0.93 μg · min−1 · year−1, P = 0.67) or BMI (−0.25 μg · min−1 · year−1 per 1 unit increase in BMI, P = 0.49) and change in AER over time. When these two variables were included in the fully adjusted mixed-effects model, they did not meaningfully alter the association between the highest tertile of sICAM-1 and change in AER (6.2 μg · min−1 · year−1 increase, P = 0.02); neither of these covariates have a significant associations with change in AER, so they were not included in the final model.
In addition to the significant effect of sICAM-1, we also observed that the intensive treatment group experienced a lower rate of increase in AER excretion by 5.3 μg · min−1 · year−1 (P = 0.01) compared with the conventional treatment group. Each additional year of diabetes duration was independently associated with a significant 0.09 μg · min−1 · year−1 increase in AER (P < 0.001), and each percent increase in A1C at baseline was directly associated with a 2.6 μg · min−1 · year−1 increase in AER (P = 0.01). The covariates of baseline SBP, DBP, pulse pressure, and BMI were not significantly associated with change in AER over time in univariate analyses and did not influence the results when included in the fully adjusted models. Inclusion of baseline AER in the multivariable mixed-effects regression model did not meaningfully alter the associations between the highest tertile of sICAM (5.4 μg · min−1 · year−1 increase in AER, P = 0.04) or the other covariates. There were 725 participants in the primary prevention group and 642 in the secondary prevention group. In the fully adjusted mixed-effects regression models, the rate of AER increase was 1.44 μg · min−1 · year−1 in the primary prevention group and 8.71 μg · min−1 · year−1 in the secondary prevention group. A test for the interaction term of the highest tertile of ICAM-1 and prevention group yielded a significant P value of 0.005.
Further analysis of the 1,367 participants without microalbuminuria at baseline (AER <40 mg/24 h) showed that 97 developed new sustained microalbuminuria. Using proportional hazards regression, we observed a significantly higher incidence of sustained microalbuminuria among those with higher baseline levels of sICAM-1 (Ptrend = 0.03). The rate ratio for sustained microalbuminuria contrasting the top versus bottom tertile of sICAM-1 levels was 1.67 (95% CI 0.96–2.92). We did not observe significant associations between other biomarkers and sustained new incident microalbuminuria (supplemental online Table A4, available in an online appendix). Again, the covariates of baseline SBP, DBP, pulse pressure, and BMI were not significantly associated with incident microalbuminuria in univariate Cox proportional hazards analyses, and results were unchanged when each of these covariates was included in the fully adjusted models.
CONCLUSIONS
We examined four candidate inflammatory biomarkers in individuals with type 1 diabetes and found that only sICAM-1 levels were significantly associated with increasing AER over time as well as the development of new microalbuminuria. Furthermore, the association between baseline sICAM-1 and increasing AER over time appeared to be more pronounced in those in the secondary prevention group who already had evidence of vascular involvement (diabetic retinopathy). This may reflect the important role of sICAM-1 as a mediator of progressive microvascular disease, which often manifests as nephropathy in conjunction with retinopathy in diabetes.
Other baseline covariates that independently influenced progressive albuminuria in this study population included longer duration of diabetes and higher baseline A1C. As expected and consistent with previously reported findings for the development of sustained microalbuminuria in the DCCT study, assignment to the intensive glucose control treatment group was protective against progressive albuminuria or incident microalbuminuria.
Increases in AER began to appear after 3 years of follow-up among those with values in the highest tertile of sICAM-1 levels. In contrast, there was a gradual increase in AER over time that was statistically independent of the levels of hsCRP, sVCAM-1, and sTNFR-1. Although these increases in AER are small in magnitude, their clinical importance is supported by data showing that AER in what has been considered the “normal range” (<30 mg/24 h) confers independent and significant risk for cardiovascular events and/or death in multiple studies including population-based (8) and cardiac disease (9) cohorts. Especially relevant to our findings that sICAM predicts incident microalbuminuria is the report that microalbuminuria was strongly predictive of subsequent CVD events in the Pittsburgh Epidemiology of Diabetes Complications longitudinal follow-up study of type 1 diabetic subjects (10).
Hyperglycemia impairs vascular endothelial cell function, probably in part through oxidation of LDL and increased formation of free radicals. For example, generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells has been noted to increase by >200% between baseline and 2 h in a study of 14 healthy humans given a 75-g glucose load (11). Free radicals, in turn, stimulate the release of proinflammatory cytokines, which induce the expression of adhesion molecules, including ICAM-1 and VCAM-1 (12). This association may be mediated through hyperglycemia-induced increased nuclear factor-κB binding, as transcription for ICAM-1 is regulated by nuclear factor-κB (13).
A member of the immunoglobulin superfamily, ICAM-1 is upregulated in activated endothelial cells and is central in migration of inflammatory cells into tissues. Poor glycemic control is associated with increased expression of ICAM-1 and other markers of endothelial activation in type 1 diabetes (14). Furthermore, insulin administration has been reported to directly decrease ICAM-1 in in vitro studies of human cultured endothelial aortic cells (15) as well as in an in vivo study of human subjects in which plasma glucose was maintained at baseline levels (16). These data are consistent with our observation that those in the intensive control group of the DCCT experienced significant decreases in ICAM-1 and VCAM-1 at 3 years of follow-up (17). In considering why baseline ICAM-1 was the most consistent and significant predictor of subsequent nephropathy progression in our analyses, we hypothesize that ICAM-1 may be a key molecule in the common final pathway of endothelial cell activation, inflammation, and injury in the kidney that manifests clinically as elevated AER in the early stages of nephropathy. For example, CRP is thought to enhance expression of endothelial adhesion molecules ICAM-1 and VCAM-1 in local tissues, whereas TNF-α is thought to prime neutrophil infiltration into target organs (18). Therefore, elevated CRP and TNF-α levels may reflect a generalized and nonspecific inflammatory condition in contrast with a more restricted state of active vascular endothelial dysfunction associated with adhesion molecules ICAM-1 and VCAM-1.
As for why sICAM-1 but not sVCAM-1 was strongly associated with increasing AER, ICAM-1 appears to be constitutively expressed by endothelial cells (19), whereas acute upregulation of VCAM-1 may be central in initiation of atherosclerosis (20). Thus, elevated plasma levels of ICAM-1 may be indicative of sustained inflammatory endothelial activity and injury in the kidney that manifests clinically as increasing albuminuria excretion over time.
ICAM-1 and VCAM-1 have been directly associated with increased AER in individuals with type 1 diabetes in cross-sectional studies (7), but this is the first investigation to our knowledge to report that sICAM-1 is an independent predictor of subsequent increased urinary albumin excretion or development of new microalbuminuria. Interestingly, animal models of diabetic nephropathy have revealed that ICAM-1 knockout mice have significantly lower levels of AER in streptozocin-induced (type 1) diabetes (21). Renal tissue in both these animal models also demonstrated significantly decreased numbers of interstitial inflammatory cells in ICAM-1-deficient animals as well as a reduction in histologic kidney damage. These data from humans and animals support the concept that endothelial dysfunction plays an important role in promoting progressive renal disease in diabetes. Moreover, ICAM-1 is implicated in progression of vascular atherosclerotic disease (22). For these reasons, we hypothesize that ICAM-1 may represent an important pathogenic link between vascular endothelial dysfunction and kidney dysfunction and its associated cardiovascular risk in individuals with diabetes.
The Steno hypothesis proposed that albuminuria reflects a systemic vascular endothelial dysfunction that promotes atherosclerotic processes in type 1 diabetes, and increased albuminuria has been associated with cardiovascular and all-cause mortality in large prospective clinical investigations (8,9). In conjunction with previous reports that sICAM-1 is an independent predictor of coronary atherosclerotic disease progression but not acute myocardial infarction (23) and of progressive peripheral artery disease but not acute vascular occlusion (24), our findings that sICAM-1 is a strong predictor of increasing albuminuria over time support the theory that elevated AER is a harbinger of progressive atherosclerosis. Moreover, sICAM-1 may play a central role in the association between albuminuria and CVD.
Limitations of the current study include the small number of nonwhite participants, typical for type 1 diabetes, that may limit the generalizability of these observed associations to minority, older, or type 2 diabetic populations. The possibility of residual confounding in these multivariable models is also present, as in any observational epidemiological study. Furthermore, these data were collected before the time of widespread ACE medication use, now an established strategy in slowing progressive nephropathy in type 1 and type 2 diabetes, so the influence of therapy upon these biomarkers and albuminuria cannot be examined. Interestingly, angiotensin II promotes upregulation of ICAM-1 and VCAM-1 on endothelial cells (25), and reduction of angiotensin II levels by ACE inhibition may have a multifactorial effect in decreasing albuminuria including reduction of adhesion molecules in addition to diminishing glomerular filtration pressure and promoting profibrotic pathways. There is a further possibility that the results are influenced by differences in proportions of missing data over time. We examined the numbers of participants with missing data in each stratum of biomarker (with a special focus on years 6 to 9, for which there is higher attrition in AER data), however, and found that the missing AER data were equally distributed among all strata of each biomarker.
These limitations are balanced by some notable strengths of this prospective investigation including the relatively large and well-defined study cohort with repeated laboratory measures for up to 9 years of follow-up. The directly measured AER with repeated timed urine collections is also a strength that allowed us to examine change in AER over time and examine its relation to novel inflammatory biomarkers.
In summary, we report that higher baseline plasma ICAM-1 is independently associated with increasing AER over time as well as with incident sustained microalbuminuria in type 1 diabetes. These findings suggest that inflammation and endothelial dysfunction play important roles in progressive nephropathy in type 1 diabetes and that sICAM-1 may be a potential early biomarker that would allow for risk stratification of patients.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant K08 DK066246 (J.L.), by Juvenile Diabetes Research Foundation Grant 1-2000-646 (D.A.S.), by the Earle P. Charlton, Jr. Charitable Foundation (D.M.N.), and by a contract with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (D.M.N.). The DCCT and its follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study were conducted by the DCCT/EDIC Research Group and supported by NIH grants and contracts from the NIDDK and by the General Clinical Research Center Program, National Center for Research Resources.
Published ahead of print at http://care.diabetesjournals.org on 16 September 2008.
P.M.R. is listed as an inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in vascular disease. P.M.R. and J.E.M. are listed as coinventors on a pending patent held by Brigham and Women's Hospital that relates to inflammatory biomarkers in diabetes prediction.
This article was not prepared under the auspices of the Diabetes Control and Complication Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study and does not represent analyses or conclusions of the DCCT/EDIC study group or the National Institutes of Health. J.L. and D.A.S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ndumele CE, Pradhan AD, Ridker PM: Interrelationships between inflammation, C-reactive protein, and insulin resistance. J Cardiometab Syndr 1:190–196, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ichinose K, Kawasaki E, Eguchi K: Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol 27:554–564, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Williams MD, Nadler JL: Inflammatory mechanisms of diabetic complications. Curr Diab Rep 7:242–248, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K: The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis 21:480–485, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH: Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46:1402–1407, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Zoppini G, Faccini G, Muggeo M, Zenari L, Falezza G, Targher G: Elevated plasma levels of soluble receptors of TNF-α and their association with smoking and microvascular complications in young adults with type 1 diabetes. J Clin Endocrinol Metab 86:3805–3808, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, Feldt-Rasmussen B: Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med 17:644–649, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106:1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Lin J, Solomon CG, Jablonski KA, Rice MM, Steffes M, Domanski M, Hsia J, Gersh BJ, Arnold JM, Rouleau J, Braunwald E, Pfeffer MA: Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation 116:2687–2693, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ: Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia 50:2280–2288, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P: Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 85:2970–2973, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Pigott R, Dillon LP, Hemingway IH, Gearing AJ: Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun 187:584–589, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P: Glucose ingestion induces an increase in intranuclear nuclear factor κB, a fall in cellular inhibitor κB, and an increase in tumor necrosis factor α messenger RNA by mononuclear cells in healthy human subjects. Metabolism 55:1177–1185, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Seckin D, Ilhan N, Ertugrul S: Glycaemic control, markers of endothelial cell activation and oxidative stress in children with type 1 diabetes mellitus. Diabetes Res Clin Pract 73:191–197, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Aljada A, Dandona P: Effect of insulin on human aortic endothelial nitric oxide synthase. Metabolism 49:147–150, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S: Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86:3257–3265, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, Ridker PM, Nathan DM: Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation 111:2446–2453, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Tesfamariam B, DeFelice AF: Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol 46:229–237, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI: Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 85:199–207, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS: A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107:1255–1262, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, Nagase R, Wada J, Shikata Y, Makino H: Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52:2586–2593, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Boyle JJ: Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 3:63–68, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Albert MA, Glynn RJ, Buring JE, Ridker PM: Relation between soluble intercellular adhesion molecule-1, homocysteine, and fibrinogen levels and race/ethnicity in women without cardiovascular disease. Am J Cardiol 99:1246–1251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pradhan AD, Rifai N, Ridker PM: Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation 106:820–825, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Soehnlein O, Schmeisser A, Cicha I, Reiss C, Ulbrich H, Lindbom L, Daniel WG, Garlichs CD: ACE inhibition lowers angiotensin-II-induced monocyte adhesion to HUVEC by reduction of p65 translocation and AT 1 expression. J Vasc Res 42:399–407, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.