Abstract
The purpose of this investigation was to determine size differences between affected and unaffected upper extremities in patients with brachial plexus birth palsy (BPBP). Forty-eight patients with BPBP underwent measurements of the bilateral upper extremities. Average age at the time of evaluation was 47 months. In addition, patients or families were asked “How important is the difference in arm size and appearance to you?” Active motion was assessed using the modified Mallet classification, Toronto Test Score, and Hospital for Sick Children Active Movement Scale. Correlation between ratios of affected to unaffected limb lengths and girths and measures of active motion were assessed using Spearman’s rank correlation coefficient. Upper arm, forearm, and hand lengths of the affected limbs were, on average, 95%, 94%, and 97% of the contralateral unaffected side, respectively. Upper arm girth, forearm girth, and hand width were, on average, 97%, 98%, and 95% of the contralateral side, respectively. All differences achieved statistical significance (p < 0.01). Furthermore, over 37% of patients or families reported that limb differences were “very” or “extremely important” to them. No statistically significant correlation between age and limb length discrepancy was noted. Furthermore, there were no correlations between upper limb discrepancies and measures of active motion in individual patients. Patients with BPBP and persistent neurological deficits may expect the affected upper extremity to be on average approximately 95% the length and girth of the contralateral limb. These differences do not correlate with patient age or clinical measurements of active movement.
Keywords: Brachial plexus birth palsy, Limb discrepancy
Introduction
Despite advances in obstetrics, the incidence of brachial plexus birth palsy (BPBP) remains approximately 1 per 1,000 live births [3, 4, 9]. Established risk factors include large size for gestational age, multiparous pregnancy, difficult or prolonged labor, and history of prior child with BPBP [9]. Microsurgical nerve reconstruction, soft-tissue releases, tendon transfers, and osteotomies have all been proposed for infants and children with persistent neurological deficits and/or secondary joint deformity to improve function [9, 10].
While previous study has focused on the natural history and surgical treatment of BPBP, little is known about the relationship between BPBP and resultant morphological and aesthetic differences between affected and unaffected upper limbs [2, 3, 6, 9, 10]. The purpose of this investigation was to determine the differences in length and girth between affected and normal upper extremities in BPBP patients and to determine the relative importance these difference(s) are to patients and families.
Materials and Methods
This investigation was performed as a part of a continuing prospective study of patients with BPBP presenting to the Hand and Upper Extremity Program of the Department of Orthopedic Surgery at our institution. Forty-eight consecutive infants and children with BPBP were evaluated by clinical examination by the authors for the purposes of this investigation. (“Appendix 1”) All patients had persistent neurological deficits with upper extremity weakness (i.e., patients who demonstrated full spontaneous neurological recovery were not included in this investigation). There were 20 males and 28 females. Nineteen patients had left upper extremity involvement. Average age at the time of evaluation was 47 months (range 1 to 169 months). Due to the young age at which many of these patients were evaluated, hand dominance was often not apparent and thus not reported here.
In addition to a thorough clinical history and physical examination, all patients underwent measurements of the bilateral upper extremities to assess for upper limb discrepancy. The following parameters were measured for both the affected and unaffected limbs using palpable subcutaneous landmarks, according to the fashion of Van Heest et al. [8]: (1) upper arm length, (2) forearm length, (3) hand length, (4) upper arm girth, (5) forearm girth, and (6) hand width. Upper arm length was measured along the lateral aspect of the brachium from the tip of the acromion to the olecranon process. Forearm length was measured from the olecranon process to ulnar styloid. Hand length was measured from the level of the ulnar styloid to the tip of the long finger. Upper arm girth was determined by the maximal circumference of the brachium, typically measured at the midpoint of its proximal–distal length. Forearm girth was the measured circumference at the maximal forearm width. Hand width was measured from the base of the first web space to the ulnar border of the hand. Results for each parameter of the affected limb were quantified by calculating the percentage of the unaffected contralateral side. (For example, forearm length of the affected limb / forearm length of the unaffected limb × 100 = forearm length percentage.) Prior to the initiation of this investigation, consensus building exercises and assessment of intra and interobserver reliability were performed among the examiners.
The subjective importance of limb appearance to patients or families was also assessed. Prior to measurement of limb dimensions, patients or families were asked “How important is the difference in arm size and appearance to you?” Responses were recorded on a scale of 1 to 4, with “1” being “not important” and “4” being “extremely important.”
In addition, upper limb active motion was assessed using the modified Mallet classification, Toronto Test Score, and Hospital for Sick Children Active Movement Scale [2, 5, 7]. The modified Mallet classification provides an assessment of global shoulder function by scoring active shoulder abduction, external rotation, internal rotation, hand-to-mouth placement, and hand-to-neck placement. The Toronto Test Score provides an assessment of active elbow flexion, elbow extension, wrist extension, digital extension, and thumb extension. The Active Movement Scale provides a comprehensive evaluation of active upper extremity motion in 13 categories: shoulder abduction, adduction, and flexion; shoulder internal and external rotation; elbow flexion and extension; forearm pronation and supination; wrist flexion and extension; digital flexion and extension; and thumb flexion and extension. All three classification systems are used specifically for BPBP, and the intra and interobserver reliability of these measures of active movement have previously been established [1].
For statistical analysis, paired t test was used to compare means of continuous paired variables, with p < 0.01 deemed statistically significant. Spearman’s rank correlation coefficients were calculated to determine associations between limb length–girth differences and age and measures of active motion. Analyses were performed using Microsoft Excel 2002 (Microsoft Corporation, Redmond, WA, USA) and SPSS (version 15.0, SPSS Inc., Chicago, IL, USA) statistical software.
This investigation was approved by the Committee of Clinical Investigation of our Institutional Review Board.
Results
Objective Measurements
Upper arm lengths of the affected limbs were, on average, 95% of the contralateral unaffected side (range 78% to 100%; “Appendix 1”). Forearm lengths of the affected limbs were also, on average, 94% of the contralateral side (range 82% to 107%). Hand lengths of affected limbs were, on average, 97% of the contralateral side (range 83% to 119%). Upper limb girth was, on average, 97% of the contralateral side (range 79% to 107%). Forearm girth was, on average, 98% of the contralateral side (range 88% to 111%). Finally, hand widths were, on average, 95% of the unaffected contralateral side (range 79% to 125%). All the above differences achieved statistical significance (p < 0.01). It should be noted that in three patients (patient 5, 27, and 46), the measured component lengths or girths of the affected limb were greater than those of the unaffected extremity (“Appendix 1”).
Mean aggregate modified Mallet classification score was 14 (range 4 to 24) out of a maximum score of 25. Mean Toronto Test Score was 7.5 (range 0 to 10) out of a maximum score of 10. Mean aggregate Active Movement Scale score was 85 (range 24 to 103) of a maximum score of 105.
There was no statistically significant correlation between age and limb length discrepancy noted of any of the measured parameters. Similarly, there were no meaningful correlations between limb length or girth discrepancies and measures of active upper extremity movement. (Table 1)
Table 1.
Spearman’s rank correlation coefficients between measures of active upper limb movement (modified Mallet classification, Toronto Test Score, and Hospital for Sick Children Active Movement scale) and measures of upper limb size discrepancies.
| Modified Mallet classification | Toronto Test Score | Active Movement Scale | |
|---|---|---|---|
| Arm length | −0.082 | 0.051 | −0.086 |
| Forearm length | −0.042 | 0.174 | 0.289 |
| Hand length | −0.023 | 0.303 | 0.294 |
| Arm girth | 0.061 | 0.302 | 0.222 |
| Forearm girth | −0.162 | −0.029 | 0.065 |
| Hand girth | −0.083 | 0.084 | −0.107 |
Significant values are italicized
Subjective Evaluation
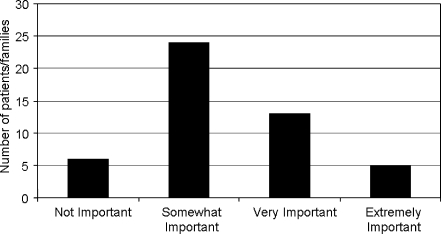
Average response to the subjective query regarding the importance of the appearance of the affected limb was 2.4 (range 1 to 4; Fig. 1). Of the 48 study subjects, six patients or families (13%) reported that size difference and appearance was “not important;” 24 patients or families (50%) reported that it was “somewhat important;” 13 patients or families (27%) reported that it was “very important;” and five patients or families (10%) reported that it was “extremely important.”
Figure 1.
Graph depicting the relative importance of differences in upper limb size and appearance in patients and families with brachial plexus birth palsy.
Discussion
Efforts to define the natural history of BPBP continue to be made in hopes of providing patients and families accurate information regarding long-term prognosis and potential benefits of surgical and other treatments. A multicenter prospective study is underway to help define the natural history and longer-term results of BPBP treatment [11].
In addition to information regarding neurological recovery, longer-term functional outcomes, and indications for operative intervention, patients and families often inquire about the effect BPBP will have on upper extremity size and appearance. These questions often arise at the initial orthopedic or microsurgical consultation, an emotionally charged setting for families and care providers alike. However, little information is currently available on this subject.
McDaid et al. [6] have previously published their analysis of 22 skeletally immature patients with BPBP. Radiographs of the involved and uninvolved humeri and forearms were obtained and relative limb lengths calculated. In their series, 21 of 22 patients (95%) demonstrated upper limb length discrepancy, with the affected extremity averaging 92% the length of the contralateral limb. Patients with upper trunk palsies had less limb length discrepancy than those with total plexus lesions. No correlation between age and percentage of limb length discrepancy was noted.
Similar findings have been noted in studies of skeletally immature patients with other neurological conditions affecting upper extremity function. Van Heest et al. [8] previously evaluated 40 children with spastic hemiplegia due to cerebral palsy for upper limb length discrepancy as well as sensory function. Interestingly, those patients with severe sensory deficits had significantly smaller upper limbs than children with mild or moderate sensibility deficits. The authors conclude that in patients with spastic hemiplegia due to cerebral palsy, upper limb length discrepancy may be a clinical marker for underlying sensory dysfunction [8, 12].
In the current investigation, length and circumference of affected upper limbs in younger patients with BPBP and persistent neurological deficits were found to be approximately 95% of that of the contralateral, unaffected limbs. While these differences were relatively small, they did achieve statistical significance and corroborate the radiographic findings of McDaid et al. Potential reasons for the observed differences include both the effect of longstanding neurological injury on muscle bulk and atrophy in addition to nerve-mediated mechanisms of limb growth and development.
Interestingly, despite the small but significant limb length and girth differences, no meaningful correlations were detected in this study between the magnitude of limb size difference and standardized measures of upper limb motion. This finding suggests that while upper limb size differences do exist, the magnitude of difference may not be utilized to estimate the amount of upper limb impairment. Similarly, patients with more limited active upper extremity movement may not necessarily have greater differences in limb size.
Despite the lack of correlation between size and upper extremity function, the majority of patients and/or families evaluated during the course of this investigation reported that potential differences in upper extremity size and appearance were “somewhat” to “extremely” important to them. This finding highlights the need for surgeons and other care providers to understand both the functional and aesthetic implications of BPBP on their patients.
There were a number of inherent limitations to this study. First, the sample size included 48 patients of varying ages. While adequate numbers of patients were evaluated to make statistically significant conclusions, additional analysis of greater number of patients of older ages would be useful to determine the relative degree of limb length and girth discrepancy at skeletal maturity. In addition, no stratification was performed according to the level or severity of brachial plexus injury, nor can any statements regarding the correlation between limb size difference and level or severity of neurological injury be made. As McDaid et al. and others have suggested, patients with total plexus lesions may have more considerable limb differences compared to those with upper trunk or more limited plexus involvement. No such conclusions may be drawn from the current investigation in this regard. Furthermore, no comparisons in upper limb length or girth were made to BPBP patients who went on to achieve full spontaneous neurological recovery, no efforts were made to use the unaffected contralateral limb as an internal control. Finally, based upon this analysis, no statements can be made on whether early microsurgical repair or plexus reconstruction—and thus presumed improved neuromuscular function—may potentially avert the observed limb differences. Future study may be directed at correlating clinical and/or radiographic measurements (e.g., cross-sectional magnetic resonance imaging) with upper extremity function, patient self-image, and overall quality of life.
In conclusion, patients with persistent neurological deficits in the setting of BPBP may expect the affected upper extremity to be on average approximately 95% the length and girth of the contralateral unaffected limb.
Acknowledgements
The authors wish to acknowledge the administrative efforts of Ms. Anne Kuo, Mrs. Laurie Travers, Ms. Rebecca Jessel, and Mr. Robert Yu in conjunction with this investigation.
This study was supported in part by grants from the Pediatric Orthopedic Society of North America and the American Society for Surgery of the Hand.
Appendix 1. Patient data
Table 2.
Patient data of affected upper extremities.
| Patient | Age at evaluation (months) | Affected side | Affected arm length (cm) | Affected forearm length (cm) | Affected hand length (cm) | Affected arm girth (cm) | Affected forearm girth (cm) | Affected hand width (cm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | L | 10 | 9.5 | 6.5 | 12.3 | 11.8 | 5 |
| 2 | 40 | L | 19 | 16 | 12 | 19 | 16 | 6 |
| 3 | 51 | R | 19 | 15 | 12 | 17 | 14 | 5.5 |
| 4 | 40 | R | 18 | 16 | 9.5 | 16 | 13.5 | 6 |
| 5 | 25 | R | 17 | 12.5 | 8.5 | 20 | 14 | 6 |
| 6 | 1 | R | 9 | 8.5 | 6 | 13 | 12 | 4 |
| 7 | 16 | L | 13 | 12 | 8.5 | 13 | 12 | 6 |
| 8 | 4 | L | 9 | 8.5 | 6.5 | 11.5 | 10 | 5 |
| 9 | 51 | R | 18 | 14 | 12 | 17 | 16 | 5.5 |
| 10 | 3 | R | 9 | 8.5 | 6.5 | 11.5 | 11.5 | 4 |
| 11 | 3 | L | 11 | 10 | 6 | 12 | 11 | 4 |
| 12 | 55 | L | 19 | 17 | 10.5 | 18 | 16 | 6.5 |
| 13 | 68 | L | 20 | 16 | 10.5 | 18 | 16 | 6 |
| 14 | 68 | R | 20 | 16 | 12 | 17 | 15 | 7 |
| 15 | 48 | L | 19 | 14.5 | 11 | 18 | 16 | 6.5 |
| 16 | 13 | L | 14 | 12 | 8 | 15 | 13.5 | 5.5 |
| 17 | 21 | L | 15 | 14 | 8.5 | 19 | 18 | 6.25 |
| 18 | 6 | L | 13 | 10 | 7.5 | 18 | 17 | 5 |
| 19 | 46 | R | 19 | 15 | 10 | 16 | 15.5 | 5.5 |
| 20 | 16 | R | 16 | 16 | 8.5 | 16 | 14.5 | 5.5 |
| 21 | 36 | R | 19 | 15 | 9 | 20 | 18 | 5.5 |
| 22 | 4 | L | 13 | 8.5 | 7.5 | 17 | 16.5 | 6 |
| 23 | 67 | R | 35 | 27 | 19.5 | 28 | 26 | 11.5 |
| 24 | 94 | R | 23 | 16.5 | 15.5 | 17 | 17.5 | 8 |
| 25 | 5 | R | 12.2 | 9 | 7 | 15 | 15 | 6.5 |
| 26 | 6 | R | 11 | 10 | 7 | 14.5 | 13 | 6.5 |
| 27 | 23 | R | 17.5 | 15 | 10 | 20 | 18.5 | 15 |
| 28 | 163 | L | 31 | 26 | 17 | 23 | 21 | 8 |
| 29 | 15 | R | 14 | 9 | 7 | 16 | 15.5 | 5.5 |
| 30 | 2 | R | 8 | 8 | 6.5 | 9 | 9 | 6 |
| 31 | 169 | L | 33 | 25 | 15 | 37 | 28 | 18 |
| 32 | 143 | R | 25 | 22 | 15 | 24 | 20.5 | 10 |
| 33 | 100 | R | 25 | 18 | 13 | 18 | 18.5 | 8 |
| 34 | 57 | R | 21 | 15 | 12.5 | 16 | 15.5 | 6.5 |
| 35 | 105 | R | 28 | 20 | 17 | 27 | 24 | 5.5 |
| 36 | 17 | R | 14.5 | 11 | 9 | 14.5 | 15 | 4.5 |
| 37 | 105 | R | 28 | 20.5 | 6 | 22 | 20 | 5.5 |
| 38 | 28 | R | 16 | 13 | 11 | 14 | 15.5 | 3 |
| 39 | 60 | R | 22 | 15 | 12 | 19 | 20 | 6 |
| 40 | 57 | R | 23 | 16 | 13.5 | 19.5 | 18.5 | 4.5 |
| 41 | 96 | L | 30.5 | 23 | 18 | 26.5 | 22.5 | 6 |
| 42 | 38 | R | 21 | 15 | 13 | 21.5 | 19.5 | 4.5 |
| 43 | 53 | R | 21.5 | 13 | 12 | 18.5 | 16.5 | 4.5 |
| 44 | 83 | L | 21 | 15 | 12.5 | 16.5 | 15.5 | 4.5 |
| 45 | 34 | R | 17.5 | 14 | 12 | 16.5 | 16 | 4 |
| 46 | 26 | L | 18.5 | 14 | 12 | 15.5 | 15.5 | 4 |
| 47 | 33 | L | 19.5 | 13 | 11.5 | 16.5 | 16 | 4.5 |
| 48 | 55 | L | 21.5 | 16 | 12.5 | 22.5 | 18.5 | 5 |
Table 3.
Patient data of unaffected upper extremities.
| Patient | Unaffected arm length (cm) | Unaffected forearm length (cm) | Unaffected hand length (cm) | Unaffected arm girth (cm) | Unaffected forearm girth (cm) | Unaffected hand width (cm) |
|---|---|---|---|---|---|---|
| 1 | 10 | 9.5 | 6.7 | 15.5 | 11.5 | 5.6 |
| 2 | 21 | 16 | 12 | 19 | 17 | 6 |
| 3 | 22 | 15 | 12 | 17 | 14.5 | 6 |
| 4 | 19 | 16 | 11 | 16 | 15 | 6 |
| 5 | 17.5 | 13 | 8.5 | 20 | 13.5 | 6 |
| 6 | 9.5 | 9 | 6 | 13.5 | 12 | 4 |
| 7 | 14 | 12 | 8.5 | 13.5 | 13 | 6 |
| 8 | 9 | 9 | 6.5 | 13 | 10 | 5 |
| 9 | 18 | 15 | 12 | 17 | 16 | 6 |
| 10 | 9 | 9 | 6.5 | 12 | 12 | 4 |
| 11 | 11 | 11 | 6 | 12 | 11 | 4 |
| 12 | 23 | 18 | 11 | 20.5 | 16 | 7 |
| 13 | 21 | 16 | 11 | 20 | 16 | 6.5 |
| 14 | 21 | 18 | 14 | 17.5 | 17 | 7 |
| 15 | 19 | 15 | 11 | 18 | 16 | 6.5 |
| 16 | 15 | 14 | 8 | 14.5 | 13.5 | 6 |
| 17 | 16 | 14 | 8.5 | 19 | 19 | 6.5 |
| 18 | 13 | 11 | 7 | 17 | 17 | 5 |
| 19 | 20 | 15 | 11 | 17 | 16 | 6 |
| 20 | 17 | 16 | 9 | 16 | 15 | 6 |
| 21 | 20 | 15.5 | 10 | 20 | 18 | 5.5 |
| 22 | 13 | 9 | 7.5 | 17 | 17 | 6 |
| 23 | 36 | 27 | 19.5 | 30 | 27 | 12.5 |
| 24 | 24 | 17.5 | 13 | 19 | 18.3 | 9 |
| 25 | 12.5 | 9 | 7 | 16 | 15 | 6.5 |
| 26 | 12.5 | 10.5 | 7 | 14.5 | 14 | 6.5 |
| 27 | 20 | 17 | 10 | 19 | 17 | 17 |
| 28 | 36 | 28 | 18 | 23 | 21 | 8.5 |
| 29 | 15 | 10 | 8 | 17 | 16 | 5.5 |
| 30 | 8 | 8 | 6.5 | 9 | 9 | 6 |
| 31 | 35 | 27 | 18 | 37 | 28.8 | 18 |
| 32 | 32 | 24 | 16.5 | 26 | 22 | 8 |
| 33 | 25 | 22 | 14 | 19 | 20 | 8 |
| 34 | 21 | 17 | 12.5 | 18 | 17 | 7 |
| 35 | 30 | 21 | 18 | 27 | 25 | 7 |
| 36 | 15 | 12 | 9.5 | 16 | 16 | 5 |
| 37 | 30 | 21 | 7 | 22 | 20.5 | 6 |
| 38 | 18 | 13.5 | 11.5 | 17 | 16.5 | 4 |
| 39 | 24 | 18 | 14 | 20.5 | 20.5 | 7 |
| 40 | 24 | 18 | 14 | 20 | 18 | 5 |
| 41 | 35.5 | 26 | 20 | 28.5 | 23 | 7 |
| 42 | 21.5 | 15.5 | 13.5 | 22 | 19.5 | 4.5 |
| 43 | 24.5 | 15.5 | 12.5 | 18.5 | 17 | 5 |
| 44 | 22.5 | 15.5 | 13 | 18 | 17 | 4.5 |
| 45 | 18.5 | 14 | 12 | 17 | 16.5 | 5 |
| 46 | 17.5 | 13 | 11.5 | 14.5 | 14 | 4 |
| 47 | 20 | 14 | 12 | 16.5 | 16.5 | 4.5 |
| 48 | 22 | 16.5 | 12.5 | 22 | 19 | 5 |
Table 4.
Patients’ affected and unaffected upper extremities data.
| Patient | Affected/ unaffected arm length (%) | Affected/ unaffected forearm length (%) | Affected/ unaffected hand length (%) | Affected/ unaffected arm girth (%) | Affected/ unaffected forearm girth (%) | Affected/ unaffected hand width (%) |
|---|---|---|---|---|---|---|
| 1 | 100 | 100 | 97 | 79 | 103 | 89 |
| 2 | 90 | 100 | 100 | 100 | 94 | 100 |
| 3 | 86 | 100 | 100 | 100 | 97 | 92 |
| 4 | 95 | 100 | 86 | 100 | 90 | 100 |
| 5 | 97 | 96 | 100 | 100 | 104 | 100 |
| 6 | 95 | 94 | 100 | 96 | 100 | 100 |
| 7 | 93 | 100 | 100 | 96 | 92 | 100 |
| 8 | 100 | 94 | 100 | 88 | 100 | 100 |
| 9 | 100 | 93 | 100 | 100 | 100 | 92 |
| 10 | 100 | 94 | 100 | 96 | 96 | 100 |
| 11 | 100 | 91 | 100 | 100 | 100 | 100 |
| 12 | 83 | 94 | 95 | 88 | 100 | 93 |
| 13 | 95 | 100 | 95 | 90 | 100 | 92 |
| 14 | 95 | 89 | 86 | 97 | 88 | 100 |
| 15 | 100 | 97 | 100 | 100 | 100 | 100 |
| 16 | 93 | 86 | 100 | 103 | 100 | 92 |
| 17 | 94 | 100 | 100 | 100 | 95 | 96 |
| 18 | 100 | 91 | 107 | 106 | 100 | 100 |
| 19 | 95 | 100 | 91 | 94 | 97 | 92 |
| 20 | 94 | 100 | 94 | 100 | 97 | 92 |
| 21 | 95 | 97 | 90 | 100 | 100 | 100 |
| 22 | 100 | 94 | 100 | 100 | 97 | 100 |
| 23 | 97 | 100 | 100 | 93 | 96 | 92 |
| 24 | 96 | 94 | 119 | 89 | 96 | 89 |
| 25 | 98 | 100 | 100 | 94 | 100 | 100 |
| 26 | 88 | 95 | 100 | 100 | 93 | 100 |
| 27 | 88 | 88 | 100 | 105 | 109 | 88 |
| 28 | 86 | 93 | 94 | 100 | 100 | 94 |
| 29 | 93 | 90 | 88 | 94 | 97 | 100 |
| 30 | 100 | 100 | 100 | 100 | 100 | 100 |
| 31 | 94 | 83 | 83 | 100 | 97 | 100 |
| 32 | 78 | 92 | 91 | 92 | 93 | 125 |
| 33 | 100 | 82 | 93 | 95 | 93 | 100 |
| 34 | 100 | 88 | 100 | 89 | 91 | 93 |
| 35 | 93 | 95 | 94 | 100 | 96 | 79 |
| 36 | 97 | 92 | 95 | 91 | 94 | 90 |
| 37 | 93 | 98 | 86 | 100 | 98 | 92 |
| 38 | 89 | 96 | 96 | 82 | 94 | 75 |
| 39 | 92 | 83 | 86 | 93 | 98 | 86 |
| 40 | 96 | 89 | 96 | 98 | 103 | 90 |
| 41 | 86 | 88 | 90 | 93 | 98 | 86 |
| 42 | 98 | 97 | 96 | 98 | 100 | 100 |
| 43 | 88 | 84 | 96 | 100 | 97 | 90 |
| 44 | 93 | 97 | 96 | 92 | 91 | 100 |
| 45 | 95 | 100 | 100 | 97 | 97 | 80 |
| 46 | 106 | 108 | 104 | 107 | 111 | 100 |
| 47 | 98 | 93 | 96 | 100 | 97 | 100 |
| 48 | 98 | 97 | 100 | 102 | 97 | 100 |
Footnotes
This study was supported in part by grants from the Pediatric Orthopedic Society of North America and the American Society for Surgery of the Hand.
References
- 1.Bae DS, Waters PM, Zurakowski D. Reliability of three classification systems measuring active motion in brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85:1733–8. [DOI] [PubMed]
- 2.Clarke HM, Curtis CG. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11:563–80. [PubMed]
- 3.Greenwald AG, Schute PC, Shiveley JL. Brachial plexus birth palsy: a 10-year report on the incidence and prognosis. J Pediatr Orthop. 1984;4:689–92. [DOI] [PubMed]
- 4.Hardy AE. Birth injuries of the brachial plexus: incidence and prognosis. J Bone Joint Surg Br. 1981;63:98–101. [DOI] [PubMed]
- 5.Mallet J. Primaute du traitement de l’epaule—methode d’expression des resultats. Rev Chir Ortho. 1972;58S:166–8. [PubMed]
- 6.McDaid PJ, Kozin SH, Thoder JJ, et al. Upper extremity limb-length discrepancy in brachial plexus palsy. J Pediatr Orthop Am. 2002;22:364–6. [DOI] [PubMed]
- 7.Michelow BJ, Clarke HM, Curtis CG, et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675–81. [PubMed]
- 8.Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. J Hand Surg Am. 1993;18:278–81. [DOI] [PubMed]
- 9.Waters PM. Obstetric brachial plexus injuries: evaluation and management. J Am Acad Orthop Surg. 1997;5:205–14. [DOI] [PubMed]
- 10.Waters PM. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop Am. 2005;25:116–26. [DOI] [PubMed]
- 11.Waters PM, Bae DS. Brachial plexus birth palsy: rationale for a multi-center prospective study. Semin Plast Surg. 2004;18:377–84. [DOI]
- 12.Waters PM, Van Heest A. Spastic hemiplegia of the upper extremity in children. Hand Clin. 1998;14:119–34. [PubMed]