SUMMARY
The identity of the viral RNA recognized directly during infection by diverse host innate immune receptors has been under debate. Here we examined the population of virus-derived siRNAs (viRNAs) in Drosophila challenged by Flock house virus (FHV), which are processed from an unidentified viral precursor to guide specific viral immunity. The results show that replication of FHV positive-strand RNA genome produces an approximately 400-bp dsRNA from the 5′-terminus that serves as the major substrate of Dicer-2 for processing into viRNAs. ViRNAs are loaded in Argonaute-2 and the loaded viRNAs are methylated at their 3′-ends. Notably, FHV-encoded RNAi suppressor B2 protein interacts with both viral dsRNA and RNA replicase and inhibits production of the 5′-terminal viRNAs. Our findings therefore provide a cell biology model in which small RNA-directed viral immunity is induced during the initiation of viral progeny (+)RNA synthesis and is suppressed by B2 inside the viral RNA replication complex.
INTRODUCTION
Small RNAs of 21 to 24 nucleotides (nt) long mediate viral immune responses in many eukaryotic hosts (Baulcombe, 2004; Ding and Voinnet, 2007; Waterhouse et al., 2001). The viral immunity pathway in plants and invertebrates overlaps the RNA silencing/RNA interference (RNAi) pathway and begins with production of virus-derived siRNAs (viRNAs) by Dicer nuclease, which then guide specific antiviral silencing by Argonaute protein in an RNA-induced silencing complex (RISC). In mammals, silencing of viral mRNAs by viral and cellular miRNAs also plays a key role in pathogenesis and immunity (Gottwein and Cullen, 2008; Lecellier et al., 2005; Otsuka et al., 2007; Pfeffer et al., 2004; Sullivan et al., 2005; Umbach et al., 2008).
siRNAs are processed by Dicer from perfect dsRNA whereas production of miRNAs involves recognition of structured stem-loop regions in a single-stranded (ss) RNA precursor (Hannon, 2002; Hammond, 2005). In organisms such as plants and C. elegans that encode RNA-directed RNA polymerase (RDR) genes, however, a target ssRNA may be converted first to dsRNA before being diced into siRNAs. Recent genetic studies have shown that the siRNA-producing Dicers, Dicer-2 (DCR2) of Drosophila melanogaster and Dicer-like 2 (DCL2), DCL3 and DCL4 of Arabidopsis thaliana, are required for the biogenesis of viRNAs from several distinct positive-strand (+) RNA viruses (Bouche et al., 2006; Deleris et al., 2006; Diaz-Pendon et al., 2007; Fusaro et al., 2006; Galiana-Arnoux et al., 2006; Wang et al., 2006). A genetic requirement in viral immunity has also been established for several additional genes from the canonical dsRNA-siRNA RNAi pathway that are dispensable for the function of miRNAs in A. thaliana, D. melanogaster and Caenorhabditis elegans (Ding and Voinnet, 2007). A key role for the dsRNA-siRNA pathway in the RNAi-mediated viral immunity implicates viral dsRNA as the trigger of the immunity, which is supported by the cloning and sequencing of approximately equal ratios of (+) and (-) strand viRNAs from two plant (+)RNA viruses and a fungal (+)RNA virus (Ho et al., 2007; Yoo et al., 2004; Zhang et al., 2008b) since (+)RNA viruses accumulate 60-100 fold higher viral (+)RNAs than (-)RNAs in the infected cells. However, the viral RNA synthesis machinery is associated with intracellular membrane structures and it is unclear when and where antiviral Dicer(s) may gain access to the transient dsRNA region of the viral replicative intermediates (vRI-dsRNA) embedded in these membrane structures. Indeed, the molecular nature of the viral RNA precursor of viRNAs is still under debate because viRNAs accumulated in plants infected with four distinct plant (+)RNA viruses as well as two subviral pathogenic ssRNAs, correspond predominantly to the polarity of the genomic RNA (Du et al., 2007; Ho et al., 2007; Itaya et al., 2007; Molnar et al., 2005). In addition, host genes implicated in antiviral silencing include cellular RDR genes that control dsRNA synthesis from ssRNA targets and others that have dual function in both miRNA and siRNA pathways (Ding and Voinnet, 2007).
RNAi-mediated viral immunity fails to restrict productive infection when the genome of pathogenic viruses encodes a protein capable of suppressing RNAi, designated viral suppressor of RNAi (VSR). Diverse plant and animal viruses including those with a genome of (+)RNA, (-)RNA, dsRNA, ssDNA or dsDNA, have been shown to encode VSRs. VSRs of viruses from different families generally share no sequence or structural similarity even if they may exhibit a similar biochemical function (Li and Ding, 2006). Use of well-defined transgene silencing models in plants together with studies in vitro has identified many distinct mechanisms of VSRs. These include inhibition of dicing and siRNA assembly into RISC by sequestering dsRNA and siRNA, respectively, direct targeting of Argonaute protein (AGO) by protein-protein interaction, and suppression of RNA silencing spread (Ding and Voinnet, 2007; Mlotshwa et al., 2008). Less is known about how VSRs suppress antiviral silencing induced by their cognate viruses during the course of infection. Most of the plant VSRs were known to enhance virus accumulation in the inoculated single cells (protoplasts), promote cell-to-cell virus movement in the inoculated leaves or facilitate the phloem-dependent long distance virus spread prior to their identification as VSRs (Diaz and Ding, 2008). Recent studies began to address how various VSR activities might account for their known roles in the distinct steps during host infection. For example, use of VSR-deficient viral mutants and A. thaliana mutants defective in specific RNA silencing pathway components has shed new light on how VSRs facilitate long distance virus spread in plants (Deleris et al., 2006; Diaz-Pendon et al., 2007).
Flock house virus (FHV) and Nodamura virus (NoV), both natural pathogens of insects, are members of the Nodaviridae, contain a 4.5 kb bipartite (+)RNA genome and have been used as a model to study RNA replication (Venter and Schneemann, 2008). RNA2 encodes CP precursor and RNA1 encodes B2 and the viral RNA-dependent RNA polymerase (RdRP), protein A, the only viral protein needed for RNA replication. Thus, RNA1 replicates in absence of RNA2 whereas RNA2 replication is RNA1-dependent. Unlike protein A and CP that use the genomic RNAs as mRNA, B2 is translated from RNA3, which is a subgenomic RNA templated by RNA1. FHV infection of Drosophila cells induces the formation of membranous vesicles called “spherules” on the outer mitochondrial membrane in which protein A is localized and RNA replication occurs (Kopek et al., 2007; Venter and Schneemann, 2008). Infection of Drosophila with FHV induces the RNAi immunity in the DCR2/AGO2 canonical RNAi pathway and requires expression of the VSR B2 (Galiana-Arnoux et al., 2006; Li et al., 2002; Li et al., 2004; Lu et al., 2005; van Rij et al., 2006; Wang et al., 2006). Studies in vitro indicate a dual function for B2, which forms an all α-helix homodimer that binds to both long dsRNA and siRNA to inhibit siRNA production and siRNA assembly into RISC, respectively (Chao et al., 2005; Lu et al., 2005; Sullivan and Ganem, 2005).
Here we describe a strongly biased production of an approximately equal ratio of (+) and (-) viRNAs targeting the 5′-terminal region of the genomic RNA in Drosophila cells abortively infected with a B2-deficient mutant of FHV, indicating that initiation of the progeny (+)RNA synthesis triggers processing of the 5′-terminal nascent vRI-dsRNA into viRNAs. In cells successfully infected with FHV, B2 acts as a structural component of the viral RNA replication complex to inhibit production of viRNAs, including the 5′-terminal viRNAs, even though B2 is dispensable for RNA replication. Our findings thus establish a model on the induction and suppression of the small RNA-directed viral immunity in Drosophila during the course of infection.
RESULTS
B2 inhibits DCR2-dependent production of viral siRNAs
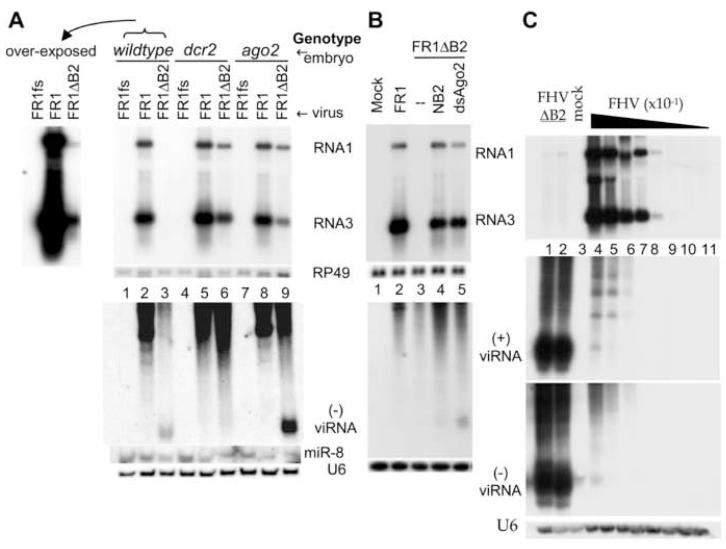
We have previously developed a protocol for examining the self replication of FHV RNA1 in Drosophila embryos by microinjection of full-length FHV RNA1 (FR1) synthesized in vitro(Wang et al., 2006). In this system, B2 plays a specific role in the suppression of antiviral RNAi in the DCR2-AGO2 RNAi pathway because unlike FR1, FR1ΔB2, carrying substitutions that abolish B2 translation, replicated to readily detectable levels in dcr-2L811fsX and ago-2414 mutant embryos, but accumulated to extremely low levels in wildtype embryos (Wang et al., 2006). We scaled up embryo microinjection in this study to analyze the effect of B2 on the production of viRNAs during FHV RNA replication, which was not investigated in previous studies (Galiana-Arnoux et al., 2006; Li et al., 2002; van Rij et al., 2006; Wang et al., 2006; Zambon et al., 2006).
Abundant accumulation of viRNAs was detected in ago-2414 embryos injected with FR1ΔB2, but viRNAs were not detectable in dcr-2L811fsX embryos injected with FR1ΔB2 (Figure 1A, lower panel, compare lanes 6 and 9), although replication of FR1ΔB2 was as robust in dcr-2L811fsX embryos as in ago-2414 embryos (Figure 1A, upper panel, compare lanes 6 and 9). viRNAs were also detected in FR1ΔB2-injected wildtype embryos albeit at a markedly reduced level compared to that in ago-2414 embryos (Figure 1A, lower panel, compare lanes 3 and 9), despite of the fact that FR1ΔB2 replicated to extremely low levels in wildtype embryos and detection of FR1ΔB2 replication required overexposure as compared to that in either ago-2414 or dcr-2L811fsX embryos (Figure 1A). Thus, these data indicate an essential role of DCR2 in the biogenesis of viRNAs. In contrast, neither AGO2 nor DCR1 may contribute significantly to viRNA biogenesis since viRNAs were undetectable in dcr-2L811fsX embryos in which DCR1 is wildtype and production of miRNAs is not affected (Lee et al., 2004) and abundant viRNAs were detected in ago-2414 embryos. Notably, we found that despite of the robust viral RNA replication in embryos of all the three genotypes following FR1 injection, viRNAs were below the level of detection not only in dcr-2L811fsX embryos but also in both the wildtype and ago-2414 embryos (Figure 1A, lower and upper panels, lanes 2, 5 and 8). Thus, expression of B2 during viral RNA replication strongly inhibited the DCR2-dependent production of viRNAs in Drosophila embryos.
Figure 1.
DCR2-dependent production of viRNA is inhibited by B2. (A, B) Accumulation of viral RNA1 and RNA3 (upper panel) as well as viRNAs (lower panel) 48 hours after micro-injection into embryos (A) or transcriptional induction in S2 cells (B) of FHV RNA1 (FR1), B2-deficient RNA1 (FR1ΔB2) or replication-defective FR1fs, which contains a frameshift mutation in the viral RdRP gene. Top left of panel A shows an overexposure of lanes 1-3. In panel B, pFR1ΔB2 was co-transfected with an additional plasmid encoding B2 of NoV (lane 4), or dsRNA targeting AGO2 (lane 5) or LacZ (lane 3). viRNAs were detected by eleven 40-nt oligos hybridizing to the (-)-strand of the coding region for ORF B2. (C) Accumulation of viral RNA1 and RNA3 as well as (+) and (-) viRNAs in S2 cells 72 hours after inoculation with virions of a B2-deficient FHV mutant (FHVΔB2, lanes 1-2) or FHV in a series of 10-fold dilutions (lanes 4-11). The same samples were analyzed for (+) and (-) viRNAs in two identical gels by using oligo probes targeting the (+) and (-)-strand of the 5′-terminal region of RNA1. Equal loading was monitored by probing for RP49 or U6.
We next examined the production of FHV siRNAs in cultured Drosophila cells transfected by a plasmid that directs an inducible transcription of either FR1 (pFR1) or FR1ΔB2 (pFR1ΔB2) using a Schneider 2 cell line not contaminated with FHV or Drosophila C virus (Li et al., 2002). As shown previously (Li et al., 2002; Li et al., 2004), FR1ΔB2 does not replicate to detectable levels in S2 cells (Figure 1B, upper panel, lane 3) unless antiviral RNAi is suppressed by co-transfection with either AGO2 dsRNA (dsAgo2) to deplete AGO2 or a plasmid directing expression of B2 (Figure 1B, upper panel, lanes 4 and 5). Production of viRNAs was detected in S2 cells co-transfected with pFR1ΔB2 and dsAgo2 in which viral RNA replication occurred in the absence of B2 (Figure 1B, lower panel, lane 5). However, viRNAs was undetectable following viral RNA replication in the presence of B2 in S2 cells transfected with pFR1 (Figure 1B, lower panel, lane 2). In addition, replication of FR1ΔB2 in S2 cells that co-expressed B2 of Nodamura virus (NoV) from a plasmid produced much less viRNAs as compared to the rescue of FR1ΔB2 by AGO2 depletion (Figure 1B, lower panel, compare lanes 4 and 5). Thus, as found in embryos, expression of B2 during viral RNA replication in S2 cells also markedly reduced the production of viRNAs. Without interference of B2 in viRNA production, the expected correlation between viral RNA replication and viRNA production was observed: higher levels of FR1ΔB2 replication in ago-2414 embryos and AGO2-depleted S2 cells resulted in with higher levels of viRNA accumulation as compared to that in wildtype embryos and S2 cells (Figure 1, compare lanes 3 and 9 of Figure 1A and lanes 3 and 5 of Figure 1B).
We further examined the production of FHV siRNAs in Drosophila S2 cells infected directly with virions of either the cloned isolate of FHV or its B2-deficinet mutant, FHVΔB2. As expected, FHVΔB2 replicated to extremely low levels in the infected cells in the absence of viral suppression of the RNAi immunity, as compared to those in cells infected with much diluted wild type FHV inoculum (Figure 1C, upper panel, compare lanes 1 and 2 with lanes 4-8). However, extremely abundant accumulation of viRNAs was detected in FHVΔB2-infected cells whereas little viRNAs were produced during the high level viral RNA replication in the presence of B2 (Figure 1C, lower panels, compare lanes 1 and 2 with lanes 4-8). The ratio of viRNAs to the genomic RNAs was at least 100-fold higher in FHVΔB2-infected cells than that in FHV-infected cells, indicating that aborted infection of FHVΔB2 was associated with highly abundant production of viRNAs. Thus, B2 also inhibited viRNA production in FHV-infected Drosophila cells, consistent with the results obtained from self replication of FHV RNA1 in absence of RNA2.
B2 expression markedly reduced, but did not eliminate, the production of viRNAs in S2 cells because viRNAs were detected in S2 cells infected with wildtype FHV (Figure 1C, lower panels, lanes 4-5) and inoculation with a higher multiplication of infection yielded more abundant accumulation of viRNAs (eg, Figure 1A in Li et al., 2002). However, viRNAs became inactive in antiviral silencing and unable to inhibit the robust replication of FHV (Figure 1C, lane 4), which directed expression of B2 that binds to viral siRNAs (see below, supplemental Figure 3B), in contrast to potent antiviral silencing directed by low levels of viRNAs when B2 was not expressed (Figure 1A, lane 3). This indicates that B2 suppresses the antiviral activity of viRNAs in addition to inhibiting siRNA production, as suggested by previous in vitro studies using synthetic dsRNA and siRNAs (Chao et al., 2005; Lu et al., 2005; Sullivan and Ganem, 2005).
viRNAs are loaded in AGO2 and methylated at their 3′-ends
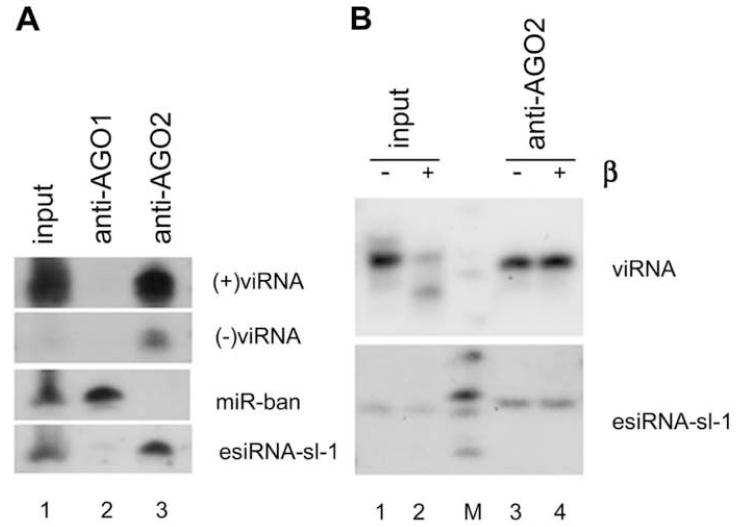
We noted that FR1ΔB2 replicated to high levels in both ago-2414 embryos and AGO2-depleted S2 cells in despite of the abundant accumulation of viRNAs, but a much lower level of viRNAs was sufficient to direct viral clearance in either wildtype embryos or S2 cells where AGO2 was active (Figure 1, compare lanes 3 and 9 of 1A and lanes 3 and 5 of 1B). Thus, AGO2 is required for the antiviral activity of viRNAs whereas AGO1 does not appear to play a role in the viral immunity in the absence of AGO2. In support of this view, we found that viRNAs of both polarities were co-immunoprecipitated from FHVΔB2-infected S2 cells by a monoclonal anti-AGO2 antibody (Figure 2A, lane 3), but not by a monoclonal anti-AGO1 antibody (Figure 2A, lane 2). As expected from previous studies (Kawamura et al., 2008; Siomi et al., 2008), our control experiments showed that immunoprecipitated AGO1, but not AGO2, was associated with miRNA-bantam (miR-ban) (Figure 2A, lanes 2 and 3); similarly, immunoprecipitated AGO2, but not AGO1, was associated with the endogenous (endo) siRNA, esiRNA-sl-1 (Figure 2, lanes 2 and 3).
Figure 2.
Characterization of viRNAs in the infected cells. (A) viRNAs are associated with AGO2 but not AGO1 in infected Drosophila cells. AGO1 and AGO2 were immunoprecipitated respectively by specific antibodies four days after inoculation with FHVΔB2 virions and bound small RNAs were analyzed by Northern blot hybridizations using probes specific to (+) and (-) viRNAs, miR-ban and esiRNA-sl-1. (B) viRNAs loaded in AGO2 were resistant to peridate oxidation and beta elimination treatments (β) whereas viRNAs in the input before immunoprecipitation were partially sensitive. The samples used were identical to lanes 1 and 3 of (A).
Drosophila miRNAs loaded in AGO1 show sensitivity to peridate oxidation and beta elimination treatments. In contrast, PIW-interacting RNAs (piRNAs) and endosiRNAs are resistant to these treatments because these small RNAs loaded in PIWI and AGO2 proteins, respectively, are methylated at their 3′-ends by the Drosophila orthologue of the A. thaliana HEN1 (Siomi et al., 2008; Yu et al., 2005). We found that viRNAs co- immunoprecipitated with AGO2 was resistant to peridate oxidation and beta elimination treatments as was esiRNA-sl-1, whereas viRNAs in the input prior to coimmunoprecipitation was partially sensitive to the treatments (Figure 2B, compare lanes 2 and 4). As expected, miR-ban co-immunoprecipitated with AGO1 was sensitive to peridate oxidation and beta elimination treatments (data not shown). These findings indicate that viRNAs loaded in AGO2 are methylated at their 3′-ends whereas a portion of viRNAs in the input are unmethylated at the 3′-ends. Thus, it is likely that the unmethylated viRNAs are free floating since no viRNA was co-immunoprecipitated with AGO1.
Profiling viRNAs produced during infection
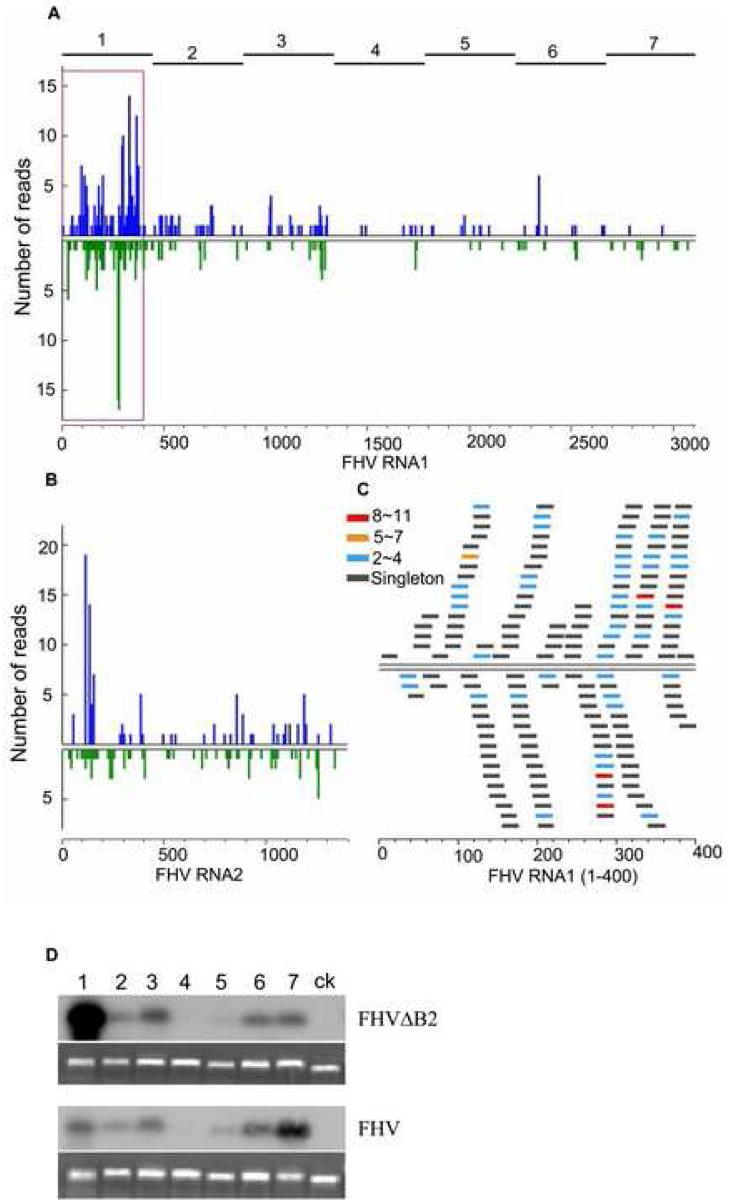
To profile viRNAs produced in infected cells without the interference of B2, we next sequenced the small RNAs from Drosophila cells infected with FHVΔB2. The small RNA library made from S2 cells four days after infection with FHVΔB2 virions was deposited into one of the four regions of a Pico Titer Plate for pyrosequencing. In total, 4371 small RNAs of 18-28 nucleotides in length were obtained. These include 106 known Drosophila miRNAs (2.4%) and 1177 FHV-specific small RNAs (27%) if one nucleotide mismatch was allowed (Supplemental Table 1). We considered only the 834 small RNAs that are 100% identical or complementary to the bipartite RNA genome of FHV (Genbank Accession No. NC_004146 and NC_004144) representing approximately 20% of the total small RNAs sequenced (Figure 3A/3B), as FHVΔB2 virions used were derived from the cloned FHV isolate (Li et al., 2002). Many FHV small RNAs were sequenced 2-4 times (Figure 3C) and most (89%) were 20 to 22 nucleotides long with a major peak at 21 nucleotides (60.3%) (Supplemental Figure 1A). The length distribution of the sequenced FHV small RNAs was similar to that of the cloned siRNAs processed in vitro from synthetic dsRNA by Drosophila embryo Dicer extracts and the recently sequenced Drosophila endogenous siRNAs (Elbashir et al., 2001; Siomi et al., 2008). Thus, we conclude that the sequenced FHV small RNAs corresponded to the viRNAs detected by Northern hybridizations.
Figure 3.
Examining the population of viRNAs produced in Drosophila cells. (A, B) Profiles of viRNAs cloned by the 5′-ligation independent method from S2 cells abortively infected with FHVΔB2. Number of viRNA reads was plotted to the positive-(top) and negative- (bottom) strand of RNA1 (A) and RNA2 (B) with 5-nt windows. (C) A close-up view of the distribution and abundance of (+) and (-) viRNAs in the 5′-terminal 400-nt region of RNA1. 264 reads were from this region including 153 (+)viRNAs (58%, top) and 111 (-)viRNAs (42%, bottom). Counts of distinct viRNAs are shown by color-coded bars. (D) Relative abundance of viRNAs targeting the 7 evenly divided regions of RNA1 (shown on top of each lane and of the graph in panel A) produced in S2 cells infected with FHV (bottom panel) or FHVΔB2 (top panel). Equal amount (300 ng) of the seven FHV RNA1-specific DNA fragments and a control LacZ fragment was fractionated and hybridized to the labeled total 20- to 24-nt viRNAs gel purified from approximately 300 μg of total RNA extracted from S2 cells 4 days after inoculation with virions of FHV or FHVΔB2. Equal loading was shown by staining with ethidium bromide.
The sequenced viRNAs from FHVΔB2-infected Drosophila cells exhibited three notable features. First, 479 and 356 viRNAs were mapped to the 3.1-kb long RNA1 and the 1.4-kb long RNA2, respectively. Thus, the density of viRNAs is higher for RNA2 (254 viRNAs/kb) than RNA1 (154 viRNAs/kb). It is possible that this reflects a higher copy number for both the (+) and (-) strands of RNA2 than RNA1 in the FHV-infected cells (Kopek et al., 2007).
Second, 57% and 43% of the sequenced viRNAs were mapped to the (+) and the (-) strands of either RNA1 or RNA2, respectively (Figure 3A). Since the genomic RNAs of FHV accumulate to approximately 100-fold higher in the infected cells than the antigenomic RNAs, (Kopek et al., 2007), presence of an approximately equal ratio of (+) and (-) viRNAs in the small RNA library indicates that vRI-dsRNA, rather than structured regions of viral (+) and (-) ssRNAs, serves as the substrate of DCR2.
Third, we observed an incomplete bias in the positions of viRNAs mapped on the viral genomic and antigenomic RNAs. Strikingly, more than 60% of the sequenced RNA1-specific viRNAs were clustered in the 5′-terminal region of about 400 nucleotides long (Figure 3A; supplemental Figure 1C). 58% and 42% of these 5′-terminal viRNAs corresponded to the genomic and antigenomic RNA1, respectively (Figure 3C), similar to the ratio found for the total viRNAs sequenced. The (+)viRNAs targeting the 5′-terminal 200-nt region of RNA2 were also very abundant; in contrast to RNA1, however, the (-) viRNAs were distributed more uniformly along the entire genomic RNA2 (Figure 3B; supplemental Figure 1C).
We next focused on the RNA1-specific viRNAs because RNA1 self-replicates unlike RNA2, which depends on RNA1 for replication in an undefined manner. We examined the distribution pattern of RNA1-specific viRNAs in Drosophila cells infected by FHVΔB2 by blot hybridizations using an established protocol (Szittya et al., 2002). Briefly, DNA fragments representing the seven evenly divided regions of RNA1 (445 nt except the 3′-terminal fragment which is 8 nt shorter; Figure 3A/3D) were synthesized by PCR, and equal amounts of each DNA fragment were probed with the 32P-labeled 20- to 24-nt small RNAs isolated from Drosophila cells abortively infected with FHVΔB2 virions. The results showed firstly an extremely low density of viRNAs targeting the middle region of RNA1, which was supported by the pyrosequencing results (Figure 3A/3D, fragment 4). Secondly, the 5′-terminal fragment (fragment 1) produced by far the strongest signal among the 7 fragments of RNA1 (Figure 3D, upper panel with the number above each lane corresponding to one of the seven regions of RNA1 indicated at the top of Figure 3A). This indicates that a large proportion of viRNAs produced in FHVΔB2-infected cells corresponded to the 5′-terminal region of RNA1, which is thus in agreement with the results from pyrosequencing (Figure 3A). Like all of the (+)RNA viruses, FHV accumulates approximately 100-fold higher viral (+)RNAs than (-)RNAs in the infected cells by multiple initiation of the progeny (+)RNA synthesis on the 3′-terminus of the antigenomic RNA template (Kopek et al., 2007).Thus, the strong biased production of an approximately equal ratio (+) and (-) viRNAs targeting the 5′-terminal 400-nt region of RNA1 further identifies the dsRNA region of the viral replicative intermediates (vRI-dsRNA) between the nascent, 5′-terminal region of the progeny (+)RNA1 and the 3′-terminal region of the (-)RNA1 template formed during initiation of the progeny (+)RNA synthesis as the predominant source of viRNAs.
Probing the same panel of RNA1-specific DNA fragments with small RNAs isolated from FHV-infected cells (Figure 3D, lower panel) revealed two major differences in viRNA production to that observed in FHVΔB2-infected cells. First, viRNAs corresponding to the 5′-terminal fragment of RNA1 were not more abundant than viRNAs corresponding to fragments 2, 3, 6 and 7 of RNA1 (Figure 3D, lower panel), in contrast to the strong bias for the 5′-terminal viRNAs in FHVΔB2-infected cells. This indicates that the massive production of viRNAs targeting the nascent vRI-dsRNA during initiation of the progeny (+)RNA synthesis was inhibited by B2 in FHV-infected cells. Second, we reproducibly observed a modestly increased production of viRNAs corresponding to the 3′-terminal fragment of RNA1 as compared to the other fragments of RNA1 in FHV-infected cells (Figure 3D, lower panel). By contrast, the 3′-terminal viRNAs were of a similar abundance to viRNAs corresponding to fragments 2, 3 and 6 of RNA1 in FHVΔB2-infected cells (Figure 3D, upper panel). As the 437-nt fragment 7 includes the 3′-terminal region of 387 nucleotides identical in sequence to RNA3, these the 3′-terminal viRNAs could be triggered by the synthesis of either RNA3 templated by (-)RNA1 or (-)RNA1 from the incoming (+)RNA1, which was not effectively blocked by B2.
B2 interacts with viral duplex RNAs in infected Drosophila cells
One interpretation for the observed suppression of 5′-terminal viRNA production by B2 is that B2 is located in close proximity to the nascent vRI-dsRNA during RNA replication. Both FHV and NoV B2 proteins (FB2 and NB2) bind long dsRNA and siRNA in vitro and inhibit both in vitro processing of long dsRNA into siRNAs and RNAi induced by siRNA (Chao et al., 2005; Lu et al., 2005; Sullivan and Ganem, 2005). FB2 and NB2 mutants containing Gln substitution at the conserved Arg (R→Q; Supplemental Figure 2) were defective in RNA binding and in the suppression of both dicing in vitro and antiviral RNAi in the infected S2 cells (Lu et al., 2005; Supplemental Figure 2), indicating that nodoviral B2 acts by sequestering dsRNA. By comparing these in vitro and in vivo activities, we found the single R→Q substitution was more effective in eliminating the VSR activity of NB2 than that of FB2 (Lu et al., 2005; Supplemental Figure 2).
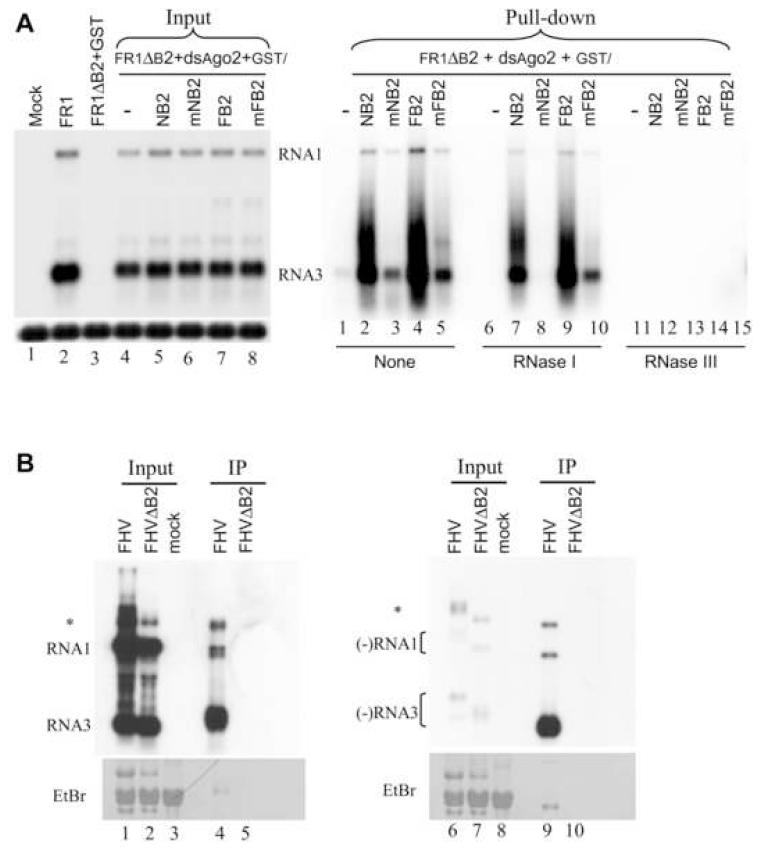
We next performed GST pull-down assay to investigate if B2 interacts with vRI-dsRNA and viRNAs during viral RNA replication. In this assay, GST-tagged wildtype and mutant B2 proteins were expressed during replication of FR1ΔB2 in S2 cells treated with AGO2 dsRNA. Depletion of AGO2 ensured similarly robust replication of the VSR-deficient FR1ΔB2 (Figure 4A, lanes 5-8 of left panel) as only two of the four fusion proteins examined (GST-FB2 and GST-NB2) were active VSRs even through Western blot analysis revealed similar expression levels for all fusion proteins (Supplemental Figure 3A). Lysates obtained two days after initiation of viral RNA replication were incubated with glutathione beads and RNAs bound to the beads were fractionated in polyacrylamide and agarose gels for Northern blot detection of small and large RNAs, respectively. Both (+) and (-)-strand viRNAs of approximately 21 nt, but not host miRNAs, were pulled down and markedly enriched by GST-NB2, but not by GST-mNB2 (Supplemental Figure 3B). This indicates that B2 complexes with viRNAs produced during viral replication in Drosophila cells.
Figure 4.
Detection of B2-dsRNA complexes in infected Drosophila cells. (A) Northern blot analysis of viral RNAs in S2 cells before (input lanes of left panel) and after (right panel) GST pulldown. S2 cells were co-transfected with pFR1ΔB2 and dsRNA of AGO2 plus a plasmid expressing GST alone (lane 1 of left panel and lanes 1, 6 and 11 of right panel), GST-tagged wildtype (lane 5 and 7 of left panel and lanes 2, 4, 7, 9, 12 and 14 of right panel) or (R→Q) mutant B2 (lane 6 and 8 of left panel and lanes 3, 5, 8, 10, 13 and 15 of right panel) of FHV and NoV. 5% of total RNA before GST pulldown was analyzed in the left panel. Right panel shows the RNA pulled down by GST with treatment of RNase I (lanes 6-10), RNase III (lanes 11-15) or without RNase treatment (lanes 1-5). (B) Northern blot analysis of viral RNAs in mock-infected S2 cells and S2 cells infected with virions of FHV or FHVΔB2 before (input) and after coimmunoprecipitation (IP) with the antibody to B2 of FHV. S2 cells were pre-treated with dsRNA of AGO2 before inoculation with FHVΔB2 virions, which is essential to ensure successful infection. The strand-specific probes recognized (+) and (-)-strand of FHV RNA1 and RNA3, respectively, each contained eleven 5′-labeled, 40-nt single-strand DNA oligos hybridizing to the (+) and (-)-strand of the ORF B2 coding region. The RNA species that migrated at the positions of FHV RNA1 and RNA3 were marked. Asterisk (*) indicates an RNA species that may correspond to the homodimer of RNA1 implicated in FHV replication. Methylene blue staining of the filter was shown at the bottom.
Northern blot hybridizations also detected large FHV-specific RNA molecules pulled down by both GST-NB2 and GST-FB2 (Figure 4A, lanes 2 and 4 of right panel). The two major RNA species in complex with B2 migrated approximately at the positions of FHV RNA 1 and RNA3, respectively. Two lines of evidence indicate that these viral RNA species pulled down by B2 were double-stranded. First, the B2-bound RNA species were completely degraded by treatment with the dsRNA-specific RNase III (Figure 4A, lanes 11-15 of right panel), but not by treatment with RNase I (Figure 4A, lanes 7-10 of right panel), which degrades ssRNA only. Second, much less viral RNAs were pulled down with either GST-mFB2 or GST-mNB2 (Figure 4A, lanes 3 and 5 of right panel), both of which were defective in dsRNA binding in vitro. In particular, the RNA species bound by NB2 were RNase I-resistant but RNase I treatment completely degraded the residual RNA species bound by mNB2 (Figure 4A, compare lanes 1 and 8 of right panel), which was defective in binding to dsRNA in vitro (Supplemental Figure 2C, lanes 3, 4, 7, and 8).
We next performed co-immunoprecipitation experiments with polyclonal antibody raised against GST-FB2 with lysates prepared from S2 cells 12 hours post infection with virions of either FHV or FHVΔB2. Total RNA extracted both from the lysates before co-immunoprecipitation (input) and from the co-immunoprecipitated complex (IP) was fractionated in two identical agarose gels, which were blotted and probed for positive and negative strands of FHV RNAs, respectively (Figure 4B). The two major RNA species that migrated approximately at the positions of FHV RNAs 1 and 3 were detected in the complex co-immunoprecipitated by the B2 antibody from the lysates of FHV-infected cells, but not from the lysates of FHVΔB2-infected cells (Figure 4B, compare lanes 4 and 9 with lanes 5 and 10). FHV replication produces approximately 100-fold more positive-strand RNAs than negative-strand RNAs (Kopek et al., 2007). However, in contrast to the positive-strand viral RNAs, the negative-strand viral RNAs were selectively enriched in the co-immunoprecipitated complex as compared to the extremely low level of the negative-strand viral RNAs present in the input (Figure 4B, lanes 1 and 2). These results indicate that in the infected cell B2 associates with viral dsRNA rather than viral (+) and (-)ssRNAs that were annealed subsequently into dsRNA during RNA extraction.
B2 interacts with the viral replicase in vivo
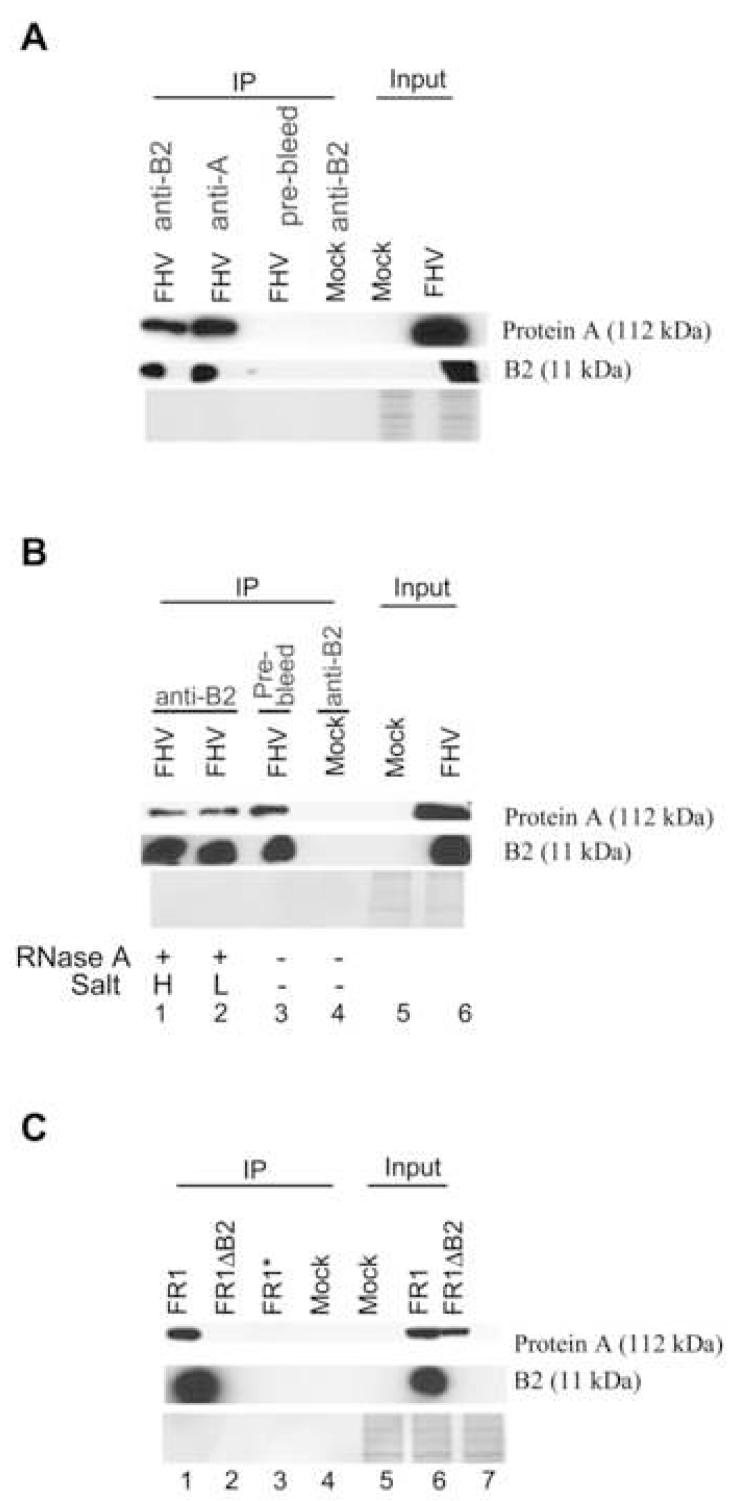
Our observation that B2 complexes with the extremely low abundant viral RNA replication intermediates in infected cells suggests that B2 might interact directly with protein A, the FHV-encoded RdRP. To test this idea, complexes were coimmunoprecipitated from the lysates of S2 cells 12 hours post infection with FHV virion by the antibody to either B2 or protein A before Western blotting analysis. Both B2 and protein A were detected in the complexes precipitated by either the B2 antibody (Figure 5A, lane 1) or the protein A antibody (Figure 5A, lane 2). However, neither protein A nor B2 was detectable in the complex precipitated from the FHV-infected cells by the pre-immune antibody (Figure 5A, lane 3) or in the complex precipitated from the mockinfected cells by the B2 antibody (Figure 5A, lane 4). These data suggest that at least a fraction of B2 is in a specific complex with the viral replicase. To investigate if B2 binds to protein A directly or indirectly via the viral RNAs, the protein A-B2 complex precipitated by the B2 antibody was subjected to treatments of RNase A in low and high salt concentrations before fractionation by denaturing polyacrylamide gel electrophoresis (Figure 5B). RNase A degrades both dsRNA and ssRNA in low salt concentration but digests ssRNA specifically in high salt concentration (Tacken et al., 2002). We found that RNase A treatment at either salt condition did not obviously disrupt the protein A-B2 complex (Figure 5A, compare lane 3 with lanes 1 and 2), indicating a direct binding of protein A by B2.
Figure 5.
Specific interaction of B2 and viral RdRP in vivo. (A, B) Coimmunoprecipitation of B2 and protein A of FHV in FHV-infected cells. Total crude protein extracts (Input) prepared 12 hours after mock inoculation or inoculation with FHV virions were immunoprecipitated (IP) with polyclonal antibody to B2 or protein A. A pre-immune antibody was used as a negative control. Western blots were analyzed with the same antibodies to detect coimmunoprecipitated proteins. The proteins coimmunoprecipitated by the B2 antibody were treated with RNase A under high (H) or low salt concentrations (L) before fractionation and Western blot analysis (lanes 1-2 of panel B). (C) Interaction of B2 and protein A in S2 cells in which RNA1 self-replicates in absence of RNA2. Total crude protein extracts (Input) prepared in S2 cells 48 hours after induction of viral RNA replication were immunoprecipitated (IP) with either polyclonal antibody to B2 (lanes 1 and 2) or the pre-immune antibody (lane 3). Mocktransfected S2 cells were used as a negative control (lane 4). As described for Figure 1B, AGO2 was depleted by dsRNA in S2 cells transfected with pFR1ΔB2 to ensure robust replication of FR1ΔB2 (lane 2).
We next investigated if B2 interacted with protein A during RNA1 selfreplication in absence of RNA2 and the capsid protein encoded by RNA2. Lysates of S2 cells 48 hours after replication of FR1 or FR1ΔB2 were immunoprecipitated by the B2 antibody. Protein A was detected in cells transfected with either pFR1 or pFR1ΔB2 whereas B2 was detected only in cells transfected with pFR1 but not with pFR1ΔB2 (Figure 5C, lanes 6 and 7). Also as expected, no viral proteins were detected in the complex precipitated by the pre-immune antibody from pFR1-transfected cells (Figure 5C, lane 3). However, Western blot analysis revealed the presence of both protein A and B2 in the complex precipitated by the B2 antibody from pFR1-transfected cells but not from pFR1ΔB2-transfected cells (Figure 5C, lanes 1and 2), indicating a specific in vivo interaction between B2 and protein A in the absence of viral coat protein.
DISCUSSION
Mechanism of induction of the small RNA-directed viral immunity
How the RNAi-mediated viral immunity is induced during the course of infection is poorly understood in both plants and invertebrate animals. This is at least in part because of the frequent use of wild-type viruses for infection that express VSRs to interfere with the induction and/or the antiviral activity of the RNAi immunity. We demonstrate in this study that following FHV challenge of Drosophila in the absence of its cognate B2, viRNAs are produced by DCR2, but DCR1 plays an undetectable role. Coimmunoprecipitation with monoclonal AGO1 and AGO2 antibodies showed that viRNAs are loaded in AGO2 whereas an association of viRNAs with AGO1 is undetectable. Loaded viRNAs are methylated at their 3′-ends, but there are unmethylated viRNAs in the infected cells, in contrast to Drosophila endo-siRNAs that are resistant to peridate oxidation and beta elimination treatments (Siomi et al., 2008). Since the Drosophila HEN1 does not methylate duplex small RNAs (Siomi et al., 2008), unmethylated viRNAs presented in the infected cells may be double-stranded possibly due to saturation of viRNA loading into AGO2. Similarly, the abundant viRNAs detected in ago-2414 embryos may be double-stranded and methylated. Pyrosequencing of viRNAs in Drosophila cells infected with FHVΔB2 further revealed an approximately equal ratio of (+) and (-) viRNAs targeting both RNA1 and RNA2. This finding identified the vRI-dsRNA as the viral precursor of viRNAs for the first time in invertebrates because (+)-and (-)-strand viral genomic RNAs accumulate asymmetrically in the infected cells. These results together show that the small RNA-directed viral immunity in Drosophila is mediated by a viRNA pathway that overlaps both the canonical dsRNA-siRNA pathway and the recently identified endo-siRNA pathway but that may not directly involve either DCR1 or AGO1 from the miRNA pathway (Hammond, 2005; Siomi et al, 2008). This conclusion is consistent with previous genetic studies that implicate the canonical RNAi pathway in antiviral silencing in several invertebrate animal species including D. melanogaster (Galiana-Arnoux et al., 2006; Keene et al., 2004; Li et al., 2002; Li et al., 2004; van Rij et al., 2006; Wang et al., 2006).
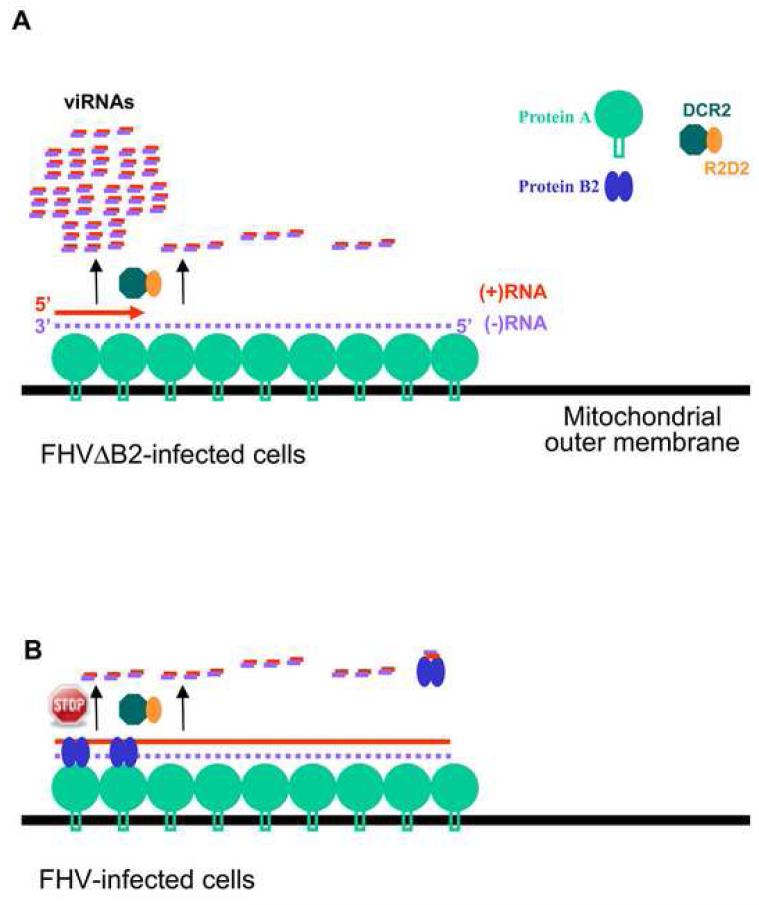
In addition to triggering antiviral RNAi in plants and invertebrates, vRI-dsRNA has been proposed as a pathogen-associated molecular pattern detected by several pattern recognition receptors such as Toll-like receptors and RIG-I-like helicases in mammals (Ishii et al., 2008). As predicted from the replication cycle of a ssRNA genome, detection of dsRNA of at least 40-bp length in the infected cells has been reported for a number of plant and animal (+)RNA viruses (Ahlquist, 2006; Weber et al., 2006). However, (+)RNA viruses may produce vRI-dsRNA during the synthesis of either the (-)RNA template from the incoming (+)RNA genome or the viral progeny (+)RNA from the (-)RNA template as well as during transcription of subgenomic RNAs from the (-)RNA template of some (+)RNA viruses. It is unknown if vRI-dsRNA synthesized from a particular step of viral RNA replication is involved in the induction of any of these dsRNA-specific innate immune responses. Examination of the viRNA population by pyrosequencing and gel blot hybridizations indicates that initiation of the FHV progeny (+)RNA1 synthesis on the 3′-terminal region of the (-)RNA template produces vRI-dsRNA molecules that are approximately 400-nt in length and are accessible to DCR2 for dicing into viRNAs. Our findings support a model for FHV and perhaps other (+)RNA viruses (Figure 6A) in which the RNAi-mediated viral immunity is triggered during the initiation of the progeny (+)RNA synthesis, which is necessary to yield the 100-fold higher viral progeny (+)RNA than (-)RNA template. We propose that dicing of the initiating vRI-dsRNA during the progeny (+)RNA synthesis is inhibitory to viral replication and that this first wave of 5′-terminal viRNAs may play a more important role in the abortive infection of Drosophila cells by FHVΔB2 than the low abundant viRNAs targeting the remaining region of RNA1.
Figure 6.
Model for the induction (A) and suppression (B) of the Drosophila RNAi immunity by FHV. Asymmetric RNA synthesis in the replication of (+)RNA viruses involves multiple initiation of the progeny (+)RNA synthesis on the low abundant (-)RNA template complexed with the viral RdRP and other host factors. The resulting dsRNA of approximate 400-nt in length formed between the 5′-terminal nascent progeny (+)RNA1 and the (-)RNA1 template in FHV-infected cells, termed the initiating vRI-dsRNA, serves as substrates of DCR2. This results in the predominant production of 5′-terminal viRNAs, thereby triggering the RNAi-mediated viral immunity and abortive infection by FHVΔB2. In addition to binding to viRNAs, B2 is part of the viral RNA replication complex by direct interactions with viral RdRP (protein A) and vRI-dsRNA and inhibits DCR2-dependent production of viRNAs, thus ensuring successful infection by FHV. We propose that sequestering the initiating vRI-dsRNA and inhibiting their processing into the 5′-terminal viRNAs by B2 play a particularly important role in the suppression of the viral immunity.
Current models envision that prior to RNA replication, protein A recruits RNA1 to the outer mitochondrial membranes, where FHV RNA replication complexes form inside spherules by self-interaction and membrane targeting of protein A (Kopek et al., 2007; Venter and Schneemann, 2008). Our observation that the initiating vRI-dsRNA during the synthesis of progeny (+)RNA1 is diced into viRNAs suggests that DCR2 is in a close proximity to the site of viral RNA replication. We did not observe any obvious phasing in the production of viRNAs including the 5′-terminal viRNAs. Thus, DCR2 may initiate siRNA processing from multiple positions of the initiating vRI-dsRNA, unlike the phased production of siRNAs from a defined end of dsRNA precursors (Chapman and Carrington, 2007). At present, it is unknown why there is an abrupt drop in viRNA abundance beyond the 5′-terminal 400 nucleotides of RNA1 and why the density of viRNAs targeting the rest of RNA1, the middle region in particular, is very low. We propose that there might be polarity of FHV RNA1 synthesis in the viral replication complex so that vRI-dsRNA formed during the initiation of RNA synthesis is much more readily accessible to DCR2 for dicing than the internal region. Dicing of the 5′-terminal initiating vRI-dsRNA by DCR2 would also inhibit the elongation of nascent vRI-dsRNA to the rest of RNA1, further reducing the abundance of viRNAs beyond the 5′-terminal 400 nucleotides of RNA1. Consistent with this hypothesis, increased production of the 3′-terminal viRNAs of RNA1 was detected in FHV-infected cells possibly because the dicing of the 5′-terminal initiating vRI-dsRNA was suppressed by B2. Compared to RNA1, RNA2 has a higher density of viRNAs than RNA1 and the accumulation of the 5′-terminal (+) and (-)viRNAs of RNA2 is asymmetrical. It is unknown if either reflects a difference in their stability or in the biogenesis influenced by the mode of RNA2 replication, which is RNA1-dependent and exhibits complex regulatory features distinct to RNA1 (Venter and Schneemann, 2008).
Mechanism of viral suppression of the small RNA-directed viral immunity
Little is known about the cell biology of viral suppression of the small RNA-directed viral immunity during the course of infection (Ding and Voinnet, 2007). B2 is among several VSRs without which the cognate viruses are unable to replicate to readily detectable levels in single cells. Previous in vitro studies using synthetic RNAs have indicated that B2 suppresses both dicing and slicing by binding and sequestering long dsRNA and siRNA, respectively (Chao et al., 2005; Lu et al., 2005; Sullivan and Ganem, 2005). GST pulldown experiments showed that B2 binds to viRNAs in the infected cells. Thus, viRNA sequestering by B2, perhaps in a non-membrane-bound cytoplasmic fraction, may indeed play a role in the suppression of antiviral silencing (Figure 6B) as suggested previously for B2 and demonstrated for the tombusviral p19 (Vargason et al., 2003). Notably, results of this study explain why B2 gains access to the low abundant vRI-dsRNA located inside the viral RNA replication complex. Both GST pulldown experiments and co-immunoprecipitation with the B2 antibody demonstrate that B2 complexes with the vRI-dsRNA in Drosophila cells infected with FHV. B2 also interacts with the viral protein A in a manner that depends on neither binding to dsRNA nor the presence of capsid protein. We also showed that B2 expressed from its cognate virus inhibits the production of viRNAs in Drosophila cells following either RNA1 selfreplication or infection with FHV virions.
Based on these findings, we propose that although non-essential for RNA replication, B2 is a structural component of the viral RNA replication complex via interactions with both vRI-dsRNA and the viral RdRP, thereby inhibiting the dicing of of the initiating vRI-dsRNA into viRNAs (Figure 6B). In this regard, the tobamoviral VSR p126 is similar to B2 since p126 is part of the viral RNA replication complex but unlike its readthrough product p183, it is not absolutely required for viral replication (Ding et al., 2004; Komoda et al., 2003; Komoda et al., 2007; Lewandowski and Dawson, 2000). Moreover, we found that expression of B2 in FHV-infected cells inhibits the production of the abundant 5′-terminal viRNAs of RNA1, which is consistent with the model that B2 suppresses production of viRNAs in close proximity to the RNA replication complex. It is of interest to note that suppression of the dicing of the 5′-terminal initiating vRI-dsRNA of RNA1 by B2 was accompanied with a modest increase in the production of the 3′-terminal viRNAs (Figure 3D), which could be processed from vRI-dsRNA formed during the initiation of either (-)RNA1 synthesis from (+)RNA or the synthesis of RNA3 templated by RNA1. Thus, B2 does not appear to inhibit the production of viRNAs targeting the 3′-terminal region of RNA1, raising an intriguing possibility that B2 may be absent at the site where the 3′-terminal viRNAs are produced (Figure 6B).
EXPERIMENTAL PROCEDURES
Cell Culture
Drosophila S2 cell culture, plasmid DNA and dsRNA transfection, microinjection of in vitro transcripts from viral RNAs to Drosophila embryos, infection of S2 cells by FHV virions and purification of FHV virions were performed as described (Wang et al., 2006).
Plasmids
Plasmids pMT-FR1, pMT-FR1ΔB2, pMT-NR1, pMT-NR1ΔB2 were as described (Li et al., 2002; 2004). pMT-mFB2 and pMT-mNB2 were generated by introducing a point mutation by PCR substituting Arg at position 54 (FB2) or 59 (NB2) to Glu. The mutant B2 coding sequences carrying the point mutation (TCGA to TCAA for FHV and TCGG to TCAG for NoV with Arg encoded by the underlined codon whereas the 1st triplet encodes Ser of protein A) were used to replace the corresponding regions in pMT-FR1and pMT-NR1 to yield FR1mB2 and NR1mB2, respectively, without altering the sequence of protein A in the -1 reading frame. GST-B2 fusion protein expression constructs pMT-GST-FB2, pMT-GST-mFB2, pMT-GST-NB2 and pMT-GST-mNB2 were made by fusing the GST coding sequence to the 5′ end of the coding sequences of wildtype and mutant B2 proteins. All plasmids were verified by sequencing in the core facility of the UCR Institute for Integrative Genome Biology.
Construction of Small RNA Library, 454 Sequencing and Sequence Analysis
We used a previously described protocol for cloning small RNAs (Sunkar and Zhu, 2004) with minor modifications in this study. Total RNA from Drosophila S2 cells 4 days post inoculation with FHVΔB2 virions, which were amplified in S2 cells pre-treated with dsRNA of AGO2, was extracted using Trizol reagent (Invitrogen). One mg total RNA was mixed with equal volume of 4M LiCl and the mixture was kept at -20°C overnight. After centrifugation at 13,000 rpm for 10 min, the small RNA in the supernatant was precipitated by ethanol and fractionated by denaturing (8M urea) 15% polyacrylamide gel electrophoresis (PAGE). The gel slice containing small RNAs of 18 to 28 nucleotides long was excised and small RNAs were eluted with 0.3 M NaCl at 4°C overnight before phenol/chloroform extraction and ethanol precipitation. The small RNA was dephosphorylated before ligation to the 3′ linker. After the addition of the 5′ linker, the small RNAs were amplified by reverse transcription — PCR and the DNA products of about 100 bp were run on a Bioanalyzer (Agilent, Santa Clara, California, USA) to determine quality and quantity. The sample was diluted to 2 × 105 molecules/μl. For emulsion PCR, 2.5 μl sample was added to a reaction mix plus 450,000 capture breads. This ratio favors getting a single copy of library per capture bead in the emulsified reaction. The resulting products were recovered by breaking emulsions and then enriching for beads containing amplified products. Approximately 40,000 enriched beads were deposited into one region of a Pico Titer Plate and run on a Genome Sequencer FLX (Roche, Basel, Switzerland).
The sequences of FHV RNA1 and RNA2 (Genbank Accession No. NC_004146 and NC_004144) were downloaded from NCBI. The adaptor sequences were masked with cross_match, and only these reads that contain both the 5′ and 3′ adaptors were kept. After removing the adaptor sequences, the reads (18-28 nt in length) were mapped to either RNA1 or RNA2 with BLASTN. Only reads that are 100% identical or complementary to FHV RNA1 or RNA2 were kept for further analyses. All other analyses were carried out with in-house scripts.
Northern Blot Analysis
RNA extraction and enrichment of small RNAs by LiCl were as described above, and Northern blot hybridizations to detect high and low molecular weight RNAs was done as described previously (Guo and Ding, 2002; Li et al., 2002; Li et al., 2004). To detect (+) and (-)-strand viRNAs, two sets (11 per set) of complementary DNA oligos of 40 nt long each that target the B2 coding region and two sets (13 per set) of DNA oligos of 40 nt long that target the 5′-terminal 400-nt of RNA1 were used. Based on the sequenced viRNAs, four DNA oligos complementary to nucleotide 106-126, 327-347, 363-383 and 369-389 of FHV RNA1 were synthesized and used as probes for the detection of 5′-terminal (+)viRNAs.
DNA Blot Analysis
The protocol reported by Szittya et al. 2002 was modified for analyzing the relative abundance of viRNAs targeting seven evenly divided regions of RNA1 (445 nt except fragment 7 that was 437 nt). The seven DNA fragments and one segment of LacZ gene (467 nt) used as negative control were amplified by PCR and 300 ng of each fragment was run on the 1.2% agrose gel. After denaturing and neutralization by soaking, the DNA was blotted and hybridized to the small RNAs isolated from infected S2 cells. The small RNAs of 20-24 nt size range were isolated as described above from S2 cells 3 days post-inoculation with FHV or FHVΔB2 virions, dephosphorylated, labeled by γ-32P-ATP, and used for hybridization.
GST pull-down assay
S2 cells were co-transfected with pMT-FR1ΔB2, dsRNA of AGO2 and a plasmids expressing one of the following proteins: FB2, mFB2, NB2 or mNB2. Cells were collected three days after transfection and were subjected to pulldown assay as described in http://www.bioprotocol.com with some modifications. Briefly, cells were lysed in NETN (20 mM Tris-HCl, pH 7.5, 0.05% Nonidet P-40, 1 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF) with 1 M NaCl followed by sonication (50% pulsar, 15 seconds for 6 times and 30 seconds interval). Cellular extracts were incubated with Glutathione Sepharose 4B Beads (GSA4B, Amersham, Sweden) for 1 hour on an orbital shaker at 4°C. The pulled down complexes were collected through centrifugation at 1000g for 1 minute, washed successively with NETN containing decreasing concentrations of NaCl, and eluted by the elution buffer (4mM MgCl2 , 300mM KCl, 20 mM HEPES-KOH, pH 7.6, 0.05% Nonidet P-40, 1 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF). The pulled down complexes were treated with proteinase K at 65°C for 20 minutes. The RNAs in complexes were extracted by phenol/chloroform extraction and ethanol precipitation for the detection of viRNAs. For detecting the high molecular weight RNAs in the pulled down complexes, 1/3 of the RNAs obtained was treated with RNase I or RNase III (NEB) before they were subjected to Northern blot analysis to detect high or low molecular weight viral RNAs.
Antibodies
Rabbit polyclonal antisera against FHV protein A (RdRP) and the monoclonal anti-AGO1 and anti-AGO2 antibodies were generous gifts from Dr. Paul Ahlquist (Miller et al., 2001) and Mikiko C. Siomi (Miyoshi et al., 2005), respectively. The immunogen used to generate the polyclonal antisera against FHV B2 protein in rabbits was a fusion protein of GST and FB2 purified using GSA4B beads according to manufacturer’s instructions. Polyclonal antibody against GST was from Santa Cruz Biotechnology.
Co-immunoprecipitation
5×107 S2 cells grown in 100 mm dish were infected with FHV virions at a multiplicity of infection (MOI) 3, collected at 12 h post-inoculation (hpi), and lysed in the IP buffer containing 25 mM Tris-HCl, pH 7.5, 150mM NaCl, 1.5 mM MgCl2, 1% NP40, 1 mM DTT and the protease inhibitor cocktail (Roche, CA). For detecting B2-RdRP complexes, the lysates from mock-inoculated cells or FHV-infected cells were cleared by centrifugation for 15 minutes at 20,000g at 4°C and incubated with the pre-immune antibody, or rabbit polyclonal antibody against FB2-GST or FHV RdRP overnight on an orbital shaker. The immunocomplexes were captured by incubating the lysates with protein A agarose (Upstate, CA) for additional 4 h. The agarose beads were collected and washed four times with ice-cold IP buffer. The immune complexes bound to the agarose beads were subjected to RNase A treatment as described (Tacken et al., 2002) with minor modifications. Briefly, the immunocomplexes precipitated by the B2 antibody were treated with 700 μg/ml RNase A at 37°C for 45 minutes in 10 mM Tris-HCl (pH 7.5) containing either 10 mM of MgCl2 to degrade both ssRNA and dsRNA or 100 mM of MgCl2 to degrade ssRNA only (Tacken et al., 2002). The agarose beads were washed three times with IP buffer before the immunocomplexes eluted and analyzed by Western blotting. Fractionation of B2 proteins was carried out in 15% SDS-PAGE and of protein A (RdRP) in 7.5% SDS-PAGE. Co-IP was carried out similarly from S2 cells mocktransfected, S2 cells transfected with pFR1 or pFR1ΔB2 using either the pre-immune antibody or FB2-GST antibody.
To detect B2-dsRNA complexes, lysates were prepared from S2 cells 12 hpi with FHV. To use as controls, S2 cells were mock-inoculated and S2 cells pre-treated with dsRNA of AGO2 (dsAgo2) were also infected with FHVΔB2 virions. Immunocomplexes were co-immunoprecipitated with the rabbit polyclonal antibody to FB2-GST as mentioned above and collected as described (Niranjanakumari et al., 2002) with some modifications. Briefly, beads were washed successively with high (1M NaCl) and low (250mM NaCl) salt IP buffer (25 mM Tirs-HCl, pH 7.5, 1% NP-40, 0.1% SDS, 1mM EDTA, 0.2 mM PMSF) and the immunocomplexes bound to beads were eluted in 100 ul of elution buffer (25 mM Tirs-HCl, pH 7.5, 5 mM EDTA, 10mM DTT and 1%SDS). RNA was extracted and subjected to Northern blot analysis as described above.
Supplementary Material
Acknowledgement
We wish to thank Paul Ahlquist for the antibody to protein A, Mikiko Siomi for the antibodies to AGO1 and AGO2, and ALN Rao for a crude FHVΔB2 virion preparation used in the early studies. This project was supported by NIH grant AI052447 (to SD).
Footnotes
Accession Number
The GEO accession number for the viral small RNAs from this study is GSM306489
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahlquist P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat Rev Microbiol. 2006;4:371–382. doi: 10.1038/nrmicro1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Bouche N, Lauressergues D, Gasciolli V, Vaucheret H. An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J. 2006;25:3347–3356. doi: 10.1038/sj.emboj.7601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman EL, Carrington JC. Specialization and evolution of endogenous small RNA pathways. Nat Rev Gene. 2007;7:844–96. doi: 10.1038/nrg2179. [DOI] [PubMed] [Google Scholar]
- Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat Struct Mol Biol. 2005;12:952–957. doi: 10.1038/nsmb1005. [DOI] [PubMed] [Google Scholar]
- Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O. Hierarchical Action and Inhibition of Plant Dicer-Like Proteins in Antiviral Defense. Science. 2006;313:68–71. doi: 10.1126/science.1128214. [DOI] [PubMed] [Google Scholar]
- Diaz-Pendon JA, Li F, Li WX, Ding SW. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell. 2007;19:2053–2063. doi: 10.1105/tpc.106.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pendon JA, Ding SW. Direct and indirect roles of viral suppressors of RNA silencing in pathogenesis. Ann Rev Phytopath. 2008;46:303–326. doi: 10.1146/annurev.phyto.46.081407.104746. [DOI] [PubMed] [Google Scholar]
- Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XS, Liu J, Cheng NH, Folimonov A, Hou YM, Bao Y, Katagi C, Carter SA, Nelson RS. The Tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Mol Plant Microbe Interact. 2004;17:583–592. doi: 10.1094/MPMI.2004.17.6.583. [DOI] [PubMed] [Google Scholar]
- Du QS, Duan CG, Zhang ZH, Fang YY, Fang RX, Xie Q, Guo HS. DCL4 targets Cucumber mosaic virus satellite RNA at novel secondary structures. J Virol. 2007;81:9142–9151. doi: 10.1128/JVI.02885-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes & Development. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro AF, Matthew L, Smith NA, Curtin SJ, Dedic-Hagan J, Ellacott GA, Watson JM, Wang MB, Brosnan C, Carroll BJ, Waterhouse PM. RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep. 2006;7:1168–1175. doi: 10.1038/sj.embor.7400837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe. 2008;3:375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HS, Ding SW. A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J. 2002;21:398–407. doi: 10.1093/emboj/21.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM. Dicing and Slicing: The core machinery of the RNA interference pathway. FEBS Lett. 2005 doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Ho T, Wang H, Pallett D, Dalmay T. Evidence for targeting common siRNA hotspots and GC preference by plant Dicer-like proteins. FEBS Lett. 2007;581:3267–3272. doi: 10.1016/j.febslet.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Itaya A, Zhong X, Bundschuh R, Qi Y, Wang Y, Takeda R, Harris AR, Molina C, Nelson RS, Ding B. A structured viroid RNA serves as a substrate for dicer-like cleavage to produce biologically active small RNAs but is resistant to RNA-induced silencing complex-mediated degradation. J Virol. 2007;81:2980–2994. doi: 10.1128/JVI.02339-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Saito K, Kin T, Ono Y, Asai K, Sunohara T, Okada TN, Siomi MC, Siomi H. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–797. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- Komoda K, Mawatari N, Hagiwara-Komoda Y, Naito S, Ishikawa M. Identification of a ribonucleoprotein intermediate of tomato mosaic virus RNA replication complex formation. J Virol. 2007;81:2584–2591. doi: 10.1128/JVI.01921-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopek BG, Perkins G, Miller DJ, Ellisman MH, Ahlquist P. Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol. 2007;5:e220. doi: 10.1371/journal.pbio.0050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Tsuda S, Tamai A, Meshi T. Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J Virol. 2003;77:11016–11026. doi: 10.1128/JVI.77.20.11016-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- Lewandowski DJ, Dawson WO. Functions of the 126- and 183-kDa proteins of tobacco mosaic virus. Virology. 2000;271:90–98. doi: 10.1006/viro.2000.0313. [DOI] [PubMed] [Google Scholar]
- Li F, Ding SW. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu Rev Microbiol. 2006;60:503–531. doi: 10.1146/annurev.micro.60.080805.142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HW, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- Li WX, Li H, Lu R, Li F, Dus M, Atkinson P, Brydon EW, Johnson KL, Garcia-Sastre A, Ball LA, et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc Natl Acad Sci U S A. 2004;101:1350–1355. doi: 10.1073/pnas.0308308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingel A, Simon B, Izaurralde E, Sattler M. The structure of the flock house virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition. EMBO Rep. 2005;6:1149–1155. doi: 10.1038/sj.embor.7400583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Maduro M, Li F, Li HW, Broitman-Maduro G, Li WX, Ding SW. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature. 2005;436:1040–1043. doi: 10.1038/nature03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC. Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 2005;19:2837–2848. doi: 10.1101/gad.1370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlotshwa S, Pruss G, Vance V. Small RNAs in viral infection and host defense. Trends Plant Sci. 2008;13:375–82. doi: 10.1016/j.tplants.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Molnar A, Csorba T, Lakatos L, Varallyay E, Lacomme C, Burgyan J. Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J Virol. 2005;79:7812–7818. doi: 10.1128/JVI.79.12.7812-7818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjanakumari S, Lasda E, Brazas R, Garcia-Blanco MA. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26:182–190. doi: 10.1016/S1046-2023(02)00021-X. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Kang YJ, Jiang Z, Du X, Cook R, et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Siomi MC, Saito K, Siomi H. How selfish retrotransposons are silenced in Drosophila germline and somatic cells. FEBS Lett. 2008;582:2473–8. doi: 10.1016/j.febslet.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Sullivan CS, Ganem D. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J Virol. 2005;79:7371–7379. doi: 10.1128/JVI.79.12.7371-7379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–686. doi: 10.1038/nature03576. [DOI] [PubMed] [Google Scholar]
- Sunkar R, Zhu JK. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–783. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargason JM, Szittya G, Burgyan J, Hall T. M. Tanaka. Size selective recognition of siRNA by an RNA silencing suppressor. Cell. 2003;115:799–811. doi: 10.1016/s0092-8674(03)00984-x. [DOI] [PubMed] [Google Scholar]
- Venter PA, Schneemann A.Recent insights into the biology and biomedical applications of Flock House virus Cell Mol Life Sci 2008 2008Jun2 [Epub ahead of print] 10.1007/s00018-008-8037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Aliyari R, Li WX, Li HW, Kim K, Carthew R, Atkinson P, Ding SW. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse PM, Wang MB, Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411:834–842. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BC, Kragler F, Varkonyi-Gasic E, Haywood V, Archer-Evans S, Lee YM, Lough TJ, Lucas WJ. A systemic small RNA signaling system in plants. Plant Cell. 2004;16:1979–2000. doi: 10.1105/tpc.104.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–5. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Segers GC, Sun Q, Deng F, Nuss DL. Characterization of hypovirus-derived small RNAs generated in the chestnut blight fungus by an inducible DCL-2-dependent pathway. J Virol. 2008;82:2613–2619. doi: 10.1128/JVI.02324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.