Abstract
OBJECTIVE—While women with prior gestational diabetes mellitus (GDM) are more likely to display features of the metabolic syndrome, including hypertension, in the years after delivery, it is unclear whether these components are also present before pregnancy. We examined the relationship between blood pressure (BP) measured before and during early pregnancy (<20 weeks) and the risk of GDM in a nested case-control study.
RESEARCH DESIGN AND METHODS—Case (n = 381) and control (n = 942) subjects were selected from a cohort of women delivering between 1996 and 1998 and screened for GDM between 24 and 28 weeks’ gestation. GDM was defined by the National Diabetes Data Group criteria. BP and covariates data were obtained by review of the medical records. Women were categorized according to BP levels recommended by the American Heart Association outside of pregnancy: <120/80 mmHg (normal), 120–139/80–89 mmHg (prehypertension), and ≥140 and/or ≥90 mmHg or use of antihypertensive medications (hypertension).
RESULTS—During early pregnancy, women with prehypertension had a small increased risk of GDM (odds ratio [OR] 1.56 [95% CI 1.16–2.10]), and women with hypertension had a twofold increased risk of GDM (2.04 [1.14–3.65]) compared with women with normal BP after adjusting for age, race/ethnicity, gestational week of BP, BMI, and parity. Similar results were seen among the subset of women with BP levels measured before pregnancy (1.44 [0.95–2.19] for prehypertension and 2.01 [1.01–3.99] for hypertension).
CONCLUSIONS—Clinicians should be aware that women presenting with hypertension may warrant early screening or intervention to prevent GDM.
Type 2 diabetes and hypertension are both components of the metabolic syndrome and commonly occur together in individuals. A recent study of initially healthy middle-aged women found that blood pressure (BP) predicted the development of incident type 2 diabetes independent of BMI and other known diabetes risk factors (1). Several studies have shown that women with a history of gestational diabetes mellitus (GDM) are more likely to have features of the metabolic syndrome, including high BP, in the years after delivery (2–5). It is unclear whether elevated BP before or during early pregnancy is associated with the development of GDM.
Crowther et al. (6) showed that treatment of mild-to-moderate levels of glucose intolerance in midpregnancy effectively reduced both perinatal and maternal complications. Therefore, identifying additional variables that predict the development of GDM may help identify women who would benefit from early screening and, if needed, early treatment of pregnancy hyperglycemia to prevent perinatal complications. Because BP is a vital sign that is measured at each medical visit, it would be an easy and inexpensive clinical characteristic that could be used to identify women at risk of GDM. We therefore evaluated the relationship between BP before and during early pregnancy (<20 weeks’ gestation) and risk of GDM in a nested case-control study among women who delivered singleton live infants at a large U.S. group practice prepaid health plan and received uniform screening and a standardized diagnostic test for GDM.
RESEARCH DESIGN AND METHODS
Setting
The study setting was the Kaiser Permanente Medical Care Program of Northern California (KPMCP-NC), which at the time provided comprehensive medical services through 15 hospitals and 23 outpatient clinics to over 3 million members located in a 14-county region in northern California. The KPMCP-NC membership represents ∼30% of the surrounding population, and it is representative of the population living in the same geographic area demographically, ethnically, and socioeconomically, except that the KPMCP-NC membership underrepresents the very poor and the very wealthy (7).
Cohort identification
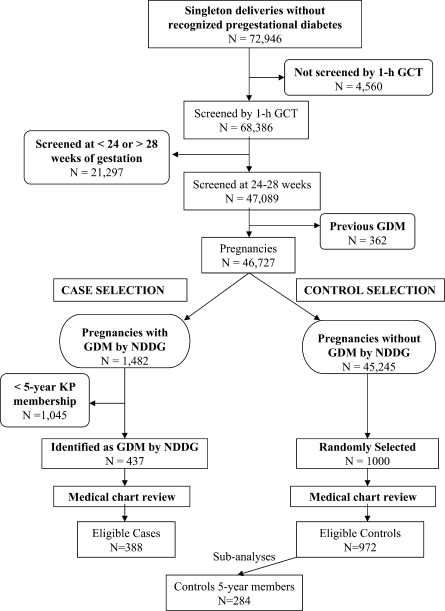
The methods used to identify this cohort have been described in detail elsewhere (8). Briefly, we identified all pregnancies that resulted in a singleton live birth between 1 January 1996 and 30 June 1998 among women without recognized diabetes before the index pregnancy. All pregnancies resulting in a singleton live birth, screened for GDM between 24–28 weeks’ gestation, and without a diagnosis of GDM in a prior pregnancy were eligible to be selected for this study (Fig. 1). Briefly, we had 46,727 singleton pregnancies that were screened for GDM at 24–28 weeks, according to the earliest ultrasound. All 437 GDM case subjects (according to the NDDG criteria) that had been in the health plan for at least 5 years were selected for medical chart review, and 388 were included in the study. A total of 1,000 control subjects were randomly selected among 45,245 pregnancies with normoglycemia; 972 met the eligibility criteria after medical chart review, and 29% were members of the health plan for at least 5 years and served as control subjects for the subanalyses on “prepregnancy” BP levels and risk of GDM.
Figure 1.
GCT, glucose challenge test; NDDG, National Institute of Diabetes and Digestive and Kidney Diseases.
Medical chart review
Trained medical records abstractors completed chart review on the selected case and control subjects and confirmed if criteria for inclusion were met and if any of the exclusion criteria were present.
Case definition
Women were classified as having GDM at the index pregnancy if two or more of the four plasma glucose values obtained during the 100-g 3-h OGTT were abnormal according to the NDDG criteria (9): fasting ≥105 mg/dl (5.8 mmol/l), 1 h ≥190 mg/dl (10.5 mmol/l), 2 h ≥165 mg/dl (9.1 mmol/l), and 3 h ≥145 mg/dl (8.0 mmol/l). All plasma glucose measurements were performed using the hexokinase method by the regional laboratory of Kaiser Permanente Northern California. This laboratory participates in the College of American Pathologists’ accreditation and monitoring program.
Data collection
Trained medical chart abstractors reviewed all available medical records to obtain information on subjects’ measured BP before and during early pregnancy and potential confounders. Abstractors recorded the first BP measured during a prenatal visit provided it was performed before 20 weeks’ gestation, according to the earliest ultrasound. For pregravid BP, the BP measured closest to but before the last menstrual period and no more than 5 years before pregnancy was recorded. The BP measured before pregnancy was not recorded if it was from an emergency or urgent-care visit. Information on use of BP medications was obtained from the medical chart.
Other information obtained on potential confounders during the medical record review included any body weights measured before pregnancy in a nonpregnant state. Last menstrual period before the index pregnancy, gestational week at the earliest ultrasound used to calculate gestational age at screening for GDM, marital status, family history of diabetes, family history of hypertension, self-reported prepregnancy weight, weight at first prenatal visit, parity, and height were abstracted from the prenatal form completed at the first prenatal visit. Prepregnancy weight was defined as the last recorded weight found in the chart before the woman's last menstrual period for the index pregnancy. For the 14.4% of women for whom these data were not available, the self-reported prepregnancy weight on the prenatal form was used. Prepregnancy BMI was calculated as prepregnancy weight in kilograms divided by the square of height in meters. BMI at first prenatal visit was calculated as weight in kilograms measured at first prenatal visit divided by the square of height in meters. Women's self-reported race/ethnicity and education were obtained by linkage to the electronic birth certificate database. Gestational age at first prenatal visit with a BP measurement was calculated from the earliest ultrasound performed before 24 weeks’ gestation.
Exposure definition
For both prepregnancy BP and BP at first prenatal visit, women were classified into three predefined BP categories according the American Heart Association's criteria: <120 mmHg for systolic and <80 mmHg for diastolic BP (subsequently call normal BP), 120–139 mmHg for systolic and/or 80–89 mmHg for diastolic (subsequently called prehypertension), and at least 140 mmHg for systolic or at least 90 mmHg for diastolic or a prescription for antihypertensive treatment found in the medical chart (subsequently referred to as hypertension) (10).
Missing data
Women for whom information on either early pregnancy or prepregnancy BP was unavailable were excluded from that particular analysis. BP measured during the first prenatal visit was missing for 7 (1.8%) case subjects and 30 (3.1%) control subjects.
BP before pregnancy was not abstracted for 185 of the control subjects. Among case and control subjects who had BP before pregnancy abstracted, these data were missing for 6 (1.5%) case subjects and 186 (23.6%) control subjects. Information on BP before pregnancy was missing for a larger proportion of control subjects in part because the question was not initially included on the questionnaire at the beginning of the study (11) that provided data on control subjects.
A total of 381 case subjects and 942 control subjects remained for the analysis of early pregnancy BP, and 381 case subjects and 289 control subjects remained for the subanalysis of prepregnancy BP.
Statistical methods
Unconditional logistic regression was used to obtain odds ratios (ORs) as estimates of the relative risk of GDM in relation to category of BP. Women with normal BP were used as the reference group. To assess confounding, we entered covariates into a logistic regression model one at a time and then compared the adjusted and unadjusted odds ratios (12). Final logistic regression models included covariates that altered unadjusted odds ratios for BP by at least 10% as well as those covariates of a priori interest (i.e., parity). Variables evaluated for confounding were maternal age, race/ethnicity, pregravid BMI (kilograms divided by the square of height in meters), parity, maternal education in years, family history of diabetes (yes/no), and family history of hypertension (yes/no). BMI at first prenatal visit when the BP was measured and gestational week at BP measurement were also included in the adjusted model assessing BP during early pregnancy. For the analysis of BP before pregnancy and GDM, we adjusted for prepregnancy BMI and time between BP measurement and pregnancy. To assess the potential modifying effects of BMI (overweight ≥25 kg/m2 vs. not overweight <25 kg/m2), age (<30 vs. ≥30 years), family history of diabetes (first- or second-degree versus none), parity (one or more live births vs. none), and race/ethnicity (non-Hispanic white vs. African American, Asian, and Hispanic women), we examined interaction terms and repeated analyses within these subgroups. This study was approved by the human subjects committee of the Kaiser Foundation Research Institute.
RESULTS
Characteristics of women with GDM and control subjects are presented in Table 1. Women with GDM were more likely to be Hispanic, Asian, or from other nonwhite racial groups; to be older than 35 years; to have ≤12 years of education; to have a family history of diabetes; to have two or more prior live-births; and to be overweight or obese at the first prenatal visit. GDM case subjects were also more likely to have hypertension or prehypertension both before and early in pregnancy. Mean gestational age at first BP measurement was 11.5 weeks for case subjects and 11.7 weeks for control subjects. Pregravid BP was measured on average 9.7 ± 12.3 months (mean ± SD) before the woman's last menstrual period for case subjects and 8.1 ± 8.5 months for control subjects.
Table 1.
Selected characteristics of case and control subjects
| GDM case subjects* | Control subjects | |
|---|---|---|
| n | 381 | 942 |
| Age (years) | ||
| <25 | 26 (6.8) | 229 (24.3) |
| 25–29 | 52 (13.6) | 226 (24.0) |
| 30–34 | 132 (34.6) | 303 (32.2) |
| ≥35 | 171 (44.9) | 184 (19.5) |
| Race/ethnicity | ||
| Non-Hispanic white | 168 (44.1) | 502 (53.3) |
| Hispanic | 75 (19.7) | 166 (17.6) |
| Asian | 47 (12.3) | 82 (8.7) |
| African American | 29 (7.6) | 90 (9.6) |
| Other | 58 (15.2) | 99 (10.5) |
| Unknown | 4 (1.0) | 3 (0.3) |
| Marital status | ||
| Never married | 54 (14.2) | 226 (24.0) |
| Married | 300 (78.7) | 689 (73.1) |
| Widowed, divorced, or separated | 16 (4.2) | 21 (2.2) |
| Unknown | 11 (2.9) | 6 (0.6) |
| Education (years) | ||
| ≤12 | 142 (37.3) | 392 (41.6) |
| 13–15 | 124 (32.5) | 267 (28.3) |
| 16 | 73 (19.2) | 159 (16.9) |
| ≥17 | 37 (9.7) | 115 (12.2) |
| Unknown | 5 (1.3) | 9 (1.0) |
| Parity | ||
| 0 | 148 (38.8) | 405 (43.0) |
| 1 | 125 (32.8) | 343 (36.4) |
| ≥2 | 108 (28.3) | 194 (20.6) |
| Family history of diabetes | ||
| First-degree relative | 87 (22.8) | 104 (11.0) |
| Second-degree relative | 98 (25.7) | 204 (21.7) |
| None | 154 (40.4) | 561 (59.6) |
| Unknown | 42 (11.0) | 73 (7.7) |
| Family history of hypertension | ||
| First-degree relative | 113 (29.7) | 233 (24.7) |
| Second-degree relative | 41 (10.8) | 100 (10.6) |
| None | 168 (44.1) | 532 (56.5) |
| Unknown | 59 (15.5) | 77 (8.2) |
| BMI at first prenatal visit (kg/m2) | ||
| <20 | 14 (3.7) | 98 (10.4) |
| 20.1–24.9 | 96 (25.2) | 423 (44.9) |
| 25.0–29.9 | 126 (33.1) | 250 (26.5) |
| ≥30 | 142 (37.3) | 149.0 (15.8) |
| Unknown | 3 (0.8) | 22.0 (2.3) |
| Blood pressure during pregnancy | ||
| Normal | 197 (51.7) | 668 (70.9) |
| Prehypertenison | 147 (38.6) | 240 (25.5) |
| Hypertension | 37 (9.7) | 34 (3.6) |
Data are n (%).
GDM equals NDDG criteria. Normal BP: ≤120/80 mmHg; prehypertension: 120–139/80–89 mmHg; and hypertension: ≥140 and/or ≥90 mmHg or use of antihypertensive drugs.
After adjustment for age, race/ethnicity, parity, BMI, family history of diabetes, and gestational age at BP measurement, GDM risk increased among women with prehypertension and to a stronger degree among women with hypertension during early pregnancy (OR 1.41 [95% CI 1.04–1.90] and 2.08 [1.17–3.69], respectively) (Table 2). We examined the interaction terms between covariates presented in Table 2 and BP in the association with GDM risk; however, none of the interaction terms were statistically significant and the P values ranged from 0.25 for age and BMI to 0.91 for family history of diabetes. We also reran the analysis after excluding 11 case subjects and 8 control subjects who were taking antihypertensive medications, and the associations did not change significantly.
Table 2.
ORs and 95% CI for GDM associated with GDM risk factors and BP during the first prenatal visit
| Case subjects | Control subjects | Crude OR (95% CI) | Adjusted OR* (95% CI) | |
|---|---|---|---|---|
| n | 381 | 942 | ||
| Blood pressure | ||||
| Normal | 197 (51.7) | 668 (70.9) | 1.00 | 1.00 |
| Prehypertension | 147 (38.6) | 240 (25.5) | 2.08 (1.60–2.60) | 1.56 (1.16–2.10) |
| Hypertension | 37 (9.7) | 34 (3.6) | 3.69 (2.26–6.04) | 2.04 (1.14–3.65) |
| Age ≥30 years | 308 (79.4) | 502 (51.7) | 3.54 (2.57–4.88) | |
| BMI ≥25 kg/m2 | 276 (56.4) | 426 (44.7) | 2.43 (1.82–3.25) | |
| Nonwhite | 219 (56.4) | 458 (47.1) | 1.77 (1.34–2.34) | |
| First- or second-degree relative with diabetes | 189 (54.7) | 320 (35.7) | 1.73 (1.32–2.28) | |
| 1+ live births | 238 (61.3) | 556 (57.2) | 0.77 (0.57–1.04) |
Data are n (%) unless otherwise indicated.
ORs from multivariate model adjusted for age, BMI at first prenatal visit, race/ethnicity, family history of diabetes, parity, and gestational week at BP measurement.
Stratified analyses suggested that the association between elevated early pregnancy BP and GDM was stronger among women known to be at high risk for GDM. Women who were overweight or obese (BMI ≥25.0 kg/m2) before pregnancy presenting with hypertension during the first trimester of pregnancy had an almost threefold increased risk of developing GDM (OR 2.76 [95% CI 1.46–5.23]), whereas the corresponding OR for women who were not overweight was 1.26 (95% CI 0.35–4.35).
In the subsample of women who had been members of KPMCP-NC for 5 years before pregnancy and with information on BP before pregnancy and smoking status, GDM risk was associated with an increased risk among women with prehypertension and hypertension, and the magnitude of the association was similar to that found with BP during pregnancy (Table 3).
Table 3.
ORs and 95% CI for GDM associated with BP before pregnancy
| Case subjects | Control subjects | Crude OR (95% CI) | Adjusted OR* (95% CI) | |
|---|---|---|---|---|
| n | 381 | 289 | ||
| Blood pressure | ||||
| Normal | 152 (39.9) | 329 (54.7) | 1.00 | 1.00 |
| Prehypertension | 181 (47.5) | 230 (38.3) | 1.66 (1.20–2.30) | 1.44 (0.95–2.19) |
| Hypertension | 48 (12.6) | 42 (7.0) | 2.18 (1.26–3.79) | 2.01 (1.01–3.99) |
Data are n (%) unless otherwise indicated. Data are from the subset of women who were members of KPNC 5 years before pregnancy.
Adjusted for age, race/ethnicity, prepregnancy BMI, parity, smoking status, family history of diabetes, and time between BP measurement and pregnancy.
We also reran the analysis after excluding 11 case subjects and 8 control subjects who took antihypertensive medications before pregnancy, and the associations did not change.
CONCLUSIONS
In this study, women with hypertension either during the 5 years before pregnancy or during the first trimester of pregnancy had a twofold increased risk of developing GDM during pregnancy. While attenuated, these associations persisted after adjusting for BMI, suggesting that the association is independent of BMI. However, the association between BP and GDM was stronger among women who were overweight (BMI ≥25.0 kg/m2).
A recent study among initially healthy women found high BP was associated with a twofold increased risk of developing type 2 diabetes during 4 years of follow-up after adjusting for known predictors of diabetes (1). Data on BP before or during pregnancy in relation to the occurrence of GDM are sparse. Our results are generally consistent with the one previous study of BP and risk of GDM (13). Lao and Ho (13) examined first-trimester BP and risk of GDM among 131 high-risk Chinese women and found that systolic BP above the median (109 mmHg) had a fourfold increased risk of GDM (OR 4.20 [95% CI 1.97–8.94]). While the magnitude of the association they found was greater than our findings, they examined only high-risk women referred to a clinic providing antenatal care, categorized women according to a dichotomous cutoff for systolic BP, and had limited information on confounders.
This study is the first to our knowledge to examine BP and GDM in an ethnically diverse population of women who underwent uniform screening for GDM. This study has several strengths, including the ability to assess measured BPs obtained pregravid and risk of GDM. A clear definition of GDM was based on objective measures of pregnancy glycemia among a cohort with universal screening performed at 24–28 weeks’ gestation. Given the extensive chart review, we were also able to identify and exclude women with recognized preexisting diabetes before pregnancy. Finally, we were able to control for several important potential confounding factors.
This study also has several limitations. During normal pregnancy, BP steadily decreases up to 21 weeks’ gestation and then increases during the second half of pregnancy (14). Our assessment of hypertension during pregnancy probably only captured severe case subjects in whom BP remained elevated even during early pregnancy. Other limitations of this study include the reliance on a single measure of BP, which may be influenced by external and internal stimuli, such as physical activity, diet, and emotional state. For the main analysis, we lacked information on smoking, a potential confounder; however, in a subanalysis limited to women who were members of the health plan for 5 years before pregnancy, we had smoking history and it did not confound the association. For the analysis of pregravid BP and GDM, we were missing information on BP on a large portion of control subjects; however, the magnitude of the association between early pregnancy BP and GDM was similar and had very little missing data. Use of certain types of antihypertensive medications may be associated with risk of type diabetes (15); however, sensitivity analyses excluding the small number of women who used antihypertensive drugs pregravid did not change our results.
Several studies have examined the metabolic syndrome or its components 2–11 years after pregnancy, and in most of these studies, mean BP levels were significantly higher among the women with prior GDM compared with women who had normal glycemia during pregnancy (3–5,16). We found the association between high BP and GDM was stronger among overweight or obese women, suggesting that these two components of the metabolic syndrome may interact synergistically to produce adverse metabolic effects that predispose to GDM during pregnancy.
There are several common pathogenic pathways to hypertension and GDM that may be underlying the association between the two conditions. Insulin resistance has been shown to be a contributing factor to both chronic (17) and gestational hypertension (18), and it is known to be involved in the pathogenesis of GDM (19). Endothethial dysfunction has been found in women with GDM both during (20) and after (21) pregnancy and is also closely related to hypertension (22). Finally, markers of inflammation such as C-reactive protein have been associated with increased BP levels (23), and elevated early pregnancy CRP levels have been related to increased risk of GDM (24).
In summary, our data suggest that women presenting with high BP, especially those who are overweight, are at increased risk of developing GDM during pregnancy. Clinicians should be aware that this subgroup of women may warrant the initiation of early screening or dietary and exercise interventions to prevent the development of GDM.
Acknowledgments
This research was supported by grant R01 DK 54834 from the National Institute of Diabetes and Digestive and Kidney Diseases and a Research Award from the American Diabetes Association.
Published ahead of print at http://care.diabetesjournals.org on 22 September 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ: Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J 28:2937–2943, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Lauenborg J, Mathiesen E, Hansen T, Glumer C, Jorgensen T, Borch-Johnsen K, Hornnes P, Pedersen O, Damm P: The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 90:4004–4010, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Verma A, Boney CM, Tucker R, Vohr BR: Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J Clin Endocrinol Metab 87:3227–3235, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Kousta E, Efstathiadou Z, Lawrence NJ, Jeffs JA, Godsland IF, Barrett SC, Dore CJ, Penny A, Anyaoku V, Millauer BA, Cela E, Robinson S, McCarthy MI, Johnston DG: The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia 49:36–40, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Meyers-Seifer CH, Vohr BR: Lipid levels in former gestational diabetic mothers. Diabetes Care 19:1351–1356, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS: Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352:2477–2486, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Krieger N: Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 82:703–710, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedderson MM, Ferrara A, Williams MA, Holt VL, Weiss NS: Androgenicity of progestins in hormonal contraceptives and the risk of gestational diabetes mellitus. Diabetes Care 30:1062–1068, 2007 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association: Gestational diabetes mellitus. Diabetes Care 21:S60–S61, 1998 [Google Scholar]
- 10.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J: Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 114:82–96, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ferrara A, Weiss NS, Hedderson MM, Quesenberry CP Jr, Selby JV, Ergas IJ, Peng T, Escobar GJ, Pettitt DJ, Sacks DA: Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia 50:298–306, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Rothman KJ, Greenland S: Modern Epidemiology. Philadelphia, Lippincott-Raven Publishers, 1998
- 13.Lao TT, Ho LF: First-trimester blood pressure and gestational diabetes in high-risk Chinese women. J Soc Gynecol Investig 10:94–98, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hermida RC, Ayala DE, Mojon A, Fernandez JR, Alonso I, Silva I, Ucieda R, Iglesias M: Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension 36:149–158, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL: Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus: Atherosclerosis Risk in Communities Study. N Engl J Med 342:905–912, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Albareda M, Caballero A, Badell G, Rodriguez-Espinosa J, Ordonez-Llanos J, de Leiva A, Corcoy R: Metabolic syndrome at follow-up in women with and without gestational diabetes mellitus in index pregnancy. Metabolism 54:1115–1121, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S: Insulin resistance in essential hypertension. N Engl J Med 317:350–357, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Carpenter MW: Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care 30(Suppl. 2):S246–S250, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Buchanan TA, Xiang AH: Gestational diabetes mellitus. J Clin Invest 115:485–491, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, Stamatelopoulos SF: Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care 21:2111–2115, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Gokce N, Holbrook M, Duffy SJ, Demissie S, Cupples LA, Biegelsen E, Keaney JF Jr, Loscalzo J, Vita JA: Effects of race and hypertension on flow-mediated and nitroglycerin-mediated dilation of the brachial artery. Hypertension 38:1349–1354, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE: Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53:693–700, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Qiu C, Sorensen TK, Luthy DA, Williams MA: A prospective study of maternal serum C-reactive protein (CRP) concentrations and risk of gestational diabetes mellitus. Paediatr Perinat Epidemiol 18:377–384, 2004 [DOI] [PubMed] [Google Scholar]