Abstract
Transcriptional silencing of genes transferred into hematopoietic stem cells poses one of the most significant challenges to the success of gene therapy. If the transferred gene is not completely silenced, a progressive decline in gene expression as the mice age often is encountered. These phenomena were observed to various degrees in mouse transplant experiments using retroviral vectors containing a human β-globin gene, even when cis-linked to locus control region derivatives. Here, we have investigated whether ex vivo preselection of retrovirally transduced stem cells on the basis of expression of the green fluorescent protein driven by the CpG island phosphoglycerate kinase promoter can ensure subsequent long-term expression of a cis-linked β-globin gene in the erythroid lineage of transplanted mice. We observed that 100% of mice (n = 7) engrafted with preselected cells concurrently expressed human β-globin and the green fluorescent protein in 20–95% of their RBC for up to 9.5 mo posttransplantation, the longest time point assessed. This expression pattern was successfully transferred to secondary transplant recipients. In the presence of β-locus control region hypersensitive site 2 alone, human β-globin mRNA expression levels ranged from 0.15% to 20% with human β-globin chains detected by HPLC. Neither the proportion of positive blood cells nor the average expression levels declined with time in transplanted recipients. Although suboptimal expression levels and heterocellular position effects persisted, in vivo stem cell gene silencing and age-dependent extinction of expression were avoided. These findings support the further investigation of this type of vector for the gene therapy of human hemoglobinopathies.
Keywords: gene therapy, thalassemia, sickle cell disease, retrovirus, green fluorescent protein
Gene silencing is commonly defined as a state of complete transcriptional repression that is epigenetically inherited through subsequent cell divisions (1). This phenomenon has great impact on gene therapy, as transferred genes may become silenced to various degrees, depending on the presence of cis-acting elements opposing the forces of silencing (e.g., enhancers, locus control regions, matrix attachment sites, insulators), the sites of chromosomal integration, and the state of differentiation the cells initially transduced and of their subsequent progeny (2). During development, a widespread wave of global silencing takes place at the time of implantation (3). Subsequently, progressive tissue-specific gene activation occurs (4). In preimplantation embryos, embryonic stem cells, and hematopoietic stem cells (HSC), transferred genes are exposed to a high frequency of gene silencing (5). Irreversible HSC gene silencing remains one of the main obstacles to the gene therapy of hematological disorders.
The discovery of DNase 1 hypersensitive sites (HS) far upstream of the human β-globin gene cluster with properties of an enhancer in at least HS2 greatly improved the prospects of gene therapy for the human hemoglobinopathies (6, 7). The term locus control region (LCR) subsequently was coined to describe the properties of a 21-kb DNA fragment encompassing the previously identified DNase 1 HS sites, and this β-LCR was reported to confer position-independent, copy number-dependent expression on a cis-linked human β-globin gene in transgenic mice bearing multiple integrated copies (8). The β-LCR became a source of great hope for the gene therapy of β-globin gene disorders because its proposed “dominant chromatin opening” function should shield the β-globin gene from silencing, and the activity of the LCR could be narrowed down to short HS cores small enough to be incorporated in gene transfer vectors (reviewed in ref. 9).
Engraftment of mice with marrow transduced with [β-globin/LCR] retroviral vectors demonstrated that high levels of expression could be achieved in CFU-S colonies of transplanted mice, yet with complete lack of position-independent expression (10–12). In long-term transplantation experiments, differences between vectors were observed. Rivella et al. (12) found complete gene silencing in HSC, whereas we observed long-term (>8 mo) expression in a significant fraction of transplanted mice, with maintenance of expression in secondary transplants (11); however, the remaining mice also were silenced at the stem cell level (9). These results were attributed to the use of “micro” or “nano” β-LCR, which, it was argued, does not recapitulate the function of the whole β-LCR and is especially sensitive to silencing triggered by retroviral sequences (13). However, the original contention that the LCR differs completely from enhancers has become doubtful in light of several exceptions to their alleged dominant chromatin opening property, especially at single integrated copy. These exceptions include silencing of an LCR-driven gene by plasmid DNA (14), adeno-associated virus (15) or retroviral elements (5), insertion near heterochromatin regions (13), and combination with heterologous genes (LacZ) (14) or even β-globin genes lacking 3′ flanking regions (5).
Certain retroviral vector derivatives, such as the murine stem cell virus (MSCV), have a decreased propensity to be silenced in HSC (16). We previously have shown that their use in combination with the ex vivo preselection of transduced HSC on the basis of expression of either a cell surface marker (CD24) or the green fluorescence protein (GFP) ensures long-term expression of retrovirally transduced genes in engrafted mice, presumably by isolating HSC without initial gene silencing (16, 17).
Here, we have investigated whether ex vivo preselection of HSC transduced with a complex retroviral vector, in which GFP is driven by the CpG island containing phosphoglycerate kinase (PGK) promoter juxtaposed to the β-globin/LCR segment, can prevent stem cell gene silencing. We observed that all mice engrafted with preselected cells concurrently expressed human β-globin and GFP, long term, in 20–95% of their red cells. Stem cell gene silencing and age-dependent extinction of expression were, therefore, avoided, although pan- and heterocellular position effects persisted.
Materials and Methods
Retroviral Vector Design and Production.
The MSCV vector (18) and the hepatitis B virus posttranscriptional regulatory element (HBPRE) (19) were kindly provided by R. Hawley (Holland Laboratory, Rockville, MD) and T. Yen (University of California, San Francisco, CA), respectively. The red-shifted humanized variant of the enhanced green fluorescent protein (EGFP) gene (17) was obtained from CLONTECH. DNA sequence of the vectors will be provided on request. The AM-12 (20) and GP + E-86 (21) packaging cells were kindly provided by A. Bank (Columbia University, New York). Replication competent retrovirus (RCR)-free viral supernatants were generated by standard methods (22). EGFP-positive cells were sorted by using a FACStar+ (Becton Dickinson) equipped with a 5-W argon and a 30-mW helium neon laser.
Animals.
Eight- to 12-week-old (C57BL/6Ly-Pep3b × C3H/HeJ)F1 [PepC3F1] and (C57BL/6J × C3H/HeJ)F1 [B6C3F1] mice were used as donor and recipient mice, respectively. All animals were bred from parental mice obtained from the Jackson Laboratory.
Bone Marrow Transduction, ex Vivo Selection, and Transplantation.
Donor PepC3F1 mice were injected 4 days previously with 150 mg/kg 5-fluorouracil (5-FU). Bone marrow (BM) cells were prestimulated for 2 days in DMEM (GIBCO/BRL) supplemented with 15% FCS (HyClone), 10 ng/ml of human IL-6, 6 ng/ml of murine IL-3, and 100 ng/ml of murine steel factor and were exposed to viral supernatants for 2 days on fibronectin (Sigma)-coated Petri dishes in the presence of 5 μg/ml protamine sulfate (Sigma). All growth factors were used as diluted supernatants from transfected COS cells prepared at the Terry Fox Laboratory (Vancouver). EGFP-positive cells were isolated by sorting on a FACStar (Becton Dickinson) 2 days after infection and injected i.v. into recipient B6C3F1 mice given 900 cGy (110 cGy/min 123Cs g-rays) of whole-body irradiation.
FACS Analysis.
Peripheral RBC and WBC were analyzed on a FACScan flow cytometer (Becton Dickinson). Reconstitution of recipient mice with donor cells was assessed by staining WBC with a biotinylated anti-Ly5.1 antibody (PharMingen) followed by phycoerythrin (PE)-conjugated streptavidin (Southern Biotechnology Associates). The proportion of EGFP-positive WBC and RBC was assessed by FACS with or without removal of RBC, respectively, by using Ficoll (Amersham Pharmacia). The proportion of cells expressing human β-globin protein was assessed by FACS analysis of RBC that had been fixed and stained with a biotinylated anti-human β-globin antibody (Biolabs, Northbrook, IL) and streptavidin-PE.
Southern Blot Analysis.
Southern blot analysis was performed by using standard methods (22). The EGFP gene, labeled with 32P by random priming, was used as a probe.
Quantification of Human Globin Gene Expression.
Human β-globin RNA and protein in RBC were quantified by RNase protection assay and HPLC, respectively. Both RNase protection (10) and HPLC (23) were performed as described.
Results
Vector Design, Production, and Characterization.
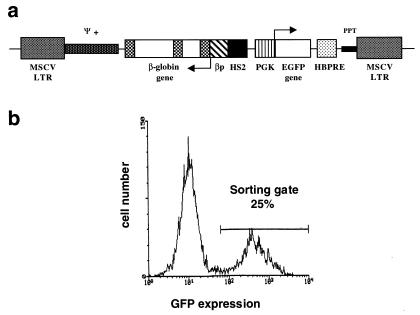
A diagram of the MSCVβ-globin/HS2/EGFP vector is shown in Fig. 1a. To prevent splicing of the viral transcript before virion packaging, the β-globin gene was inserted in reverse orientation with respect to the direction of viral transcription. In addition, an internal deletion of β-globin intron 2 and several point mutations were introduced to eliminate unwanted splice sites and polyadenylation signals, as described (10). The natural polyadenylation signal of the human β-globin gene was replaced by a more potent synthetic polyadenylation signal, based on that of the rabbit β-globin gene (24).
Figure 1.
(a) Diagram of the MSCVβ-globin/HS2/GFP vector. Arrows indicate transcription initiation sites. MSCV LTR, murine stem cell virus long terminal repeat; ψ+, extended packaging signal; βp, β-globin promoter; HS2, hypersensitive site 2; PGK, phosphoglycerate kinase promoter; HBPRE, the hepatitis B virus posttranscriptional regulatory element; PPT, polypurine track. (b) Flow cytometric profile of bone marrow cells 48 h postinfection with MSCVβ-globin/HS2/EGFP virus supernatant. The sorting gate used to select EGFP-positive cells is shown.
To enable us to address conditions in which gene silencing and position effects may be more prominent, we chose to place the β-globin gene cassette under the control of the HS2 element of the human β-globin LCR rather than a more complete LCR fragment. Viral producer cells were generated by using the ecotropic packaging cell line GP + E-86. The producer clone used in subsequent studies yielded titers of 3 × 105 colony-forming units per ml, as determined by transfer of EGFP expression to NIH 3T3 cells, and transmitted a full-length provirus (data not shown).
Bone Marrow Transduction and ex Vivo Isolation of Transduced Stem Cells that Escape Initial Gene Silencing.
PepC3F1 and B6C3F1 mice were chosen as donors and recipients, respectively, based on their allelic differences at the Ly5 locus: PepC3F1 mice are Ly5.1/Ly5.2 heterozygotes, whereas B6C3F1 mice are homozygous Ly5.2. These differences allowed us to quantify precisely the levels of hematopoietic reconstitution of recipient mice (Ly 5.2) with donor (Ly 5.1) cells.
Donor PepC3F1 mice were injected i.v. with 5-FU to stimulate HSC division. Marrow cells were cultured for 48 h in medium containing IL-3, IL-6, and steel factor before exposure to retroviral supernatants. FACS analysis of marrow 2 days postinfection showed 15–25% EGFP-positive cells (Fig. 1b). In pilot experiments, we found that the percentage of reconstitution with EGFP-positive cells of mice transplanted with nonselected marrow was lower or equal to the initial gene transfer efficiency (15–25%). Southern blot analysis of hematopoietic tissues of recipient mice showed that this was because of stem cell competition with both nontransduced and transduced, yet silenced, donor cells (data not shown). Thus, to enable the transplantation of only those transduced cells in which the transgene was not initially silenced, EGFP-positive cells were selected by FACS, and 1.5–3.0 × 105 selected cells were injected into each of seven lethally irradiated recipient B6C3F1 mice.
Kinetics of Reconstitution with Donor-Derived Cells.
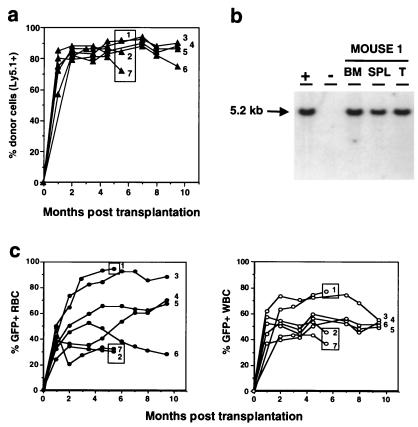
All mice were kept for long-term studies and monitored at regular intervals by quantitative FACS and RNase protection analysis of peripheral blood samples. In addition, three mice were killed 5.5 mo posttransplantation to enable Southern blot analysis of bone marrow, spleen, and thymus DNA. As early as 5 wk posttransplantation, the vast majority of WBC were Ly5.1-positive, demonstrating that they were derived from the transplanted donor marrow (Fig. 2a). The proportion of Ly5.1-positive WBC remained stable between 70% and 90% for a minimum of 9.5 mo posttransplantation, the longest time point tested (Fig. 2a). More importantly, all mice showed the presence of EGFP-positive RBC and WBC as early as 1 wk posttransplantation and reached stable levels between 2 and 3 mo posttransplantation (Fig. 2c).
Figure 2.
Evolution of the reconstitution of peripheral blood cells with MSCVβ-globin/HS2/EGFP retrovirus-transduced, EGFP-positive cells. (a) Reconstitution kinetics of peripheral blood cells of recipient mice with donor-derived (Ly5.1+) cells. The proportion of Ly5.1+ peripheral blood leukocytes in each transplant recipient is shown. Boxed circles identify those animals that were killed at 5.5 mo posttransplantation for Southern blot analysis. (b) Southern blot analysis of a representative mouse killed 5.5 mo posttransplantation. DNA was isolated from bone marrow (BM), spleen (SPL), and thymus (T); +, DNA from the GP + E-86 MSCVβ-globin/HS2/EGFP viral producer shown to have X copies of integrated provirus; −, DNA from unmanipulated GP + E-86 packaging cells. DNA was digested with SacI and proviral integration assessed by probing with the EGFP gene. Left margin, Molecular size marker. (c) Reconstitution kinetics of the peripheral blood of recipient mice with EGFP-positive red (Left) and white (Right) blood cells.
Evidence of Transduction of Totipotent Stem Cells and Long-Term Reconstitution with Transduced Cells.
At 5.5 mo posttransplantation, up to 93% of RBC (range 30–93%) and 75% of WBC (range 38–75%) were EGFP-positive, as determined by quantitative FACS analysis. These levels remained relatively stable for a period of 9.5 mo, the longest time point tested (Fig. 2c).
Southern blot analysis of DNA obtained from bone marrow, spleen, and thymus of mice killed at 5.5 mo posttransplantation showed that the vast majority of cells within these organs harbored intact recombinant provirus (Fig. 2b, and data not shown). The average number of proviral copies per cell, after normalizing for DNA loading and chimerism, was 1.
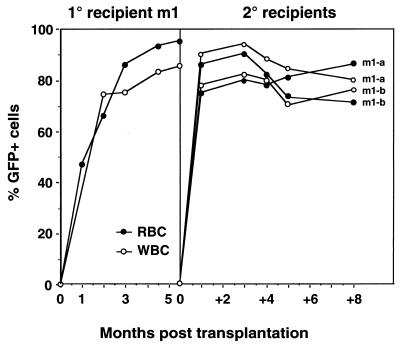
To provide stronger evidence for transduction of HSC and to determine whether EGFP expression would be maintained for extended periods of time in vivo, bone marrow from one primary recipient of MSCVβ-globin/HS2/EGFP-transduced marrow killed 5.5 mo posttransplantation was injected into two secondary lethally irradiated mice. The proportion of EGFP-positive RBC and WBC ranged from 74% to 90% and 73% to 94%, respectively, and was maintained for a minimum of 8 mo posttransplantation, the longest time point tested (Fig. 3). Southern blot analysis of DNA obtained from bone marrow, spleen, and thymus of these secondary recipients confirmed that the majority of cells within these organs harbored intact recombinant provirus (data not shown). In addition, to quantify better the proportion of marrow cells that contained intact provirus, bone marrow from the three primary recipients killed at 5.5 mo posttransplantation was i.v.-injected into secondary lethally irradiated mice to generate day-12 spleen colonies. Southern blot analysis of 43 individual spleen colonies showed that all 43 (100%) contained intact recombinant provirus (data not shown), demonstrating that the vast majority of bone marrow cells harbored the EGFP and human β-globin transgenes.
Figure 3.
Reconstitution kinetics of the peripheral blood of secondary recipient mice with EGFP-positive cells. Shown is the proportion of EGFP-positive RBC and WBC, as determined by FACS, in the primary recipient m1 (Left) and the two secondary recipients (Right) at various time points posttransplantation.
Long-Term Human β-Globin Expression in All Transplanted Mice: Avoidance of Stem Cell Gene Silencing and Time-Dependent Extinction of Expression; Persistence of Position-Dependent Effects.
Expression of human β-globin in murine RBC was assessed by RNase protection assay and FACS and HPLC analyses at various time points posttransplantation.
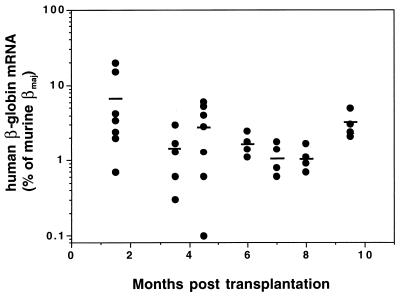
Human β-globin mRNA was detected in RBC from all transplant recipients at all time points tested (range 0.1–20% of endogenous mouse beta-major mRNA) (Fig. 4). These levels remained relatively constant over extended periods posttransplantation, averaging 3.1% (SD 1.3%) of endogenous mouse β-major at 9.5 mo, the last time point analyzed (Fig. 4).
Figure 4.
Human β-globin mRNA expression in RBC of transplanted mice. Expression of human β-globin mRNA in RBC of recipient mice was quantified by RNase protection assay 1–9.5 mo posttransplantation. Expression levels of human β-globin mRNA are shown as a percentage of endogenous murine β-major mRNA levels and were corrected for the proportion of GFP-positive RBC based on the concordance between β-globin and GFP-expressing cells (see Fig. 5). Individual mice are represented by circles; mean expression values are indicated by horizontal lines.
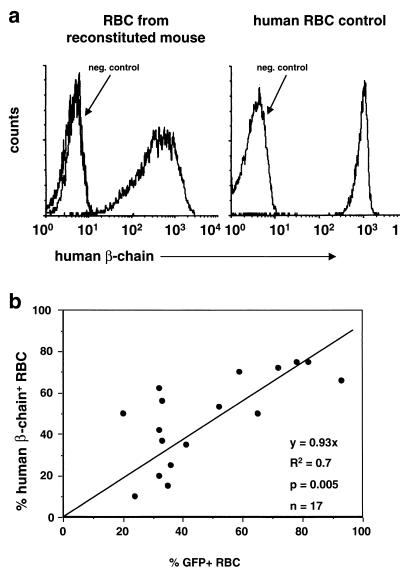
Furthermore, human β-globin protein was detected in RBC from all mice at all time points tested by intracellular staining of RBC with a human β-globin chain-specific mAb (Fig. 5a). The proportion of human β-chain-positive RBC ranged from 10% to 78% (Fig. 5). Human β-globin protein was not detected in WBC (data not shown). The presence of human β-globin protein in RBC was confirmed by HPLC analysis in both primary and secondary recipients analyzed at 4.5 or 2 mo posttransplantation, respectively (data not shown). Together, these data demonstrate sustained, high-level expression of human β-globin mRNA and protein in all transplant recipients and a lack of time-dependent extinction of globin transgene expression. However, position-dependent effects on human β-globin gene expression were still observed.
Figure 5.
Expression of human β-globin chains in RBC of reconstituted mice and correlation between EGFP and human β-globin expression. (a) FACS analysis of RBC from a representative mouse 5 mo posttransplantation (Left) and a human control (Right), using a mAb specific for human β-globin. RBC from an unmanipulated mouse were used as a negative control. (b) Correlation between the proportion of RBC expressing EGFP and the proportion of RBC expressing human β-globin in reconstituted mice. The proportion of EGFP-positive and human β-globin-positive RBC was assessed separately by FACS between 1 and 5 mo posttransplantation. Each transplant recipient was analyzed twice; three recipients were analyzed three times.
Concurrent but Heterocellular Expression of GFP and β-Globin with Position-Effect Variegation.
A strong correlation between β-globin and EGFP expression in RBC from transplanted mice was observed (range 37–75% vs. 30–90% for β-globin and GFP, respectively, R2 = 0.7), suggesting that the provirus was either capable of expressing both transcriptional units (β-globin and EGFP) or none at all (Fig. 5b).
Discussion
Although retrovirus-mediated gene transfer of a globin gene derivative into HSC holds promise for the gene therapy of thalassemias and sickle cell disease, this ultimate goal continues to pose various challenges. Recent years have seen progress in the design of stable [β-globin/LCR] retroviral vectors by preventing unwanted splicing of the full-length viral RNA and facilitating its nucleocytoplasmic export before virion packaging. The present study corroborates these findings by demonstrating reliable, high-titer transfer of an intact provirus to transduced cells.
Most current efforts are now focused on controlling the expression of the transferred β-globin gene and avoiding stem cell gene silencing, age-dependent extinction of expression, and position-dependent expression at suboptimal levels. Chromatin-mediated gene silencing is increasingly recognized as an essential mechanism of the developmental control of gene expression and the protection against genome invaders, such as viruses and transposons. It is, therefore, not surprising that evidence of gene silencing has been observed frequently in gene therapy experiments, which include transfer of [β-globin/LCR] vectors. In this study, we investigated whether preselecting HSC in which the transferred provirus is not initially silenced, on the basis of EGFP expression ex vivo would ensure maintenance of β-globin gene expression in vivo after transplantation. Simultaneously, the preselection procedure also should eliminate nontransduced stem cells from the graft.
We first demonstrate long-term reconstitution of 100% mouse transplant recipients with virtually 100% transduced cells containing an intact provirus. Evidence of gene transfer to totipotent HSC includes: (i) the presence of intact proviral DNA by quantitative Southern blot analysis in all lineages (spleen, thymus, and bone marrow) at approximately 1 copy per cell in virtually all donor-derived cells (>90%), (ii) transfer of intact proviral DNA in 100% of CFU-S analyzed, (iii) long-term (>9 mo) expression of EGFP and human β-globin in peripheral blood cells of all mice, and (iv) duplication of the above results in secondary transplants. More importantly, we demonstrate that stem cell preselection avoids both subsequent gene silencing at the stem cell level in vivo and age-dependent extinction of expression, even when HS2 alone was used rather than a more complex LCR derivative. We show that 100% of the mice expressed EGFP in their peripheral blood cells (range 20–95%) as long as they were analyzed (>9 mo) without significant decrease in expression as the mice aged. The utility of the preselection procedure was evidenced by previous failures in achieving such high gene expression levels with the same vector but omitting the step of preselection. In contrast to our previous uses of bicistronic vectors, the β-globin gene was merely cis-linked to the PGK-EGFP cassette, whose expression was used for ex vivo preselection. This suggests that selecting for provirus integration into sites of active transcription at the stem cell level on the basis of EGFP expression ex vivo exerts a positive effect on the transcription of a cis-linked gene whose expression is recruited later during erythroid differentiation. This hypothesis is corroborated by the fact that EGFP-positive WBC were negative for human β-globin expression, showing appropriate erythroid specificity, whereas EGFP and human β-globin were simultaneously expressed in RBC. Thus, one proviral transcriptional unit did not impair expression of the second as previously observed but, rather, both units were regulated concurrently.
However, human β-globin expression levels per RBC remained suboptimal, heterocellular, and not independent from the sites of chromosomal integration (0.15–20% of endogenous mouse β-major mRNA; 10–78% proportion of human β-globin expressing RBC), as was expected from the mere use of HS2 alone. In the future, inclusion of more complex LCR elements may significantly enhance expression levels but may not guarantee complete and position-independent expression (5).
Gene silencing is not merely the result of the lack of available transcriptional activators because it is greatly influenced by position effects. Chromatin modifications are an integral part of the mechanism of gene silencing (25).These alterations include CpG methylation (2, 26), histone deacetylation, and H1 histone-mediated chromatin compaction (2, 25). It is tempting to hypothesize that these molecular events also play a critical role in the suboptimal and heterocellular expression of the transferred β-globin gene and that chromatin insulators or barriers will prove to be useful remedies. Further understanding of the molecular interplay between host chromatin and [β-globin/LCR] provirus ultimately may lead to general solutions for optimal control of transgene expression with major implications for the treatment of the hemoglobinopathies and for the field of gene therapy as a whole.
Acknowledgments
We thank P. Rostena and K. Dalal for excellent technical support, G. Thornbury, R. Zapf, and G. Cameron for excellent cell sorting, and E. Bouhassira and B. Hennemann for helpful discussions. This work was supported by grants from the National Institutes of Health (HL55435 to M.F., I.M.L., C.J.E., R.K.H, and P.L.) and postdoctoral fellowships from the Swiss National Science Foundation and the Ciba-Geigy-Jubilaeums-Stiftung (to C.P.K.).
Abbreviations
- BM
bone marrow
- GFP
green fluorescent protein
- EGFP
enhanced GFP
- LCR
locus control region
- HS2
hypersensitive site 2
- HSC
hematopoietic stem cells
- MSCV
murine stem cell virus
- PGK
phosphoglycerate kinase
- PE
phycoerythrin
Footnotes
See commentary on page 5022.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100082597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100082597
References
- 1.Sun F-L, Elgin S C R. Cell. 1999;99:459–462. doi: 10.1016/s0092-8674(00)81534-2. [DOI] [PubMed] [Google Scholar]
- 2.Bird A P, Wolffe A P. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 3.Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Cedar H, Razin A. Genes Dev. 1992;6:705–714. doi: 10.1101/gad.6.5.705. [DOI] [PubMed] [Google Scholar]
- 4.Razin A, Cedar H. Microbiol Rev. 1991;55:451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne C S, Pasceri P, Singal R, Sukonnik T, Ginder G D, Ellis J. J Virol. 1999;73:5490–5496. doi: 10.1128/jvi.73.7.5490-5496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuan D, Solomon W, Li Q, London I M. Proc Natl Acad Sci USA. 1985;82:6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuan D, Solomon W, London I M, Lee D P. Proc Natl Acad Sci USA. 1989;86:2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosveld F, van Assendelft G B, Greaves D R, Kollias G. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 9.Stamatoyannopoulos G, Nienhuis A W. In: The Molecular Basis of Blood Diseases. Stamatoyannopoulos G, Nienhuis A,W, Majerus P W, Varmus H, editors. Philadelphia: Saunders; 1994. pp. 107–155. [Google Scholar]
- 10.Leboulch P, Huang G M, Humphries R K, Oh Y H, Eaves C J, Tuan D Y, London I M. EMBO J. 1994;13:3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raftopoulos H, Ward M, Leboulch P, Bank A. Blood. 1997;90:3414–3422. [PubMed] [Google Scholar]
- 12.Rivella S, Sadelain M. Semin Hematol. 1998;35:112–125. [PubMed] [Google Scholar]
- 13.Grosveld F. Curr Opin Genet Dev. 1999;9:152–157. doi: 10.1016/S0959-437X(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 14.Ellis J, Pasceri P, Tan-Un K C, Wu X, Harper A, Fraser P, Grosveld F. Nucleic Acids Res. 1997;25:1296–1302. doi: 10.1093/nar/25.6.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W Y, Bailey E C, McCune S L, Dong J Y, Townes T M. Proc Natl Acad Sci USA. 1997;94:5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawliuk R, Eaves C, Humphries R. Hum Gene Ther. 1997;8:1595–1604. doi: 10.1089/hum.1997.8.13-1595. [DOI] [PubMed] [Google Scholar]
- 17.Pawliuk R, Bachelot T, Wise R J, Mathews-Roth M M, Leboulch P. Nat Med. 1999;5:768–773. doi: 10.1038/10488. [DOI] [PubMed] [Google Scholar]
- 18.Hawley R, Lieu F, Fong A, Hawley T. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 19.Huang Z, Yen T. Mol Cell Biol. 1995;15:3864–3869. doi: 10.1128/mcb.15.7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz D, Goff S, Bank A. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 21.Markowitz D, Goff S, Bank A. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawliuk R, Kay R, Lansdorp P, Humphries R K. Blood. 1994;84:2868–2877. [PubMed] [Google Scholar]
- 23.Fabry M E, Costantini F, Pachnis A, Suzuka S M, Bank N, Aynedjian N S, Factor S M, Nagel R L. Proc Natl Acad Sci USA. 1992;89:12155–12159. doi: 10.1073/pnas.89.24.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt N, Briggs D, Gil A, Proudfoot N J. Genes Dev. 1989;3:1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- 25.Tyler J K, Kadonaga J T. Cell. 1999;99:443–446. doi: 10.1016/s0092-8674(00)81530-5. [DOI] [PubMed] [Google Scholar]
- 26.Singal R, Ginder G D. Blood. 1999;93:4059–4070. [PubMed] [Google Scholar]