Abstract
OBJECTIVE—It has been argued that the relationship between depression and diabetes is bi-directional, but this hypothesis has not been explicitly tested. This systematic review examines the bi-directional prospective relationships between depression and type 2 diabetes.
RESEARCH DESIGN AND METHODS—A search was conducted using Medline for publications from 1950 through 2007. Reviewers assessed the eligibility of each report by exposure/outcome measurement and study design. Only comparative prospective studies of depression and type 2 diabetes that excluded prevalent cases of depression (for diabetes predicting depression) or diabetes (for depression predicting diabetes) were included. Two sets of pooled risk estimates were calculated using random effects: depression predicting type 2 diabetes and type 2 diabetes predicting depression.
RESULTS—Of 42 full-text publications reviewed, 13 met eligibility for depression predicting onset of diabetes, representing 6,916 incident cases. Seven met criteria for diabetes predicting onset of depression, representing 6,414 incident cases. The pooled relative risk (RR) for incident depression associated with baseline diabetes was 1.15 (95% CI 1.02–1.30). The RR for incident diabetes associated with baseline depression was 1.60 (1.37–1.88).
CONCLUSIONS—Depression is associated with a 60% increased risk of type 2 diabetes. Type 2 diabetes is associated with only modest increased risk of depression. Future research should focus on identifying mechanisms linking these conditions.
Depression and diabetes are highly prevalent in the U.S. Over 6.5% of the U.S. adult population has been diagnosed with diabetes (1), and the incidence of type 2 diabetes is increasing, in part due to the national increase in obesity. Approximately 16% of U.S. adults will suffer major depressive disorder at some point in their lives, and this proportion is greater when other forms of depressive disorder, such as dysthymia and minor depression, are included (2). Thus, the hypothesis that depression and diabetes are causally related deserves attention from researchers and policy-makers alike.
Depression is associated with poor health behaviors (i.e., smoking, physical inactivity, caloric intake) that increase risk of type 2 diabetes (3). Depression is also related to central obesity and potentially to impaired glucose tolerance (4). Depression is associated with physiological abnormalities, including activation of the hypothalamic-pituitary-adrenal axis, sympathoadrenal system, and pro-inflammatory cytokines, which can induce insulin resistance and contribute to diabetes risk (5). Diabetes may increase risk of depression because of the sense of threat and loss associated with receiving this diagnosis and the substantial lifestyle changes necessary to avoid developing debilitating complications. Also, studies suggest that late-life depression is associated with a history of vascular disease, including diabetes (6). In sum, evidence suggests that the exposure/outcome relationship between these conditions is bi-directional and may change over the life course.
Previous reviews have explored the relationship between depression and diabetes using retrospective (7) and prospective (8) studies, but none have assessed this relationship from a lifespan perspective by simultaneously examining its bi-directionality. Retrospective studies often use lifetime prevalence measures and thus they do not inform questions of temporality. The search criteria used in previous reviews have been relatively conservative and may have missed studies in which depression or diabetes was not the primary exposure of interest. While both depression and diabetes are more common among certain demographic groups (i.e., women and African Americans, respectively), it is unclear whether this relationship varies across such groups. We therefore undertook a new review to synthesize the current evidence of the prospective relationships between depression and type 2 diabetes and provide a more reliable risk estimate and comprehensive picture of these relationships over the lifespan.
RESEARCH DESIGN AND METHODS
Search strategy
We conducted literature searches using MEDLINE with the three limits “publication date from 1 January 1950 to 31 December 2007,” “English language,” and “human subjects” and combinations of the medical subject headings “Diabetes Mellitus” or “Diabetes Mellitus, Type 2,” “Depression” or “Depressive Disorder,” “Risk Factors,” and “Prospective Studies” or “Longitudinal Studies.” The reference lists of previous meta-analyses and selected articles were screened.
Selection criteria
To be included, a report had to 1) have a prospective design, 2) include cases of probable type 2 diabetes (i.e., studies that examined only type 1 diabetes or diabetes before age 30 years were excluded), 3) provide enough data to generate a relative risk estimate, and 4) exclude prevalent cases of either depression (for diabetes predicting depression onset) or diabetes (for depression predicting diabetes onset). In the event of multiple publications, only the most recent manuscript for a particular study population was included.
Data extraction
Two reviewers used a custom data abstraction form to evaluate and summarize selected articles. Abstracted information included authors, year, location, source of participants, sample composition, assessment of diabetes/depression, and matching and/or statistical adjustment for potential confounders. If multiple risk estimates (with error measurements) were presented in a given manuscript (e.g., nested multivariable models), the estimate that most closely adjusted for only demographic characteristics (e.g., age, sex, race, socioeconomic indicators, and marital status/household composition) was selected. We chose this approach because some studies adjusted for prominent effect modifiers (i.e., family history, health behaviors, adiposity) while others did not, and thus the interpretation of the pooled value using the most-adjusted estimates from each study is misleading, for it is neither an estimate of the direct nor the total effect (9).
Statistical analysis
The estimate from each study was used to generate a pooled relative risk using random effects. Two separate analyses were conducted: depression predicting type 2 diabetes and type 2 diabetes predicting depression. Random-effects modeling explicitly accounts for unmeasured variability across the values using the DerSimonian and Laird method, resulting in a pooled estimate with a wider confidence interval relative to the fixed-effects models (10). We evaluated heterogeneity in the estimates using the Cochrane Q statistic. If a study only presented stratified risk estimates (i.e., by sex), these estimates were combined using random effects and then that pooled estimate was used for the meta-analysis. For studies that presented graded relationships (e.g., low, medium, high depressive symptoms), only the estimate for the highest category was selected. Forest plots of the estimates and 95% CIs, with the weight of each point estimate indicated by the relative size of the marker, were used to visualize the range of effects. We used subgroup analyses to explore potential variability in the relationships by demographic characteristics (e.g., age, sex, race) and conducted sensitivity analyses to assess the robustness of our results (11). We used Egger's test and funnel plots to assess publication bias. If such bias was evident, we used the trim and fill approach (12) to generate a pooled estimate that accounted for the unpublished negative findings. All analyses were conducted using Stata v9.0 (StataCorp, College Station, TX), and statistical significance was set a priori at P ≤ 0.05.
RESULTS
Study selection
A total of 21,190 original-research articles were retrieved by the searches (the titles of which were examined by two independent reviewers). Manuscripts that did not reference either depression or diabetes in the title (or specifically referred to type 1 diabetes) were excluded at this phase, and titles that referred to “development,” “risk/incidence,” or “comorbidity/co-occurrence” and similar terms were selected for additional review (n = 1,168). From this set, 42 articles were retrieved for full abstraction. Of these, 24 articles did not meet selection criteria and were excluded (see Supplemental Fig. 1 and supplemental search results in the online appendix available at http://dx.doi.org/10.2337/dc08-0985). Ten studies were excluded for failing to remove prevalent cases of depression/diabetes at baseline. One was excluded for using a measure of “burnout” rather than depression. Ten reports were excluded because they did not provide enough data to generate a risk estimate. Two studies used the same sample (13,14), and only the most recent publication (Arroyo et al. [14]) was retained. Two studies used samples selected for the presence of specific diabetes or depression risk factors (antipsychotic medications [15] and coronary heart failure [16]), respectively). We determined that these samples would introduce bias and heterogeneity into the pooled estimate and therefore excluded them. However, we conducted sensitivity analyses to assess the effect of this decision. The remaining 18 articles (two of which examined both depression predicting diabetes and diabetes predicting depression) were retained for analysis and are described in Tables 1 and 2.
Table 1.
Comparative prospective studies of depression and incident type 2 diabetes
| Author | Follow-up | Incident n/Total n | Sample source | Sample composition* | Depression assessment | Diabetes assessment | Selected estimate (95% CI)† | Statistical adjustment for selected estimate† |
|---|---|---|---|---|---|---|---|---|
| Eaton et al. (17) | 13 years | 89/1,920 | Population based | Age: ≥18 years | Diagnostic Interview Schedule | Self-report | 2.23 (0.90–5.55) | Age, sex, race, and BMI |
| 63% F | ||||||||
| 34% B | ||||||||
| Kawakami et al. (26) | 8 years | 41/2,380 | Occupation based | Age: ≥18 years | Zung Self-Report Depression Scale | OGTT | 2.32 (1.06–5.08) | Age |
| Only men | ||||||||
| 100% A | ||||||||
| Stellato et al. (21) | 9 years | 54/1,156 | Population based | Age: 40–70 years | CES-D | Self-report | 3.09 (1.34–7.12) | Free testosterone, SHBG, hypertension, heart disease, and BMI |
| Only men | ||||||||
| 97% W | ||||||||
| Carnethon et al. (18) | 15.6 years | 369/6,190 | Population based | Age: 25–74 years | General Well-Being Scale | Self-report, MRD, death certificate | 2.52 (1.73–3.67) | Age, race, and sex |
| 59% F | ||||||||
| 15% B | ||||||||
| Arroyo et al. (14) | 4 years | 973/72,178 | Occupation based | Age: 45–72 years | Short-Form 36 | Self-report | 1.55 (1.27–1.90) | Age |
| Only women | ||||||||
| ∼100% W | ||||||||
| Everson-Rose et al. (19) | 3 years | 96/2,662 | Population based | Age: 42–52 years | CES-D | Self-report, FPG | 1.66 (1.05–2.61) | Age, study site, race, education, and medication use |
| Only women | ||||||||
| 47% W | ||||||||
| Golden et al. (22) | 6 years | 721/11,615 | Population based | Age: 48–67 years | Vital Exhaustion Questionnaire | Self-report, FSG | 1.63 (1.31–2.02) | Age, sex, race, study site, and education |
| 56% F | ||||||||
| 22% B | ||||||||
| Kumari et al. (27) | 10.5 years | 361/10,308 | Occupation based | Age: 35–55 years | General Health Questionnaire | Self-report, OGTT | 1.14 (0.83–1.57) | Age, sex, length of follow-up, ethnicity, electrocardiogram abnormalities, and employment grade |
| 44% F | ||||||||
| ∼95% W | ||||||||
| Palinkas et al. (28) | 8 years | 79/971 | Population based | Age: ≥50 years | BDI | OGTT, FPG, non-FPG | 2.50 (1.29–4.87) | Age, sex, physical activity, and BMI |
| 57% F | ||||||||
| 100% W | ||||||||
| van den Akker et al. (20) | 15 years | 3,245/68,004 | Clinic network | Age: ≥20 years | International Classification of Health Problems in Primary Care (ICHPPC-2) | MRD | 1.04 (0.84–1.28) | Age, sex, BMI, socioeconomic status, and interaction of depression*age*sex |
| 58% F | ||||||||
| Mallon et al. (23) | 12 years | 88/2,663 | Population based | Age: 45–65 years | Self-reported dysphoria | Self-report | 1.47 (0.48–4.47) | Age |
| 53% F | ||||||||
| Carnethon et al. (24) | 8 years | 147/4,681 | Population based | Age: ≥65 years | CES-D | Diabetes medication use or FPG | 1.63 (1.12–2.36) | Age, race, and sex |
| 59% F | ||||||||
| 13% B | ||||||||
| Engum (25) | 10 years | 653/37,291 | Population based | Age: ≥30 years | Anxiety and Depression Index | Self-report, confirmed with FPG | 1.51 (1.27–1.80) | Age, sex, education, and marital status |
| 55% F |
Racial/ethnic composition not provided for some studies: A, Asian; B, African American; F, female; W, non-Hispanic white. BDI, Beck Depression Inventory; CES-D, Centers for Epidemiologic Studies Depression Scale; FPG, fasting plasma glucose; MRD, medical record diagnosis; OGTT, 75-g oral glucose tolerance test; SHBG, sex hormone–binding globulin.
Selected estimate refers to estimate that is most closely adjusted for only demographic characteristics (age, sex, race/ethnicity, socioeconomic indicators, and marital status) and is the estimate used in the pooled analyses and are depicted in Figure 1. Estimates for Mallon et al. (23) and Kumari et al. (24) derived from pooled random-effects models across sex.
Table 2.
Comparative prospective studies of type 2 diabetes and incident depression
| Author (year) | Follow-up | Incident n/Total n | Sample source | Sample composition* | Depression assessment | Diabetes assessment | Selected estimate (95% CI)† | Statistical adjustment of selected estimate† |
|---|---|---|---|---|---|---|---|---|
| Palinkas et al. (28) | 8 years | 118/971 | Population-based | Age: ≥50 years | BDI | 75-g oral glucose tolerance test, FPG, non-FPG | 0.73 (0.41–1.30) | Age, sex, physical activity, and BMI |
| 57% F | ||||||||
| 100% W | ||||||||
| Polsky et al. (37) | 6 years | 571/8,387 | Population-based | Age: 51–61 years | CES-D | Self-report | 1.17 (0.98–1.41) | Age, sex, race, marital status, education, wealth, income, self-rated health, disability, baseline CES-D, and chronic conditions |
| 52% F | ||||||||
| 87% W | ||||||||
| Brown et al. (38) | 12 years | 2,534/88,776 | Population-based health care registry | Age: ≥20 years | 1) Rx for antidepressant and 2) depression-related claim (ICD-9 code 296, 309, or 311) within ±3 months of Rx | 1) Two or more diabetes-related claims within a 2-year period (ICD-9 code 250), or 2) diabetes-related hospitalization, or 3) hypoglycemic prescription | 1.04 (0.94–1.15) | Age, sex, number of MD visits, comorbid arthritis, cancer and vascular disease, and insulin use |
| 53% F | ||||||||
| de Jonge et al. (30) | 5 years | 231/4,803 | Population-based | Age: ≥55 years | GMS-AGECAT | Self-report | 1.42 (1.04–1.93) | Age and sex |
| 58% F | ||||||||
| Kim et al. (29) | 2 years | 63/521 | Population-based | Age: ≥65 years | GMS-AGECAT | Self-report | 1.0 (0.4–2.5) | Unadjusted |
| 55% F | ||||||||
| 100% A | ||||||||
| Engum (25) | 10 years | 2,303/7,291 | Population-based | Age: ≥30 years | Hospital Anxiety and Depression Scale–Depression subscale | Self-report, confirmed with FPG | 1.24 (0.78–1.98) | Age, sex, education, and marital status |
| 55% F | ||||||||
| Maraldi et al. (39) | 5.9 years | 594/2,522 | Population-based | Age: 70–79 years | CES-D or self-reported antidepressant use | Self-report diagnosis/ hypoglycemic medication use or FPG | 1.31 (1.07–1.61) | Adjusted for age, sex, race, study site, and baseline CES-D |
| 48% F | ||||||||
| 56% W |
Racial/ethnic composition not provided for some studies: A, Asian; B, African American; F, female; W, non-Hispanic white. BDI, Beck Depression Inventory; CES-D, Centers for Epidemiologic Studies Depression Scale; FPG, fasting plasma glucose; GMS-AGECAT, Geriatric Mental State B3 diagnostic schedule with application of the Automated Geriatric Examination for Computer-Assisted Taxonomy algorithm.
Selected estimate refers to estimate that is most closely adjusted for only demographic characteristics (age, sex, race/ethnicity, socioeconomic indicators, and marital status) and is the estimate used in the pooled analyses and is depicted in Figure 1.
Depression predicting type 2 diabetes
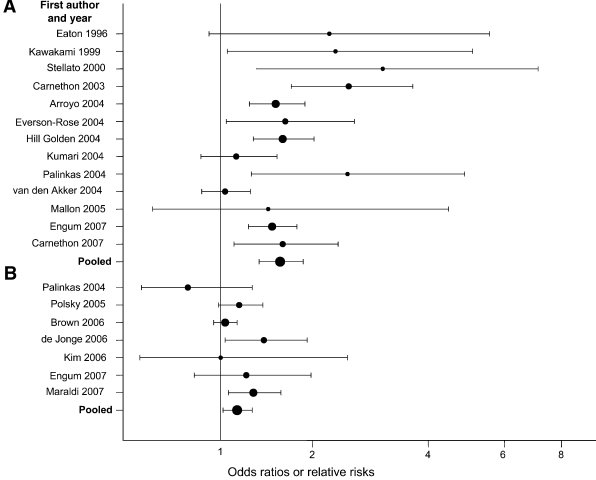
The results from 13 studies of depression predicting incident diabetes, representing 6,916 cases of diabetes, are consistent (Fig. 1A). Even in those instances in which the association was not statistically significant, the trend for depression to increase risk of subsequent diabetes was present. Assessment of heterogeneity indicated that the random effects model was appropriate (Cochrane Q statistic [13 d.f.]: 37.63, P < 0.001), generating a pooled relative risk (RR) of 1.60 (95% CI 1.37–1.88).
Figure 1.
Forest plot of prospective studies of depression and type 2 diabetes. A: Baseline depression predicting incident type 2 diabetes. B: Baseline diabetes predicting incident depression.
Measurement of depression and diabetes status varied across the reports. Most used nondiagnostic measures of depressive symptoms and distress (i.e., Centers for Epidemiologic Studies for Depression Scale, General Health Questionnaire). Several studies supplemented self-report measures of diabetes with physiologic measures (i.e., fasting plasma glucose). The consistency of this relationship across different means of depression and diabetes assessment indicates that the finding is unlikely to be a spurious result of measurement error.
Type 2 diabetes predicting depression
Only seven studies investigated the association between type 2 diabetes and risk of depression (Table 2), representing 6,414 incident cases (Fig. 1B). There was no evidence of heterogeneity (Q statistic [6 d.f.]: 9.17, P < 0.16); however, we present the random effects estimates to be consistent with the above analyses (the fixed-effects estimates did not differ substantially or change the interpretation of the findings; data not shown). There is only modest evidence to support the hypothesis that type 2 diabetes is a “depressogenic” condition that increases risk developing depression (RR 1.15, 95% CI 1.02–1.30).
Moderating influences
We conducted a series of random effects subgroup analyses to explore whether the relation for depression predicting diabetes varied across demographic groups. The RR from studies that reported a mean (or median) age of <50 years (17–19) or that provided age-stratified results (20) was 1.96 (P < 0.001), while the RR derived from studies with mean/median age ≥50 years (14,21–25) or that provide age-stratified results was more modest (RR 1.50, P < 0.001) (two studies [26,27] did not provide mean/median age). The RR for women (derived only from six studies that excluded men [14,19] or provided sex-stratified estimates [20,23,25,27]) was 1.26 (95% CI 0.95–1.67). The RR for men (derived from six studies that excluded women (21,26) or provided sex-stratified estimates) was 1.57 (1.24–1.99). The RR for studies that included primarily (>95%) whites (14,21,27,28) was 1.65 (P < 0.004). The RR for studies that included at least 10% African Americans (17–19,22,24) was similar (RR 1.79, P < 0.001) (four studies that either did not report racial/ethnic composition [20,23,25] or included only Asians [26] were excluded).
Sensitivity analysis
We conducted a series of sensitivity analyses to assess the robustness of our findings. First, we assessed the influence of selecting the demographic-adjusted estimate for the main analyses by pooling estimates from the most- and least-adjusted models across the studies. For studies of depression predicting diabetes, the least-adjusted RR was 1.61 (95% CI 1.38–1.88), and the most-adjusted (all 13 of which adjusted for adiposity) RR was 1.40 (1.21–1.61). For diabetes-predicting depression, the least-adjusted pooled RR was 1.20 (1.06–1.36) and the most-adjusted (only two of which adjusted for adiposity) RR was 1.09 (1.01–1.18). Second, we assessed the impact of excluding the Miller et al. (15) and Havranek et al. (16) studies (excluded because of sample selection criteria, described above). For depression predicting diabetes, inclusion of the antipsychotic medication use sample (15) slightly attenuated the pooled estimate (RR 1.54, P < 0.001). For diabetes-predicting depression, inclusion of the heart failure sample (16) did not alter the pooled estimate (RR 1.17, P < 0.012). We conducted analyses excluding studies that did not adjust for age (21,29), which slightly attenuated both sets of results (data not shown). For depression predicting diabetes, studies that used physiologic testing or clinical records to supplement diabetes measurement reported slightly smaller effects (RR 1.58) than those that relied only on self-report (RR 1.63), although both were statistically significant (P < 0.001). Similarly, for diabetes-predicting depression, studies with clinical measures reported smaller effects (RR 1.11) than self-report only (RR 1.16), but these results were no longer statistically significant (P < 0.26). Finally, because both depression and diabetes can have extended prodromal periods, we excluded four samples that had short (≤5 years) follow-up periods (14,19,29,30). After excluding these studies, diabetes was no longer a significant predictor of depression (RR 1.09, 95% CI 0.96–1.25), but depression remained predictive of incident diabetes (1.64, 1.34–2.00).
Assessment of publication bias
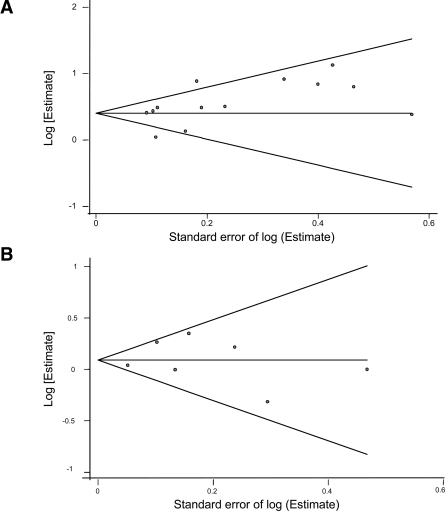
We found suggestive but not statistically significant evidence of publication bias for studies of depression predicting diabetes (Begg's corrected rank coefficient 1.40, P < 0.161, and Egger's test, P < 0.089) (Fig. 2) consistent with a previous meta-analysis (8). Using the fill and trim approach (12), which imputes estimates from hypothetical negative unpublished reports, the publication-bias corrected risk estimate remained statistically significant (RR 1.42, 95% CI 1.20–1.67). There was no evidence, either from visual inspection of the funnel plot or the Begg's coefficient (0.30, P < 0.76) of publication bias regarding studies of diabetes-predicting depression.
Figure 2.
Begg's funnel plot with 95% confidence limits. A: Depression predicting incident of type 2 diabetes. B: Type 2 diabetes predicting incident depression.
CONCLUSIONS
There is a strong and robust association between depression and incidence of type 2 diabetes, but only a weak relation between diabetes and risk of depression. Early retrospective studies had suggested that the relationship between depression and diabetes was bi-directional, but prospective analyses were necessary to understand its natural history. In contrast to the reports of depression predicting diabetes, there was no evidence of heterogeneity or moderation of effect among the studies of diabetes predicting depression. Diabetes is a serious metabolic disorder that has life-changing consequences for individuals affected by it. Depression is a complex condition characterized by disruptions in all facets of life—social, psychological, behavioral, and biological. The finding that depression is associated with a 60% increase in risk of developing type 2 diabetes rivals other known risk factors for this disease, such as smoking (31). Our findings suggest that there is only a modest association between diabetes and incidence of depression, but it is an understudied phenomenon, and it may be that competing risks for late-life depression (i.e., macrovascular disease, functional/cognitive decline) mask this relationship. Depression is also difficult to detect in older adults (32), and thus measurement error may partially explain why this association is so modest. The subgroup analyses suggest this relationship may vary by age and sex, and studies should focus on identifying groups in which this association is particularly robust to target prevention efforts.
Risk factor epidemiology can inform prioritization of prevention efforts through the notion of population-attributable risk (33). The population-attributable risk describes the magnitude of reduction in a given outcome expected if the effect of the risk factor were eliminated, assuming the risk factor is a necessary cause of the outcome. It is a function of the strength of the association and the prevalence of the risk factor. For example, atypical antipsychotic medications are associated with ∼30% increased risk of type 2 diabetes (15), but use of these agents is rare in the general population (prevalence <1%), and thus the associated population-attributable risk is on the order of 0.5%. In contrast, the risk for diabetes associated with depression reported above coupled with a prevalence of 16% (2) is associated with a population-attributable risk of 9% because depression is much more common.
This analysis has strengths and limitations. The primary strengths are the expansive literature search and the explicit assessment of the bi-directionality of the depression-diabetes relationship, which previous reviews have not systematically evaluated. We also conducted sensitivity analyses to assess the robustness of our findings. The primary limitation stems from the quality of the included studies. There was evidence of heterogeneity and potential publication bias among the studies of depression predicting diabetes, and while we conducted a relatively broad search, by limiting it to only one database, we may have missed some reports. However, inclusion of the hypothetical missing negative studies still resulted in a statistically significant, albeit attenuated, pooled estimate of elevated risk of diabetes. Importantly, even this attenuated risk estimate was of greater magnitude than the pooled risk estimate of diabetes predicting depression. While we attempted to generate the total effect of the depression-diabetes relationship by selecting estimates adjusted primarily for demographic characteristics, adjustment for confounders varied, and thus our pooled analyses only approximate the total effect. We did not examine the role of diabetes complications in this analysis, although there is compelling evidence that depression is associated with poorer glycemic control (34) and increased risk of complications (35).
Research should move from epidemiologic investigations that have established this association and begin the process of empirically testing causal hypotheses. Many aspects of this relationship—most notably, whether treating depression lowers the increased risk of diabetes—have yet to be examined in a controlled manner, although there is suggestive evidence that antidepressant use is associated with elevated, not lowered, risk of diabetes, possibly an example of confounding by indication (36). The studies reviewed here demonstrate the importance of early detection of depression and the important role of primary care physicians in careful monitoring of the physiological consequences and correlates of psychiatric disorders.
Acknowledgments
This study was supported by National Institute of Mental Health grants T32-MH14592, R01-MH47447, and F31-MH78443 and the Robert Wood Johnson Health and Society Scholars program.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.American Diabetes Association: Diabetes Fact Sheet. Available from http://wwwdiabetesorg/about-diabetesjsp, 2005. Accessed 1 May 2008
- 2.Kessler R, Berglund P, Delmer O, Jin R, Merikangas K, Walters EE: Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Strine T, Mokdad A, Dube S, Balluz L, Gonzalez O, Berry J, Manderscheid R, Kroenke K: The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry 30:127–137, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Weber B, Schweiger U, Deuschle M, Heuser I: Major depression and impaired glucose tolerance. Exp Clin Endocrinol Diabetes 108:187–190, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Golden SH: A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev 3:252–259, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Camus V, Kraehenbuhl H, Preisig M, Bula C, Waeber G: Geriatric depression and vascular diseases: what are the links? J Affect Disord 81:1–16, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Anderson R, Freedland K, Clouse R, Lustman P: The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24:1069–1078, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Knol M, Twisk J, Beekman A, Heine R, Snoek F, Pouwer F: Depression as a risk factor for the onset of type 2 diabetes mellitus: a meta-analysis. Diabetologia 49:837–845, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Lipsey M, Wilson D: Practical Meta-Analysis. Thousand Oaks, CA, Sage Publications, 2001
- 10.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7:177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Berlin J: Benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol 142:383–387, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R: Trim and fill: a simple funnel-plot-based approach of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Saydah S, Brancati F, Golden S, Fradkin J, Harris M: Depressive symptoms and the risk of type 2 diabetes mellitus in a US sample. Diabete Metab Res Rev 19:202–208, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Arroyo C, Hu F, Ryan L, Kawachi I, Colditz G, Speizer F, Manson J: Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care 27:129–133, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller E, Leslie D, Rosenheck R: Incidence of new-onset diabetes mellitus among patients receiving atypical neuroleptics in the treatment of mental illness. J Nerv Ment Dis 193:387–395, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Havranek E, Spertus J, Masourdi F, Jones P, Rumsfeld J: Predictors of the onset of depressive symptoms in patients with heart failure. J Am Coll Cardiol 44:2333–2338, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Eaton WW, Armenian H, Gallo J, Pratt L, Ford D: Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care 19:1097–1102, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Carnethon M, Kinder L, Fair J, Stafford R, Fortmann S: Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination Epidemiologic Follow-Up Study, 1971–1992. Am J Epidemiol 158:416–423, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Everson-Rose S, Meyer P, Powell L, Pandey D, Torrens J, Kravitz H, Bromberger J, Matthews K: Depressive symptoms, insulin resistance and risk of diabetes in women at midlife. Diabetes Care 27:2856–2862, 2004 [DOI] [PubMed] [Google Scholar]
- 20.van den Akker M, Schuurman A, Metsemakers J, Buntinx F: Is depression related to subsequent diabetes mellitus? Acta Psychiatr Scand 110:178–183, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Stellato R, Feldman H, Hamdy O, Horton E, McKinlay J: Testosterone, sex hormone-binding globulin and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 23:490–494, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Golden S, Williams J, Ford D, Yeh H, Patton Sanford C, Neito F, Brancti F: Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care 27:429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mallon L, Broman J-E, Hetta J: High incidence of diabetes in men with sleep complaints or short sleep duration. Diabetes Care 28:2762–2767, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Carnethon M, Bigs M, Barzilay J, Smith N, Vaccarino V, Bertoni A, Arnold A, Siscovick D: Longitudinal association between depressive symptoms and incident type 2 diabetes mellitus in older adults. Arch Intern Med 167:802–807, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Engum A: The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosom Res 62:31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kawakami N, Takatsuka N, Shimizu H, Ishibashi H: Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 22:1071–1076, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Kumari M, Head J, Marmot M: Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med 4:1873–1880, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Palinkas L, Lee P, Barrett-Connor E: A prospective study of type 2 diabetes and depressive symptoms in the elderly: the Rancho Bernardo Study. Diabet Med 21:1185–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Stewart R, Kim S, Yang S, Shin I, Yoon J: Vascular risk factors and incident late-life depression in a Korean population. Br J Psychiatry 189:26–30, 2006 [DOI] [PubMed] [Google Scholar]
- 30.de Jonge P, Roy J, Saz P, Marcos G, Lobo A: Prevalent and incidence depression in community-dwelling elderly persons with diabetes mellitus: results from the ZARADEMP project. Diabetologia 49:2627–2633, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Willi C, Bodenmann P, Ghali W, Faris D, Cornuz J: Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 298:2654–2664, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Gallo J, Anthony J, Muthen B: Age differences in the symptoms of depression: a latent trait analysis. J Gerontology 49:251–264, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Eaton WW: Epidemiologic evidence on comorbidity of depression and diabetes. J Psychosom Res 53:903–906, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Lustman P, Anderson P, Freedland K, de Groot M, Carney R, Clouse R: Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 23:934–942, 2000 [DOI] [PubMed] [Google Scholar]
- 35.de Groot M, Anderson R, Freedland K, Clouse R, Lustman P: Association of depression and diabetes complications: a meta-analysis. Psychosom Med 63:619–630, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Rubin R, Ma Y, Marrero D, Peyrot M, Barrett-Connor E, Kahn S, Haffner S, Price D, Knowler W: Elevated depressive symptoms, antidepressant use, and risk of developing diabetes during the Diabetes Prevention Program. Diabetes Care 31:420–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polsky D, Doshi J, Marcus S, Oslin D, Rothbard S, Thomas N, Thompson C: Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med 165:1260–1266, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Brown L, Majumdar S, Newman S, Johnson J: Type 2 diabetes does not increase risk of depression. CMAJ 175:42–46, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maraldi C, Volpato S, Penninx B, Yaffe K, Simonsick E, Strotmeyer E, Cesari M, Kirtchevsky S, Perry S, Ayonayon H, Pahor M: Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: the health, aging and body composition study. Arch Intern Med 167:1137–1144, 2007 [DOI] [PubMed] [Google Scholar]