Abstract
OBJECTIVE—The albumin-to-creatinine ratio (ACR) reflects urinary albumin excretion and is increasingly being accepted as an important clinical outcome predictor. Because of the great public health need for a simple and inexpensive test to identify individuals at high risk for developing type 2 diabetes, it has been suggested that the ACR might serve this purpose. We therefore determined whether the ACR could predict incident diabetes in a well-characterized cohort of pre-diabetic Americans.
RESEARCH DESIGN AND METHODS—A total of 3,188 Diabetes Prevention Program (DPP) participants with a mean BMI of 34 kg/m2 and elevated fasting glucose, impaired glucose tolerance, and baseline urinary albumin excretion measurements were followed for incident diabetes over a mean of 3.2 years.
RESULTS—Of the participants, 94% manifested ACR levels below the microalbuminuria range and 21% ultimately developed diabetes during follow-up. Quartiles of ACR (median [range] within quartiles: 1, 3.0 [0.7–3.7]; 2, 4.6 [3.7–5.5]; 3, 7.1 [5.5–9.7]; and 4, 16.5 [9.7–1,578]) were positively associated with age, markers of adiposity and insulin secretion and resistance, blood pressure, and use of antihypertensive agents with antiproteinuric effects and inversely related to male sex and serum creatinine. An elevated hazard rate for developing diabetes with doubling of ACR disappeared after adjustment for covariates. Within the DPP intervention groups (placebo, lifestyle, and metformin), we found no consistent trend in incident diabetes by quartile or decile of ACR.
CONCLUSIONS—An ACR at levels below the microalbuminuria range does not independently predict incident diabetes in adults at high risk of developing type 2 diabetes.
With the explosive growth of incident diabetes, type 2 diabetes has become a major international public health challenge. Moreover, an increasing number of individuals have evidence of a pre-diabetic state, which indicates significant future risk of developing diabetes. Fortunately, accumulating evidence suggests that type 2 diabetes can be delayed or prevented in individuals with pre-diabetes by either lifestyle modification or medication (1–2). However, because prevention is a fundamental public health goal, there is clearly a great need for effective strategies to identify high-risk individuals. Unfortunately, the best available risk stratification method is an oral glucose tolerance test (OGTT), which is both costly and difficult to perform in a clinical setting.
The albumin-to-creatinine ratio (ACR) in a single untimed urinary specimen is a reflection of urinary albumin excretion and is increasingly being accepted as a marker that predicts several important health outcomes, including hypertension, kidney failure, cardiovascular events, and mortality (3–5). These associations have been observed throughout the biological range, even at levels far below those previously considered to be pathological (e.g., microalbuminuria) (6).
The ACR is also closely linked to cardiometabolic risk factors, vascular disease, and insulin resistance (7–9) and might therefore play a clinically important role in predicting future onset of diabetes. Observational studies have shown an association between ACR and other markers of urinary albumin excretion and incident diabetes (10–12). In addition, observations that proteinuria-reducing therapies (e.g., ACE inhibitors and angiotensin II receptor blockers) delay progression to diabetes also support this hypothesis, albeit indirectly (13,14). However, the observational studies were heterogeneous in terms of study design and risk for incident diabetes, did not include proteinuria throughout its biologic range, and/or recruited individuals from ethnic groups distinct from the general U.S. population. In addition, a randomized clinical trial did not find that ramipril, a proteinuria-reducing agent, altered the incidence of diabetes (15).
The Diabetes Prevention Program (DPP) enrolled a large and well-characterized cohort of adults who were at high-risk for developing diabetes based on having elevated fasting glucose and impaired glucose tolerance. We tested the hypothesis that ACR, throughout its biological range, improves the prediction of future diabetes.
RESEARCH DESIGN AND METHODS
The eligibility criteria, design, and methods of the DPP have been reported elsewhere (16). In brief, eligibility criteria included age ≥25 years, BMI ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), and fasting plasma glucose levels between 95 and 125 mg/dl (lower limit did not apply to the American Indian centers) in addition to impaired glucose tolerance (IGT) by an oral glucose tolerance test (OGTT) (plasma glucose of 140–199 mg/dl 2 h after a 75-g oral glucose load). Participants were recruited from 27 U.S. study sites and were excluded if they had conditions that would impair their ability to participate or took certain medicines, including thiazide diuretics and β-blockers (16). All participants gave informed consent and signed documents approved by the institutional review board at each center. Eligible participants received standard advice on a healthy diet and physical activity and were randomly assigned to one of three additional interventions (intensive lifestyle intervention versus metformin versus matching placebo).
Measurements and laboratory tests
Development of diabetes was determined by an annual OGTT or by a semiannual fasting plasma glucose level with confirmation by a second test, using the criteria of the American Diabetes Association (21) and the World Health Organization (17). Urinary albumin excretion was estimated from a morning fasting spot urine sample by the urinary albumin-to-urinary creatinine ratio (i.e., milligrams of albumin per gram of creatinine). Urinary albumin was measured using Behring reagents on the BN II nephelometer (Dade Behring, Deerfield, IL) (interassay coefficient of variation [CV] 4.4% and intra-assay CV 4.3%). Microalbuminuria was defined using standard criteria as ACR between 30 and 300 mg/day, whereas macroalbuminuria was defined as ACR >300 mg/day. Serum and urinary creatinine concentrations were measured using Roche reagents on the Hitachi 917 autoanalyzer (Boehringer Mannheim, Mannheim, Germany) (serum creatinine: interassay CV 3.5% and intra-assay CV 3.2%; urinary creatinine: interassay CV 1.8% and intra-assay CV 1.2%).
Immunoreactive insulin was measured in plasma. Measurement methods for glucose and insulin have been published previously (18). Insulin secretion and sensitivity were expressed using glucose and insulin measured in conventional units (milligrams per deciliter and microunits per milliliter, respectively). Insulin secretion was measured using the corrected insulin response = (100 × 30-min insulin)/(30-min glucose × [30-min glucose − 70 mg/dl]) (19). Insulin sensitivity was measured using the insulin sensitivity index, which is 22.5/(fasting insulin × [fasting glucose/18.0]), the reciprocal of which is the homeostasis model assessment of insulin resistance (HOMA-IR) (20). Baseline demographic and anthropometric (i.e., BMI measured as weight in kilograms divided by height in meters and waist circumference) data were also measured.
Statistical methods
Because of its skewed distribution, ACR was analyzed both as a categorical (i.e., by quartiles) and as a continuous (i.e., after base 2 logarithm transformation) variable. Baseline characteristics were described by means ± SD for continuous variables and percentages for categorical variables. Spearman's correlation coefficients between continuous variables and ACR were reported. For categorical variables, ANOVA was used to identify differences in the base 2 logarithm-transformed ACR. Because a significant interaction (P < 0.05) was noted between treatment group and incident diabetes when ACR was divided into quartiles (but not when it was examined as a continuous variable after base 2 logarithm transformation), the former analysis was stratified by treatment arm. Cox proportional hazards models were used to evaluate the association between ACR and risk of developing diabetes by both univariate and multivariate means. The first multivariate model adjusted for the demographic factors age, sex, and race/ethnicity alone, whereas the second included demographics with the time-dependent covariates exercise and weight loss as well as baseline characteristics that were associated with ACR in a statistically significant manner (at the 0.05 level). All analyses were performed using SAS (SAS Institute, Cary, NC). Nominal P values are presented without adjustment for multiplicity of testing.
RESULTS
Descriptive data
The DPP randomly assigned 3,234 participants to one of three treatment arms (placebo, metformin, or lifestyle). Our analysis included only the 3,188 individuals with ACR data at baseline. The distribution of baseline ACR was highly skewed at the upper end. Overall, 2,997 participants (94%) had ACR levels below the microalbuminuria cutoff point of 30 mg/g, and only 14 (0.4%) had macroalbuminuria (ACR level >300 mg/g). The medians and ranges of ACR within quartiles are shown in Table 1.
Table 1.
Baseline characteristics by quartile of ACR
| Characteristics | Quartiles of ACR |
Correlation coefficient | P value* | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| n | 797 | 797 | 797 | 797 | ||
| ACR (mg/g) | 3.0 (0.7–3.7) | 4.6 (3.7–5.5) | 7.1 (5.5–9.7) | 16.5 (9.7–1578) | ||
| Age (years) | 50.0 ± 10.6 | 50.3 ± 10.3 | 50.7 ± 10.5 | 51.5 ± 11.1 | 0.04 | 0.02 |
| Sex (% male) | 41.3 | 34.1 | 25.5 | 28.4 | — | <0.01 |
| Race (as row %) | — | 0.77 | ||||
| White | 23.7 | 25.3 | 26.9 | 24.1 | ||
| Black | 31.4 | 20.2 | 21.8 | 26.7 | ||
| Hispanic | 23.0 | 26.6 | 25.9 | 24.5 | ||
| Native American | 23.0 | 30.3 | 20.4 | 26.3 | ||
| Asian | 22.4 | 22.4 | 30.6 | 24.7 | ||
| Family history of diabetes (% yes) | 70.8 | 70.9 | 68.3 | 68.4 | — | 0.94 |
| History of gestational diabetes (% yes) | 16.0 | 15.8 | 15.3 | 17.2 | — | 0.78 |
| BMI (kg/m2) | 33 ± 6 | 34 ± 7 | 34 ± 6 | 35 ± 7 | 0.09 | <0.01 |
| Waist circumference (cm) | 105 ± 13 | 104 ± 15 | 104 ± 14 | 107 ± 15 | 0.07 | <0.01 |
| Fasting glucose (mg/dl) | 107 ± 9 | 106 ± 8 | 106 ± 8 | 107 ± 8 | <−0.01 | 0.94 |
| 2-h OGTT (mg/dl) | 165 ± 17 | 164 ± 17 | 164 ± 17 | 166 ± 17 | 0.03 | 0.06 |
| Fasting insulin (pmol/l) | 25 ± 13 | 26 ± 14 | 27 ± 16 | 29 ± 17 | 0.08 | <0.01 |
| A1C (%) | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 | 6.0 ± 0.5 | 0.05 | 0.01 |
| Homeostasis model assessment of insulin resistance | 6.6 ± 3.6 | 7.0 ± 3.9 | 7.0 ± 4.4 | 7.7 ± 4.7 | 0.07 | <0.01 |
| Systolic blood pressure (mmHg) | 120 ± 14 | 122 ± 14 | 124 ± 14 | 129 ± 16 | 0.2 | <0.01 |
| Diastolic blood pressure (mmHg) | 77 ± 9 | 77 ± 9 | 78 ± 9 | 81 ± 10 | 0.1 | <0.01 |
| Smoking (%) | 7.9 | 5.5 | 5.5 | 8.8 | — | 0.27 |
| Serum creatinine (mg/dl) | 0.83 ± 0.17 | 0.78 ± 0.17 | 0.76 ± 0.16 | 0.76 ± 0.18 | −0.16 | <0.01 |
| Total cholesterol (mg/dl) | 203 ± 36 | 202 ± 37 | 205 ± 36 | 204 ± 37 | 0.02 | 0.21 |
| Triglycerides (mg/dl) | 160 ± 89 | 164 ± 91 | 160 ± 92 | 171 ± 111 | 0.01 | 0.47 |
| Medication usage (%) | ||||||
| ACE inhibitor | 6.1 | 6.5 | 8.3 | 10.2 | — | <0.01 |
| Angiotensin receptor blocker | 1.0 | 0.9 | 1.0 | 1.0 | — | 0.91 |
| Calcium channel blocker | 5.5 | 5.1 | 6.9 | 12.0 | — | <0.01 |
| Diuretic† | 1.8 | 2.0 | 1.8 | 1.9 | — | 0.50 |
| Study randomization (%) | — | 0.75 | ||||
| Lifestyle | 32.4 | 34.8 | 31.5 | 34.9 | ||
| Metformin | 35.9 | 30.2 | 32.7 | 33.4 | ||
| Placebo | 31.7 | 35.0 | 35.8 | 31.7 | ||
Data are median (25th–75th quartile) or means ± SD unless indicated otherwise.
P values are calculated using Spearman's rank correlation for continuous variables (e.g., age or BMI). For categorical variables (e.g., sex or race), P values are from ANOVA tests using base 2 logarithm of urinary albumin excretion.
Nine of the 60 participants taking diuretics at baseline were taking thiazides, which is a protocol violation.
The cross-sectional association between baseline characteristics and ACR is shown in Table 1. ACR was positively associated with age and markers of adiposity and insulin secretion and resistance, blood pressure, and use of antihypertensive agents with antiproteinuric effects and was inversely related to male sex and serum creatinine, both of which influence urinary excretion of creatinine, the denominator in the ACR. Race/ethnicity, prior gestational diabetes, family history of diabetes, serum lipids, and smoking were not significantly associated with ACR.
ACR and development of diabetes
The 3,188 participants were followed for a mean of 3.2 years (range 0–5.0 years), during which 674 (21%) developed diabetes. A test of heterogeneity revealed a significant interaction between ACR, treatment group, and diabetes risk, so the analysis was stratified by treatment group. Table 2 shows HRs for incident diabetes by ACR quartile for the unadjusted and fully adjusted stratified models. No consistent pattern was seen for either adjusted or unadjusted models or with a model adjusting for demographic characteristics alone (data not shown). When ACR was examined as a continuous variable, the unadjusted model showed a 7% increase in incident diabetes with every doubling of ACR (hazard ratio [HR] 1.07 [95% CI 1.0–1.1]), but statistical significance was lost (0.98 [0.91–1.06]) when the model was fully adjusted for the covariates baseline age, sex, race, BMI, waist circumference, fasting insulin, insulin sensitivity/secretion, systolic and diastolic blood pressure, serum creatinine, and ACE inhibitor and calcium channel blocker use and for time-dependent changes in weight and physical activity.
Table 2.
Relative hazards for developing diabetes in the DPP by quartile of baseline ACR
| Study arm | HRs (95% CI) for quartile of ACR |
|||
|---|---|---|---|---|
| 1 (0.7–3.7 mg/g) | 2 (3.7–5.5 mg/g) | 3 (5.5–9.7 mg/g) | 4 (9.7–1,578 mg/g) | |
| Placebo (n = 1,070) | ||||
| Unadjusted | 1.0 | 0.66 (0.47–0.93) | 0.81 (0.58–1.12) | 1.01 (0.72–1.37) |
| Adjusted* | 1.0 | 0.51 (0.33–0.79) | 0.68 (0.44–1.03) | 0.76 (0.50–1.15) |
| Lifestyle (n = 1,064) | ||||
| Unadjusted | 1.0 | 1.64 (0.99–2.72) | 1.57 (0.93–2.64) | 1.85 (1.12–3.06) |
| Adjusted* | 1.0 | 1.56 (0.83–2.93) | 1.15 (0.58–2.29) | 1.62 (0.84–3.14) |
| Metformin (n = 1,054) | ||||
| Unadjusted | 1.0 | 1.21 (0.82–1.78) | 1.13 (0.76–1.66) | 1.39 (0.96–2.03) |
| Adjusted* | 1.0 | 1.21 (0.76–1.93) | 1.03 (0.64–1.64) | 0.98 (0.60–1.58) |
Data are median (range). ACR is calculated as milligrams of albumin/grams of creatinine.
Adjusted for baseline: age, sex, race, BMI, waist circumference, fasting insulin, insulin sensitivity/secretion, systolic and diastolic blood pressure, serum creatinine, and ACE inhibitor and calcium channel blocker use. Also adjusted are time-dependent changes in weight and physical activity.
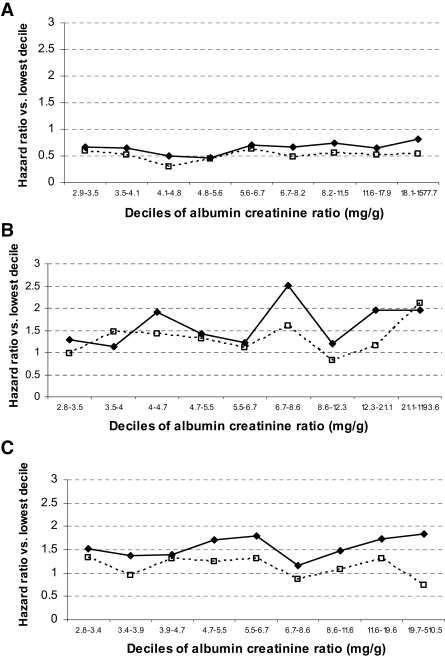
To test the possibility that examining quartiles of ACR may not have been sensitive enough to reveal an association between incident diabetes and ACR at its higher range (e.g., microalbuminuria), as suggested by other studies (10,11), HRs were examined after each DPP treatment cohort was separately divided into 10 equal groups by ACR (∼100 participants/group) (Fig. 1). No consistent pattern was observed between ACR and incident diabetes before or after full adjustment for covariates in the highest decile, in which 191 of 318 subjects had micro- or macroalbuminuria.
Figure 1.
HRs for incident diabetes in DPP by baseline urinary albumin creatinine ratio. Placebo group (A), lifestyle group (B), metformin group (C).  , unadjusted data; □, adjusted data.
, unadjusted data; □, adjusted data.
CONCLUSIONS
Identifying a simple, safe, and inexpensive tool to improve prediction of future diabetes would be an important public health achievement, especially in light of the ongoing diabetes pandemic. Preliminary results from several observational studies raise the possibility that low levels of ACR could play such a role, with further (indirect) evidence being that proteinuria-reducing antihypertensive agents are associated with a reduced risk of incident diabetes (13,14). However, in the present study, the largest study of pre-diabetic individuals to date, we did not find that ACR had any independent predictive value.
Our study hypothesis was not necessarily dependent upon a causal link between increasing ACR and onset of diabetes. For example, one possible premise is that exposure to levels of glycemia below what is conventionally considered pathological induces changes in renal handling of urinary albumin, which in turn would lead to increased ACR. Alternatively, increased ACR could simply be one of a number of early, organ-specific manifestations of insulin resistance that herald the onset of diabetes. Regardless, we felt it important to test this hypothesis, especially given prior findings.
Results from previous observational studies have supported an association between urinary albumin excretion and incident diabetes. In a longitudinal study of 2,205 American Indians, ACR of ≥30 mg/day (i.e., microalbuminuria or macroalbuminuria) predicted incident diabetes over an average of 4 years of follow-up in a combined group of men with normal or IGT (odds ratio 2.19 [95% CI 1.48–3.21]) and women with baseline IGT only (2.69 [1.41–5.21]) (11). A prospective, community-based Dutch study of 5,654 individuals with normal or impaired glucose tolerance found a stepwise increase in the 4-year risk of incident diabetes by baseline urinary albumin excretion as measured by 24-h urine collections (tertile 1, ≤6.9 mg/kg, 1.8%; tertile 2, 6.9–12.4 mg/kg, 2.3%; tertile 3, ≥12.4 mg/kg, 5.8%; P < 0.001) (12). Results were relatively unchanged when individuals with baseline IGT were excluded from the analysis. Mykkänen et al. (10) observed a significantly higher proportion of baseline microalbuminuria (44.4% vs. 30.4%, P = 0.017) in elderly Finns who developed diabetes after 3.5 years of follow-up (compared with those who did not), although there was no actual difference in mean ACR between groups. In this study, the increased odds of developing diabetes were no longer statistically significant after adjustment for fasting plasma glucose and insulin.
Our study contributes new information that has been lacking in several important ways. First, our cohort was composed exclusively of individuals who were at high risk of developing diabetes on the basis of elevated fasting glucose and IGT plus overweight or obesity (for the majority). Because our sample size was far larger than all the IGT subgroups from the previously mentioned studies combined, it is unlikely that our negative findings were related to insufficient statistical power. Interestingly, despite their elevated risk for diabetes, the great majority of our cohort did not have microalbuminuria. Second, we analyzed ACR throughout its continuous range, avoiding artificial cutoff values, such as microalbuminuria, that could limit its descriptive utility (6). On the other hand, the DPP included very few subjects with micro- or macroalbuminuria, which could have reduced the power to show an association in these ranges and the generalizability of our results. Indeed, the predictive power of ACR could have been obscured by the increased risk for development of diabetes present in the DPP cohort at baseline. The fact that ACR was so closely associated with insulin or glycemic parameters supports this hypothesis. In addition, we excluded individuals with chronic kidney disease, further reducing generalizability, and measured ACR only once at baseline. ACR can be affected by diet, physical activity, and other habits, which may have introduced some variability into our findings, although this would be limited somewhat by the uniform collection criterion (i.e., fasting morning sample). Finally, major differences between the DPP and prior studies were the ethnically and culturally diverse cohort and the fact that we detected early diabetes by annual or semiannual surveillance glucose tolerance tests using accepted criteria (17,21), thus reducing the likelihood that ACR reflected prior exposure to severe hyperglycemia.
The study hypothesis was based on the presumption that subtle damage occurs within the kidney in the pre-diabetic state—whether from chronic exposure to abnormal glycemia that is below the formal threshold for diabetes, elevated intrarenal blood pressure, or oxidative stress (22), among other causes—that manifests itself as elevated ACR. ACR, as a subtle marker of incipient damage, could in turn herald the onset of diabetes. Although we did not confirm such a relationship, this does not exclude the possibility that ACR can predict hard outcomes in this population, as it has in others.
In summary, in a population of subjects at elevated risk for diabetes, ACR below the microalbuminuria range does not predict incident diabetes.
Supplementary Material
Acknowledgments
A.N.F. is supported by the National Institutes of Health (K23 RR019615).
Published ahead of print at http://care.diabetesjournals.org on 16 September 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study Group: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, Hope Study Investigators: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286:421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL: Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Ann Intern Med 123:754–762, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Brantsma AH, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT: Urinary albumin excretion as a predictor of the development of hypertension in the general population. J Am Soc Nephrol 17:331–335, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Forman JP, Brenner BM: ‘Hypertension’ and ‘microalbuminuria’: the bell tolls for thee. Kidney Int 69:22–28, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Mykkänen L, Zaccaro DJ, O'Leary DH, Howard G, Robbins DC, Haffner SM: Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects: the Insulin Resistance Atherosclerosis Study (IRAS). Stroke 28:1710–1716, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM: Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes 47:793–800, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Palaniappan L, Carnethon M, Fortmann SP: Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens 16:952–958, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Mykkänen L, Haffner SM, Kuusisto J, Pyorala K, Laakso M: Microalbuminuria precedes the development of NIDDM. Diabetes 43:552–557, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Lee ET, Welty TK, Cowan LD, Wang W, Rhoades DA, Devereux R, Go O, Fabsitz R, Howard BV: Incidence of diabetes in American Indians of three geographic areas: the Strong Heart Study. Diabetes Care 25:49–54, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT, PREVEND Study Group. Urinary albumin excretion and its relation with C-reactive protein and the metabolic syndrome in the prediction of type 2 diabetes. Diabetes Care 28:2525–2530, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, Luomanmaki K, Dahlof B, de Faire U, Morlin C, Karlberg BE, Wester PO, Bjorck JE: Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet 353:611–616, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, Fodor G, Holman RR, Dream Trial Investigators: Effect of ramipril on the incidence of diabetes. N Engl J Med 355:1551–1562, 2006 [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Prevention Program Research Group: The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22:623–634, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553, 1998 [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Prevention Program Research Group: The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 23:1619–1629, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H: Glucose tolerance and insulin release, a mathematical approach I. Assay of the β-cell response after oral glucose loading. Diabetes 25:241–244, 1976 [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Duncan ER, Walker SJ, Ezzat VA, Wheatcroft SB, Li JM, Shah AM, Kearney MT: Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am J Physiol Endocrinol Metab 293:E1311–E1319, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.