Abstract
Background. There is little evidence for superior outcome of one dialysis therapy versus another. Still, nephrologists have to prescribe dialysis every day. It is therefore of interest to ascertain the opinion among nephrology professionals regarding which therapy they consider to be the best and to compare this to reality.
Methods. We designed a survey addressing these questions and distributed it at five international dialysis and nephrology congresses during 2007.
Results. Responses were collected from 6595 delegates, 57% physicians and 28% nurses. Peritoneal dialysis (PD) was considered the best initial dialysis therapy for a planned start in a typical patient. The dialysis treatment chosen to be best for long-term use was home/self-care dialysis applied >3 times/week. The best extracorporeal form of dialysis among European respondents was high-volume haemodiafiltration (HDF), while the Asians and Americans gave preference to high-flux haemodialysis (HD). Only 7% preferred low-flux HD. Finally, the respondents were asked what level of evidence they would require to consider one form of dialysis superior to another. The majority wanted hard evidence, i.e. improved survival, to make such a distinction.
Conclusions. The view of nephrology professionals on the value of different dialysis therapies reflects current scientific discussions. They consider PD to be the best initial therapy and frequent application of home/self-care dialysis to be the best long-term therapy. High-flux membranes are strongly preferred for any extracorporeal form of therapy, and HDF seems to be the modality of choice among Europeans. The opinions expressed are far from reality, which we interpret to show that non-medical factors have a strong impact on treatment allocation.
Keywords: frequent dialysis, haemodiafiltration, opinion, peritoneal dialysis, self-care dialysis
Introduction
Since the birth of chronic dialysis treatment almost 50 years ago, significant technological and medical advancements have influenced the way patients are dialyzed, but the basic questions about what and how much to remove from blood and how and how often to do so are still without an answer. Although major studies have been done to provide the missing evidence, the result has so far been inconclusive. Still, nephrologists have to prescribe dialysis for their patients every day, and while on one hand there is little evidence for superior outcome of one dialysis therapy versus another, treatment personalization and specific prescription for selected patients seems to be a key towards improving outcomes.
In a healthcare environment with severe economic limitations, the lack of evidence may favour treatment allocation to be dictated by non-medical factors rather than by the experience of the nephrologist or the needs of the single patient. We therefore decided to undertake an international survey among nephrology professionals to ascertain their view on the best dialysis treatment for different groups of patients and to see how this correlates with the allocation of dialysis therapy today.
Subjects and methods
A survey instrument including six questions was designed and tested for maximum clarity in a pilot survey. The first three questions asked for opinions about the best initial dialysis therapy, the best long-term dialysis therapy and the best extracorporeal dialysis therapy. The fourth question asked for the evidence required to rank dialysis therapies. The answers to these four questions are described and discussed below, while the remaining questions, being of different nature, will be reported elsewhere. Each question had three to four alternative answers and additionally the option ‘no opinion’. Only one answer was allowed per question, and when more than one box was marked, the response was placed in the ‘no opinion’ category. The response alternatives reflected the common versions of dialysis therapies, given in broad categories and defined so as to avoid confusion and exceptions. Peritoneal dialysis (PD) was described as CAPD/APD (continuous ambulatory PD/automated PD). Haemodialysis (HD) was combined with haemodiafiltration (HDF) to comprise all forms of extracorporeal dialysis.
The questionnaires were distributed during 2007, starting at the World Congress of Nephrology in Rio de Janeiro, then at the ERA-EDTA Congress in Barcelona, the EuRoPD meeting in Helsinki, the EDTNA-ERCA Conference in Florence and finally at the ASN Meeting in San Francisco. A small number of questionnaires were also collected at national meetings in France and Australia. Responses were anonymous, but information about country of origin, profession (physician, nurse, administrator or other) and experience of dialysis was requested. All information was compiled and summarized by an independent institute.
Results
We received 6595 responses to our survey (Table 1), with physicians (57%) and Europeans (59%) in majority among the respondents. When asked about their experience of dialysis, 55% of the respondents indicated that they had >10 years’ experience and only 5% gave the answer <1 year.
Table 1.
Origin and profession of respondents to dialysis opinion survey, 2007
| Congress | n | Physicians (%) | Nurses (%) | West Europe (%) | East Europe (%) | Asia (%) | Americas (%) |
|---|---|---|---|---|---|---|---|
| WCN | 1029 | 78 | 7 | 8 | 14 | 10 | 62 |
| ERA-EDTA | 2041 | 78 | 3 | 23 | 39 | 15 | 4 |
| EuRoPD | 772 | 74 | 19 | 45 | 39 | 5 | 3 |
| EDTNA-ERCA | 1634 | 3 | 82 | 54 | 35 | 1 | 1 |
| ASN | 795 | 78 | 3 | 29 | 8 | 16 | 42 |
| Other | 324 | 35 | 65 | 18 | – | 75 | – |
| Total | 6595 | 57 | 28 | 31 | 28 | 13 | 17 |
Best initial dialysis treatment
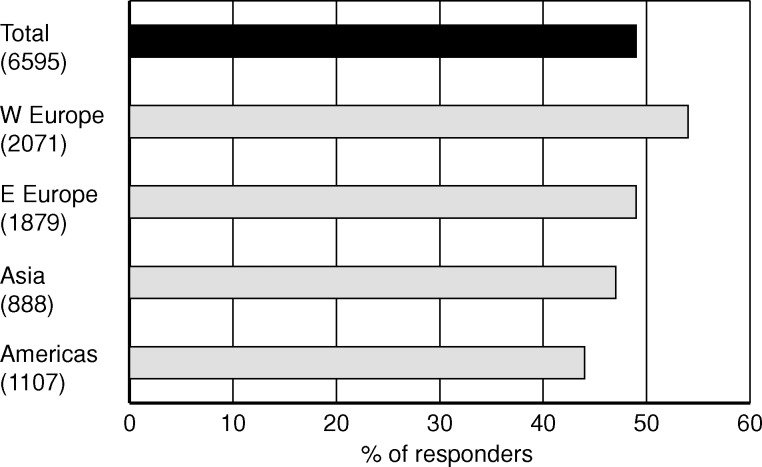
The first question addressed the initial dialysis treatment for a typical patient, aged 65 years with one comorbidity. What was considered to be best for this patient among the alternatives: CAPD/APD, in-centre HD/HDF or home/self-care HD/HDF? With self-care therapies among the options, it was assumed that the selected treatment was the patient's choice. The result shows a majority of 49% for PD to be considered the best dialysis therapy for incident patients under the given conditions (Figure 1). The support for PD was in majority in all regions and it was strongest among European physicians, 60%. Not surprisingly, the preference for PD was exceptionally strong among the participants of the EuRoPD meeting where 91% of the respondents placed PD as the best initial dialysis treatment. The other alternatives received the support of 30% for in-centre dialysis and 17% for self-care dialysis from the total group. The order between the alternatives was the same in all regions and subgroups with exception for the 539 nurses from East Europe who placed in-centre dialysis (51%) before PD (30%).
Fig. 1.
Share of nephrology professionals who chose the answer ‘CAPD/APD’ in response to the question ‘What do you consider to be the best initial dialysis treatment for a patient with planned start, today and in the near future?’
Best long-term dialysis treatment
The next question asked about the best long-term dialysis treatment for the majority of patients. The alternatives were again CAPD/APD, in-centre HD/HDF and home/self-care HD/HDF. For this question, the issue of frequency was introduced and the choice of in-centre treatment had to be given as either 3 times/week or >3 times/week. The choice of home/self-care HD/HDF was clearly indicated as >3 times/week, reflecting a common practice for home HD. Thus, there were four alternatives, two for self-care dialysis and two for in-centre dialysis. There were also two alternatives for frequent dialysis, one at home and one in-centre.
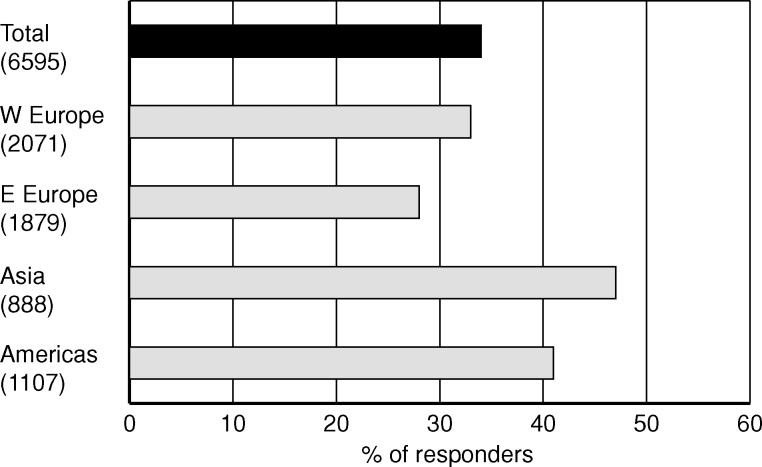
In all regions, frequent application of home/self-care dialysis was considered the best long-term dialysis treatment, and 34% of the total responses favoured this therapy (Figure 2). The responses from Asia stand out with 47% choosing this option. The other three alternatives received similar support of ∼20% each, with a non-significant difference between them.
Fig. 2.
Share of nephrology professionals who chose the answer ‘home/self-care HD/HDF >3 times/week’ in response to the question ‘What do you consider to be the best long-term dialysis treatment for the majority of patients, today and in the near future?’
The combined result shows that 56% of the nephrology professionals expressed that a self-care administered therapy (PD or HD/HDF) is better for the patients than in-centre dialysis. Considering frequency instead, the total result shows that 52% of physicians and nurses working with dialysis had the opinion that HD/HDF administered more frequently than 3 times/week is better for the patients than the current regime of 3 times/week. If we add the numbers favouring PD, 74% of the respondents clearly said that frequent or continuous dialysis is better than 3 times/week.
Best extracorporeal form of dialysis
With ∼90% of dialysis patients worldwide being treated with an extracorporeal therapy, the next question focused on the perceived benefits of the major types of this form of dialysis. To avoid confounding of the answers with biocompatibility issues, all alternatives assumed that membranes were synthetic, dialysis fluid was ultrapure and infusion solution, when required, was on-line prepared. The alternatives were low-flux HD, high-flux HD, high-volume HDF and high-volume haemofiltration (HF).
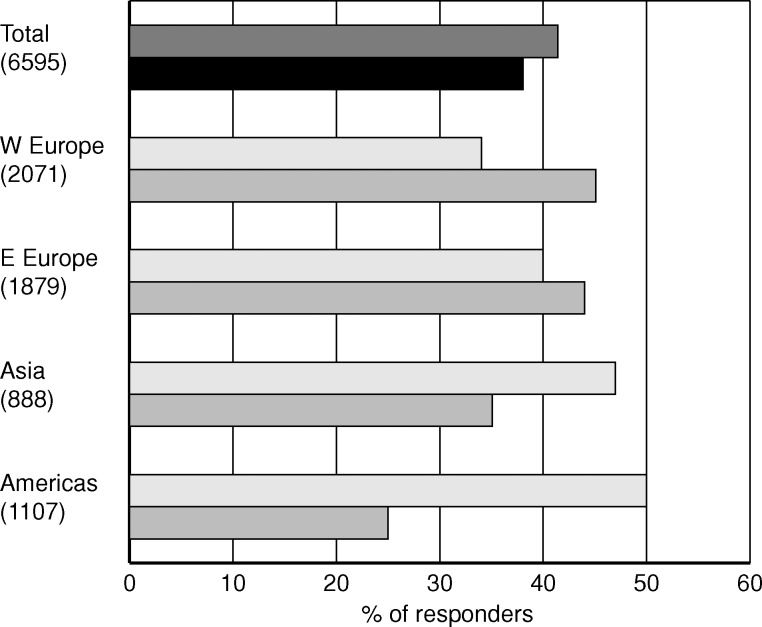
The total result shows that high-flux HD and high-volume HDF were preferred to a similar extent by ∼40% each (Figure 3). HDF was preferred to a greater extent in Europe, but moving eastwards the preference for high-flux HD grew and became dominant in Asia, and in the Americas there was clear differentiation with high-flux HD being named the best by 50%. This trend was more pronounced among physicians than among nurses. The combined support for high-flux HD and HDF was close to 80% in all regions and all subgroups, and this shows an overwhelming belief that dialysis with high-flux membranes is superior to dialysis with low-flux membranes. In fact, only 7% of the total number of respondents indicated low-flux HD as the best option.
Fig. 3.
Share of nephrology professionals who chose the answers ‘high-flux HD’ (grey/white) and ‘high-volume HDF’ (black/light grey) in response to the question ‘What do you consider to be the best extracorporeal form of dialysis?’
Minimum level of evidence
The final question addressed the view of the respondents on the minimum level of evidence they would require to consider one form of therapy superior to another. The alternatives given were to have hard evidence in the form of improved survival or to have surrogate evidence, which could be improvement of markers such as C-reactive protein or left ventricular hypertrophy. It could also be to have so-called soft evidence, i.e. studies showing better quality of life. The assumption was made that all study results should be significant and originate from randomized, controlled studies. The final option was to have own or colleagues’ experience as the base for ranking.
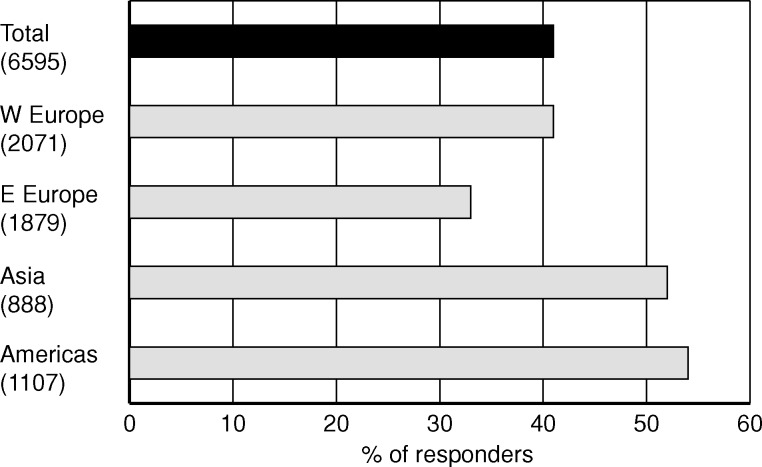
Among the four alternatives, a clear majority responded that they would require hard evidence to consider one form of dialysis superior to another (Figure 4). This was claimed by 41% of the total, and by over 50% in Asia and America. Soft evidence was the choice by 25% of the total and surrogate evidence by only 16%. The ranking of evidence levels was the same in the subgroups.
Fig. 4.
Share of nephrology professionals who chose the answer ‘hard evidence, i.e. improved survival’ in response to the question ‘In order to consider one form of dialysis superior to another, what is the minimum level of evidence you would require?’
Discussion
Today there is no randomized, controlled study of sufficient dimension showing significantly superior survival of a cross-section of patients treated with one dialysis therapy compared to another. Still, this is what a majority of the nephrology community participating in our survey say that they require to consider one therapy better than another. Yet, they have an opinion about what is best for their patients, based on their experience and the evidence available to us today. It is likely that the type of evidence we would like to obtain is almost impossible to achieve because of the lack of financial support, or simply because the design is too difficult and even a randomized controlled trial may not represent ‘real life’. With this in mind, we should consider the opinion of professionals as a tool to identify patterns of practice or ways of thinking and treasure them as important messages coming from everyday experience.
The majority of the respondents in our survey suggest PD as an optimal, initial dialysis treatment. There are many aspects in favour of this view. One of the most often quoted is the superior preservation of residual renal function in PD patients when compared to treatment with conventional haemodialysis [1]. When used for patients on the waiting list for a transplant, PD has been shown to result in a better graft outcome than HD [2]. For many years, registry data from North America have shown that survival on PD is better than on HD during the first 2–3 years of dialysis treatment [3]. Recent data from the USRDS suggest that survival on PD now shows further improvement for periods extending beyond the initial years [4]. Starting patients on PD and transferring them to HD in a timely fashion, so-called integrated therapy, has been shown to lead to a survival advantage in comparison to treatment with only HD [5]. Finally, treatment with PD delays the need for a functional vascular access, the Achilleus’ heel of all extracorporeal therapies. The result of our survey confirms these arguments, but all this is in stark contrast to reality. Global information on the treatment mode for incident dialysis patient is not available, but the USRDS shows that 6% of the new dialysis patients in 2005 were treated with PD. Thus the use of PD and the views on PD are surprisingly different.
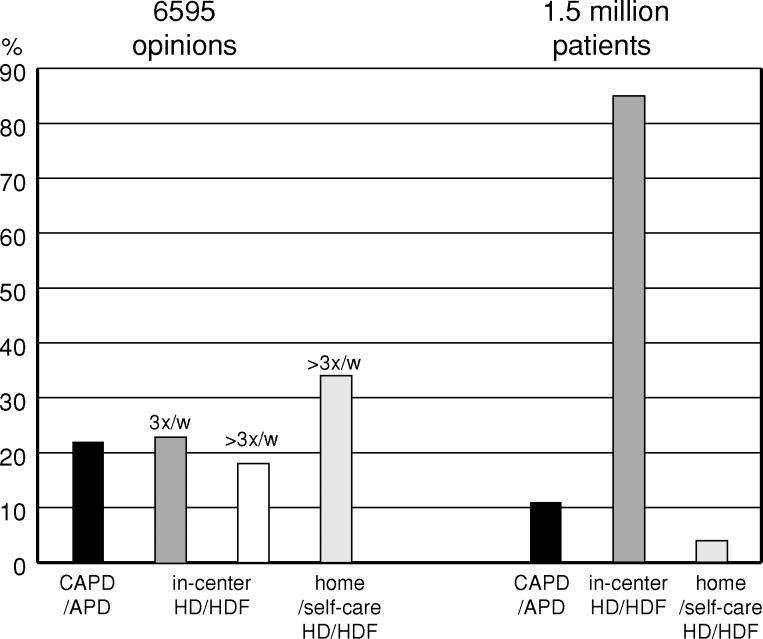
In-centre dialysis, performed 3 times/week, is today used for at least 85% of the prevalent patients worldwide, but only 23% of the 6595 respondents in our survey consider this to be the best long-term dialysis treatment (Figure 5). Instead, self-care dialysis, be it PD or HD, performed at home or in a self-care setting, is indicated as the best form of dialysis treatment for the majority of patients by 56%. In reality, even an optimistic estimation of self-care patients worldwide would give a figure of maximum 15%. A number of reasons can be found to explain this large difference. With a dialysis population of increasing age and comorbidity, the general condition of the patients may prevent them from assuming responsibility for their dialysis treatment. Patient motivation is another key factor of vital importance for the benefits of a self-care therapy to be realized [6]. Then there are all the non-medical factors against self-care therapies, including physician bias and reimbursement disincentives. Ignorance about the practice and value of self-care therapy among nephrology professionals with little or no experience of this treatment form is often quoted as a cause, but the result of our survey does not support that notion. Other surveys have also shown a positive attitude towards self-care therapies among nephrologists. In 2003, Mendelssohn published the results from a series of surveys among nephrologists in Canada, the UK and the USA [7]. When asked to indicate the preferred distribution of treatment modes for optimal quality of life and wellness, the physicians in the different countries recommended self-care therapy for 45–49% of the patients.
Fig. 5.
What is the best long-term dialysis treatment? Opinion versus reality.
During the past 10 years, the interest in self-care HD has increased in parallel with a growing number of positive reports on significant clinical benefits from treatment with frequent dialysis, performed as short sessions during daytime or long nocturnal dialysis, 5–7 times/week [8]. Already today the trips to and from the dialysis unit 3 times/week are a time-consuming burden to patients and with more frequent dialysis the practical disadvantages may offset the therapeutic benefits, unless dialysis can be organized to take place at home. Thus, the realization of more frequent dialysis is closely associated with self-care HD. The positive attitude among nephrology professionals to this form of dialysis therapy is an encouraging signal to healthcare providers and industry to develop programmes and products that enable more patients to benefit from self-care treatment.
Two recent major studies have addressed the issue of high-flux dialysis versus low-flux dialysis in a prospective, randomized, controlled design. The HEMO study found no difference in the primary outcome parameter, all cause mortality, or any of the main secondary outcomes between the groups of 921 patients treated with high-flux membranes and the 925 patients using low-flux membranes [9]. However, they did find that high-flux dialysis resulted in 20% reduced risk of cardiac death, a secondary combined outcome parameter, and 32% reduced mortality risk in the subgroup of patients treated with dialysis for >3.7 years before the start of the study. The MPO study was designed to compensate for some of the shortcomings of the HEMO study by only including incident patients and only allowing single use of filters [10]. Looking only at patients whose albumin levels were ≤4.0 g/dl at the time of enrolment in the study, they found a survival benefit of 37% for the 250 patients treated with high-flux membranes compared to the 243 patients treated with low-flux membranes. No difference in outcome was seen when all 647 study patients were considered. However, among the 150 diabetic patients, there was also a significant survival benefit from treatment with high-flux membranes. The result of the MPO study has been widely reported, but its publication as a full paper is still pending.
High-flux dialysis is HD performed with high-flux membranes. Compared to low-flux dialysis, the increased membrane permeability results in removal of an extended range of solutes and increased volumes of ultrafiltration. Because the excess ultrafiltration is compensated by backfiltration of dialysis fluid, high-flux dialysis comprises a certain amount of convective clearance with additional removal of large solutes. When compared with HDF, we could say that high-flux HD is HDF with a limited amount of convection and without the use of an external substitution solution. Therefore, it is logical to assume that benefits of high-flux dialysis might be extended when moving to HDF, which in the high-volume version provides an additional convective transport, and thus increased removal of larger solutes. This is also supported by observational data from DOPPS where HDF patients treated with exchange volumes exceeding 15 l per session showed significantly better survival than HDF patients having a lower volume of convective removal and patients on high-flux dialysis [11]. Other benefits associated with high-flux dialysis, such as improved blood pressure control, anaemia management and nutritional status and reduced levels of ß2-microglobulin, are all to a greater extent also reported with HDF [12].
Regarding the extracorporeal treatments, there are again large differences between the opinion expressed in our survey and therapies used today. We estimate that one-third of prevalent patients on HD are still treated with low-flux membranes, at least half of them cellulosic, but only 7% rated low-flux dialysis with synthetic membranes as best for the patients. The remaining two-thirds are treated with high-flux membranes, the majority in HD mode.
High-volume HDF requires the use of an on-line prepared substitution solution and is therefore only practiced in countries where this form of sterilization is approved by the authorities or recognized by the community [13]. Most European countries have a growing population of patients treated with on-line HDF and its use is spreading in Australia and parts of Asia [14]. On-line HDF is also starting to be practiced in Canada, but is still not approved in the USA. There are probably close to 50 000 patients on HDF only in Europe, the majority in Western Europe where this therapy is already long-established. The survey result therefore reflects the practical experience of HDF, with the strongest conviction about its benefits in Europe, and this falling as we move eastwards, being replaced by a preference for high-flux HD. Still, the belief in the value of high-flux membranes is equally strong everywhere.
The opinions expressed by the nephrology professionals in our survey closely reflected the ongoing scientific discussion around clinical benefits reported for new therapies and new forms of old therapies. Still, the responders expressed their belief in evidence-based medicine by indicating that a definite ranking of therapies would require evidence of superior survival. However, it is unlikely that we will ever have such evidence for PD versus HD, for in-centre HD versus home HD, for HD 3 times/week versus HD 5–6 times/week or for high-flux HD versus high-volume HDF, considering past and ongoing study efforts. The number of patients and the resources required to conduct adequately powered, prospective, randomized, controlled studies of these issues is probably out of reach for the nephrology community. Instead, we will have to look with critical eyes and statistical rigour at the data available to us [15].
We have not referred to economy once throughout this discussion, although economical issues are certainly among the reasons for reality to be so different from the opinion of the medical professionals about what is best for their patients. However, while economical limitations may explain why synthetic high-flux membranes are not used for all patients, they do not serve this purpose to justify why self-care dialysis, PD as well as HD, is not used for more patients. Furthermore, economy does not explain why the authorities in some countries do not accept controlled, stepwise ultrafiltration for the preparation of infusion solutions for convective therapies, which would allow a more cost-effective and, by many considered superior form of dialysis therapy, high-volume HDF, to be more widely practiced. So non-medical issues, besides economy, appear to play a major role in treatment allocation.
The respondents in this survey were not randomly selected and may therefore not represent an international cross-section of nephrology professionals. They do, however, make up a very large sample of physicians and nurses who attended international congresses in nephrology and dialysis during 2007, and their responses to our questions are based on long experience of dialysis, over 10 years on average. Their opinions closely reflect what is considered best practice in the nephrology community today and this would likely be a guide to how they would treat the majority of their patients provided non-medical factors did not prevent them.
In conclusion, our survey among 6595 international nephrology professionals showed that the majority considers PD to be the best initial dialysis therapy, while frequently applied, self-care dialysis, when possible in a home environment, is selected as the best long-term therapy. The best extracorporeal treatment form is dialysis with a high-flux membrane, applied either in HD or in HDF. These opinions appear to reflect the present view on best clinical practice, but they are far from the clinical reality, where non-medical factors seem to have a strong influence.
Conflict of interest statement. The first author (IL) is a full-time employee of Gambro Lundia AB.
References
- 1.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 2.Goldfarb-Rumyantzev AS, Hurdle JG, Scanding JD, et al. The role of pretransplantation of renal replacement therapy modality in kidney allograft and recipient survival. Am J Kidney Dis. 2005;46:537–549. doi: 10.1053/j.ajkd.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Hao W, Xia H, et al. Mortality risks of peritoneal dialysis and hemodialysis. Am J Kidney Dis. 1999;34:1065–1074. doi: 10.1016/S0272-6386(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 4.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2007 Annual Data Report. Am J Kidney Dis. 2008;51(Suppl 1):S1–S320. doi: 10.1053/j.ajkd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Van Biesen W, Vanholder RC, Veys N, et al. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol. 2000;11:116–125. doi: 10.1681/ASN.V111116. [DOI] [PubMed] [Google Scholar]
- 6.Ledebo I. What limits the expansion of self-care dialysis at home? Hemodialysis Int. 2008;12:S53–S58. doi: 10.1111/j.1542-4758.2008.00298.x. [DOI] [PubMed] [Google Scholar]
- 7.Mendelssohn DC. PD and the future: the role of PD in the overall management of ESRD. Blood Purif. 2003;21:24–28. doi: 10.1159/000067853. [DOI] [PubMed] [Google Scholar]
- 8.Suri RS, Nesrallah GR, Mainra R, et al. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol. 2006;1:33–42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 9.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Hannedouche T, Jacobson S, et al. The effect of membrane permeability on ESRD: design of a prospective, randomized multicentre trial. J Nephrol. 1999;12:85–88. [PubMed] [Google Scholar]
- 11.Canaud B, Bragg-Gresham JL, Marshall MR, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006;69:2087–2093. doi: 10.1038/sj.ki.5000447. [DOI] [PubMed] [Google Scholar]
- 12.Van Den Weerd NC, Penne EL, Van Den Dorpel MA, et al. Haemodiafiltration: promise for the future? Nephrol Dial Transplant. 2008;23:438–443. doi: 10.1093/ndt/gfm791. [DOI] [PubMed] [Google Scholar]
- 13.Ledebo I. On-line preparation of solutions for dialysis: practical aspects versus safety and regulations. J Am Soc Nephrol. 2002;13:S78–S83. [PubMed] [Google Scholar]
- 14.Petrie JJB, Ng TG, Hawley CM. Review article: is it time to embrace haemodiafiltration for centre-based haemodialysis? Nephrol. 2008;13:269–277. doi: 10.1111/j.1440-1797.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 15.Jager KJ, Zoccali C, MacLeod A, et al. Confounding: what is it and how to deal with it. Kidney Int. 2008;73:256–260. doi: 10.1038/sj.ki.5002650. [DOI] [PubMed] [Google Scholar]